Significant outcomes
-
Patients with coronary heart disease (CHD) and comorbid congestive heart failure (CHF) show significantly lower levels of brain-derived neurotrophic factor (BDNF) than patients with CHD without comorbid CHF, also after controlling for potential confounders and depression.
-
BDNF was significantly reduced in depressed patients with CHD compared to non-depressed patients with CHD, but this association was not significant after controlling for somatic comorbidity, platelet count, smoking status, sex, age and antidepressant treatment. We therefore suggest to carefully report and control for possible relevant confounders while investigating BDNF and depression in future research.
Limitations
-
The relatively small sample size (N = 225), the cross-sectional study design and limited generalisability to patients with CHD limit the results of the present study.
Introduction
Coronary heart disease (CHD) and depression are leading contributors to the global burden of disease (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018; Rehm & Shield, Reference Rehm and Shield2019). The two diseases are marked by a high comorbidity (Rudisch & Nemeroff, Reference Rudisch and Nemeroff2003), a worse medical prognosis for patients suffering from both diseases (Meijer et al., Reference Meijer, Conradi, Bos, Thombs, van Melle and de Jonge2011), and a dose–effect relationship between severity of depression and cardiac prognosis, such as long-term cardiac mortality (Lespérance et al., Reference Lespérance, Frasure-Smith, Talajic and Bourassa2002; Whooley et al., Reference Whooley, de Jonge, Vittinghoff, Otte, Moos, Carney, Ali, Dowray, Na, Feldman, Schiller and Browner2008). Prevalence rates for major depression in patients with CHD vary from 10 to 27%, depending on the type of depression assessment and CHD severity (Rudisch & Nemeroff, Reference Rudisch and Nemeroff2003). A similar picture exists for the association between congestive heart failure (CHF) and depression (Mbakwem et al., Reference Mbakwem, Aina and Amadi2016). Inflammation, the autonomic nervous system, the hypothalamic–pituitary–adrenal axis, endothelial dysfunction, platelet function, serotonin and polyunsaturated fatty acids have been suggested as biological factors linking depression and cardiovascular disorders (CVDs) (de Jonge et al., Reference de Jonge, Rosmalen, Kema, Doornbos, van Melle, Pouwer and Kupper2010). One biological marker particularly associated with depression and CHD is brain-derived neurotrophic factor (BDNF). BDNF is a neurotrophin that is crucial for synaptic function and neuronal plasticity (Allen & Dawbarn, Reference Allen and Dawbarn2006). Blood BDNF concentrations are known to reflect concentrations of BDNF in the brain (Sartorius et al., Reference Sartorius, Hellweg, Litzke, Vogt, Dormann, Vollmayr, Danker-Hopfe and Gass2009). In the hippocampus, BDNF plays an important role in learning and memory function (Allen & Dawbarn, Reference Allen and Dawbarn2006) and it has repeatedly been linked to depression (Brunoni et al., Reference Brunoni, Lopes and Fregni2008; Bocchio-Chiavetto et al., Reference Bocchio-Chiavetto, Bagnardi, Zanardini, Molteni, Nielsen, Placentino, Giovannini, Rillosi, Ventriglia, Riva and Gennarelli2010; Zhang et al., Reference Zhang, Wang, Sha, Zhou and Zhang2011; Molendijk et al., Reference Molendijk, Spinhoven, Polak, Bus, Penninx and Elzinga2014). Meta-analyses have demonstrated an increase in BDNF after antidepressant treatment (Brunoni et al., Reference Brunoni, Lopes and Fregni2008). Therefore, current research assumes that synaptic and neuroplasticity have an important role of in the development and treatment of depression (Brunoni et al., Reference Brunoni, Lopes and Fregni2008) via a stress-induced reduction in expression of BDNF in the limbic regions that control mood (Duman & Monteggia, Reference Duman and Monteggia2006).
Recent research has also attributed BDNF an important role in the cardiovascular system. BDNF is synthesised and released in non-neuronal cells; it has been shown to be involved in cardiovascular development (Caporali & Emanueli, Reference Caporali and Emanueli2009) and connected to several important cardiac processes, such as coronary vessel development, angiogenesis, survival of cardiomyocytes, vascular growth, vascular smooth muscle cell migration and revascularisation (Kermani et al., Reference Kermani, Rafii, Jin, Whitlock, Schaffer, Chiang, Vincent, Friedrich, Shido, Hackett, Crystal, Rafii and Hempstead2005; Pius-Sadowska & Machaliński, Reference Pius-Sadowska and Machaliński2017). Reduced BDNF concentrations have been associated with cardiovascular-related mortality, future coronary events in patients with angina pectoris, risk factors for cardiovascular dysfunction and acute coronary syndrome (ACS) (Manni et al., Reference Manni, Nikolova, Vyagova, Chaldakov and Aloe2005; Jiang et al., Reference Jiang, Liu, Zhang and Chen2011). Furthermore, BDNF seems to play a specific role in cardiac injury (Donovan et al., Reference Donovan, Miranda, Kraemer, McCaffrey, Tessarollo, Mahadeo, Sharif, Kaplan, Tsoulfas, Parada, Toran-Allerand, Hajjar and Hempstead1995; Okada et al., Reference Okada, Yokoyama, Toko, Tateno, Moriya, Shimizu, Nojima, Ito, Yoshida, Kobayashi, Katagiri, Minamino and Komuro2012). Ejiri et al. proposed that BDNF not only has a cardioprotective effect but also contributes to atherogenesis and plaque instability via BDNF-induced oxidative stress (Ejiri et al., Reference Ejiri, Inoue, Kobayashi, Shiraki, Otsui, Honjo, Takahasi, Ohashi, Ichikawa, Terashima, Mori, Awano, Shinke, Shite, Hirata, Yokozaki, Kawashima and Yokoyama2005).
A role of BDNF in physiological processes has recently also been shown outside of neurological or cardiovascular mechanisms (Wang et al., Reference Wang, Freeman, Sathish, Thompson, Pabelick and Prakash2016; Chen et al., Reference Chen, Liang, He, An, Zhao and Wu2016). Moreover, there is early evidence of sex-specific associations of BDNF and physiological outcomes (Wang et al., Reference Wang, Freeman, Sathish, Thompson, Pabelick and Prakash2016; Schmalhofer et al., Reference Schmalhofer, Markus, Gras, Kopp, Janowitz, Grabe, Groß, Ewert, Gläser, Albrecht, Eiffler, Völzke, Friedrich, Nauck, Steveling, Könemann, Wenzel, Felix, Dörr and Bahls2019)
There is growing evidence linking BDNF to CHF: CHF and the severity of its symptoms have been shown to be associated with decreased BDNF concentrations (Takashio et al., Reference Takashio, Sugiyama, Yamamuro, Takahama, Hayashi, Sugano, Izumiya, Hokimoto, Minamino, Yasuda, Anzai and Ogawa2015; Kadowaki et al., Reference Kadowaki, Shishido, Honda, Narumi, Otaki, Kinoshita, Nishiyama, Takahashi, Arimoto, Miyamoto, Watanabe and Kubota2016). Moreover, it has been shown that BDNF concentrations have a predictive value regarding future clinical outcomes in patients with CHF (Fukushima et al., Reference Fukushima, Kinugawa, Homma, Masaki, Furihata, Yokota, Matsushima, Takada, Kadoguchi, Oba, Okita and Tsutsui2015; Kadowaki et al., Reference Kadowaki, Shishido, Honda, Narumi, Otaki, Kinoshita, Nishiyama, Takahashi, Arimoto, Miyamoto, Watanabe and Kubota2016).
However, cause and effect of BDNF and cardiovascular mechanisms are currently still unclear (Ejiri et al., Reference Ejiri, Inoue, Kobayashi, Shiraki, Otsui, Honjo, Takahasi, Ohashi, Ichikawa, Terashima, Mori, Awano, Shinke, Shite, Hirata, Yokozaki, Kawashima and Yokoyama2005; Hashimoto, Reference Hashimoto2013; Bahls et al., Reference Bahls, Könemann, Markus, Wenzel, Friedrich, Nauck, Völzke, Steveling, Janowitz, Grabe and Felix2019).
A number of studies have examined BDNF’s role in the relationship between CVD and depression (Bozzini et al., Reference Bozzini, Gambelli, Boiocchi, Schirinzi, Falcone, Buzzi, Storti and Falcone2009; Liu et al., Reference Liu, Su, Duan, Wang, Liu, Feng, Wang, Liu and Zhang2014; Kang et al., Reference Kang, Bae, Kim, Shin, Hong, Ahn, Jeong, Yoon and Kim2016; Kuhlmann et al., Reference Kuhlmann, Tschorn, Arolt, Beer, Brandt, Grosse, Haverkamp, Müller-Nordhorn, Rieckmann, Waltenberger, Warnke, Hellweg and Ströhle2017, Han et al., Reference Han, Zhang, Wang, Yang, Guo, Li, Zhang, Wang, Chen, Geng and Jiang2019). It has been demonstrated that BDNF-related Val66Met polymorphism is involved in both depression and CHD. It is suggested that the met allele associated with low BDNF secretion plays a role in CHD pathogenesis and is associated with an elevated risk of depression in patients with ACS. Moreover, met allele carriers exhibited higher remission rates after antidepressant treatment and were more vulnerable to persistent depression in longitudinally designed studies (Bozzini et al., Reference Bozzini, Gambelli, Boiocchi, Schirinzi, Falcone, Buzzi, Storti and Falcone2009; Liu et al., Reference Liu, Su, Duan, Wang, Liu, Feng, Wang, Liu and Zhang2014; Kang et al., Reference Kang, Bae, Kim, Shin, Hong, Ahn, Jeong, Yoon and Kim2016). In line with this finding, lower serum concentrations of BDNF appear to be associated with the persistence but not the incidence of depressive symptoms (Kuhlmann et al., Reference Kuhlmann, Tschorn, Arolt, Beer, Brandt, Grosse, Haverkamp, Müller-Nordhorn, Rieckmann, Waltenberger, Warnke, Hellweg and Ströhle2017). The only other study to investigate BDNF blood concentration’s role in the link between a cardiovascular disease (CHF) and depression failed to find an association between BDNF concentrations and depressive symptoms (Fukushima et al., Reference Fukushima, Kinugawa, Homma, Masaki, Furihata, Yokota, Matsushima, Takada, Kadoguchi, Oba, Okita and Tsutsui2015). To date, BDNF serum concentrations in patients with CHD have not been investigated in the context of depressive symptoms.
Due to the extensive physical and mental pathologies that have been linked to BDNF, such as inflammation, cardiovascular pathologies and neurodegenerative diseases such as Alzheimer’s disease (Allen & Dawbarn, Reference Allen and Dawbarn2006; Pius-Sadowska & Machaliński, Reference Pius-Sadowska and Machaliński2017), accurately controlling for a wide range of possible physical and psychological confounders, when investigating the link between BDNF and somatic disease or BDNF and depression, appears to be important. The studies investigating links between BDNF and CVD were mainly controlled for age, sex and some physical parameters, but most of them did not control for the medical comorbidity burden. Previous studies on patients with CHD found differential associations for somatic versus cognitive-affective depressive symptoms: cognitive-affective symptoms were associated with the clinical recognition of a depressive disorder in patients with acute myocardial infarction (MI), while somatic symptoms were not. Somatic symptoms were more consistent predictors of mortality and rehospitalisation as long-term outcomes than cognitive-affective depressive symptoms (de Jonge et al., Reference de Jonge, Mangano and Whooley2007; Smolderen et al., Reference Smolderen, Spertus, Reid, Buchanan, Krumholz, Denollet, Vaccarino and Chan2009).
The aim of the present study was to investigate whether depression and somatic comorbidities are independently linked to levels of BDNF, in hospitalised patients with CHD. Specifically, we analysed the relationships between BDNF and overall depressive symptom level, overall somatic comorbidity burden, CHF and occurrence of an ACS, while controlling for pre-specified confounders
Methods
Study design and blood collection
In total, 322 hospitalised patients with CHD were recruited from two study sites (cardiac units at the Charité – Universitätsmedizin Berlin and University Hospital Münster) in Germany between December 2012 and November 2015. Patients with a documented CHD (diagnosis in the medical chart), sufficient language skills and no severe cognitive impairments or terminal disease were eligible for inclusion in this observational, cross-sectional study. A member of the study team drew blood (8.5 ml) from hospitalised patients who had provided written and informed consent; this was allowed to clot for 30–60 min and centrifuged at 3500 rpm for 15 min at 4°C. The serum was removed and stored at –20°C until the BDNF concentrations were determined. Data from 97 patients were excluded from the analyses for various reasons [withdrawn consent, suspected CHD not confirmed by diagnostic process during treatment, cognitive impairment which was not documented pre-inclusion, no completed baseline questionnaire, clotting time of less than 30 min and unreliable BDNF measurement (<0.5 ng/ml)]. A detailed flow chart of the study has been reported previously (Kuhlmann et al., Reference Kuhlmann, Tschorn, Arolt, Beer, Brandt, Grosse, Haverkamp, Müller-Nordhorn, Rieckmann, Waltenberger, Warnke, Hellweg and Ströhle2017).
BDNF determination
The serum BDNF concentrations were measured using highly sensitive and specific, fluorometric, two-site enzyme-linked immunosorbent assays (ELISA), according to the manufacturer’s instructions (Promega Inc, Mannheim, Germany) but modified for a fluorometric technique: primary anti-BDNF monoclonal antibody (Promega Inc, Cat#: G7610), anti-human-BDNF polyclonal antibody (Promega Inc, Cat#: G1641) and goat anti-chicken-IgY-alkaline phosphatase polyclonal secondary antibody (Abcam, Cat#: ab97142) were used before the enzyme reaction was started and stopped after one night of incubation in a dark, moist chamber at room temperature. The procedure has previously been described in further detail (Hellweg et al., Reference Hellweg, von Arnim, Büchner, Huber and Riepe2003; Ziegenhorn et al., Reference Ziegenhorn, Schulte-Herbrüggen, Danker-Hopfe, Malbranc, Hartung, Anders, Lang, Steinhagen-Thiessen, Schaub and Hellweg2007).
Assessment of sociodemographic variables, depressive symptoms and medical parameters
Depressive symptoms and demographic characteristics were assessed using a self-rating questionnaire that was completed either during hospitalisation or within 3 weeks after discharge. Depressive symptoms were assessed using the Patient Health Questionnaire (PHQ-9), a standard instrument which is widely used to screen for clinical depression and to measure depression severity (Kroenke et al., Reference Kroenke, Spitzer and Williams2001). Medical charts were reviewed to collect relevant medical information, including the presence of an ACS (unstable angina pectoris or MI), CHF, antidepressant medication at hospital admission, body mass index (BMI), hypertension, diabetes, dyslipidemia, left ventricular ejection fraction (LVEF), history of MI, history of revascularisation (percutaneous coronary intervention or bypass operation), length of hospital stay and platelet count. The latter was assessed to account for the links between platelet alterations and both BDNF and depression (Ziegenhorn et al., Reference Ziegenhorn, Schulte-Herbrüggen, Danker-Hopfe, Malbranc, Hartung, Anders, Lang, Steinhagen-Thiessen, Schaub and Hellweg2007; Serra-Millàs, Reference Serra-Millàs2016). Furthermore, variables were extracted from medical charts for the Charlson Comorbidity Index [CCI, (Charlson et al., Reference Charlson, Pompei, Ales and MacKenzie1987)]. The CCI was used in two variations: the CCI according to the original publication and a modified version without CHF and MI, in order to control for comorbid somatic diseases other than cardiac diseases.
Statistical analyses
Regression-based multiple imputation was used to manage missing data and predictors were selected based on associated variables (Holmes et al., Reference Holmes, Selzer, Johnston, Kelsey, Holubkov, Cohen, Williams and Detre2003; Siew et al., Reference Siew, Peterson, Eden, Moons, Ikizler and Matheny2013; Biering et al., Reference Biering, Hjollund and Frydenberg2015). The detailed procedure has been described previously (Kuhlmann et al., Reference Kuhlmann, Tschorn, Arolt, Beer, Brandt, Grosse, Haverkamp, Müller-Nordhorn, Rieckmann, Waltenberger, Warnke, Hellweg and Ströhle2017). The PHQ-9 depression scale was summed to give a total score and also divided into two subscales (cognitive-affective and somatic), in line with previous studies in cardiac patients (de Jonge et al., Reference de Jonge, Mangano and Whooley2007; Smolderen et al., Reference Smolderen, Spertus, Reid, Buchanan, Krumholz, Denollet, Vaccarino and Chan2009). In addition, patients were grouped into those with ‘elevated depressive symptoms’ versus ‘non-elevated depressive symptoms’, using a PHQ-9 score of 7 as the cut-point. This cut-point was chosen because it had shown the best trade-off between sensitivity and specificity for a clinical depression diagnosis in a larger sample of hospitalised patients with CHD who were recruited from the same study sites (Tschorn et al., Reference Tschorn, Rieckmann, Arolt, Beer, Haverkamp, Martus, Waltenberger, Müller-Nordhorn and Ströhle2019).
To compare depressed versus non-depressed patients concerning relevant sociodemographic and clinical variables, chi-square statistics were conducted for nominal variables (sex, smoking status, use of antidepressants, ACS, CHF, hypertension, diabetes, dyslipidemia, MI and revascularisation), t-tests were used for continuous variables (age, platelets, PHQ-9, serum BDNF, BMI and LVEF), and Mann–Whitney U-tests were used to compare medians (modified CCI and length of hospital stay). Correlation analyses were used to asses associations between continuous covariates (age and platelet count), and BNDF and t-tests were used to analyse dichotomous covariates (sex, smoking status and use of antidepressants) and BDNF levels. Linear regression analyses were computed to investigate the relationships between BDNF and PHQ-9, PHQ-9 subscales, and somatic comorbidity (CCI). Multiple regression analyses were used to control for possible confounders. We used logistic regression models to analyse the relationship between PHQ-9 groups (depressed vs. non-depressed) and BDNF concentrations. Likewise, two further logistic regression models were used to analyse the relationships of ACS and CHF with BDNF. All the analyses were conducted using IBM SPSS version 25. All the reported p-values are two-sided and were considered statistically significant at <0.05.
Specification of confounders
Variables were considered to be confounders if a link to both the currently analysed variables was found repeatedly in the relevant literature (e.g. BDNF and depression, ACS/CHF and BDNF, somatic comorbidity and BDNF). All the analyses investigating BDNF and depression included age, sex, smoking status, use of antidepressants, platelet count and three markers of somatic comorbidity as possible confounders: ACS, CHF and the modified CCI. The analyses to investigate BDNF and CCI included age, sex, platelet count, PHQ-9 sum score and smoking status as confounders. All the analyses investigating BDNF and CHF or ACS were adjusted for age, sex, platelet count, PHQ-9 sum score, smoking status and modified CCI. Confounder selection and adjustment is based on the definition of BDFN as the exposure variable for depression as the outcome variable. When investigating associations of CHF, ACS and CCI with BDNF, we defined BDNF as the exposure variable of CHF, ACS, and CCI as outcome variables. Collinearity diagnostics did not reveal any multicollinearity in the adjusted regression models.
Results
The sociodemographic and clinical characteristics of the overall sample (N = 225), as well as the depression groups, are shown in Table 1.
Table 1. Sample characteristics

PHQ-9, Patient Health Questionnaire-9; ACS, acute coronary syndrome; CHF, congestive heart failure; CCI, Charlson Comorbidity Index; BDNF, brain-derived neurotrophic factor; BMI, body mass index; MI, myocardial infarction; LVEF, left ventricular ejection fraction.
Chi-square statistics were used to compare nominal variables, t-tests were used to compare continuous variables and Mann–Whitney U-tests were used to compare medians.
* Length of hospital stay was reported for n = 215 patients.
Significant p values (<0.05) are highlighted in bold.
BDNF concentrations and depressive symptom severity
The link between BDNF concentrations and depressive symptoms (PHQ-9) did not reach significance (β = −0.114, CI = −0.246−0.018, p = 0.09, Table 2). A statistical trend disappeared when possible confounders were controlled for (β = −0.085, CI = −0.224−0.054, p = 0.23). This relationship was also examined by comparing depressed and non-depressed patients with CHD, grouped by the PHQ-9 cut-point of 7 via logistic regression, which revealed a statistically significant difference; BDNF concentrations in non-depressed patients were higher than in depressed patients [2.85 ng/ml vs. 3.17 ng/ml, odds ratio (OR) = 0.768, CI = 0.601–0.983, p=0.04]. This difference was no longer statistically significant after adjustment for confounders (OR = 0.820, CI = 0.624–1.078, p = 0.015).
Table 2. Associations between BDNF, covariates and depression
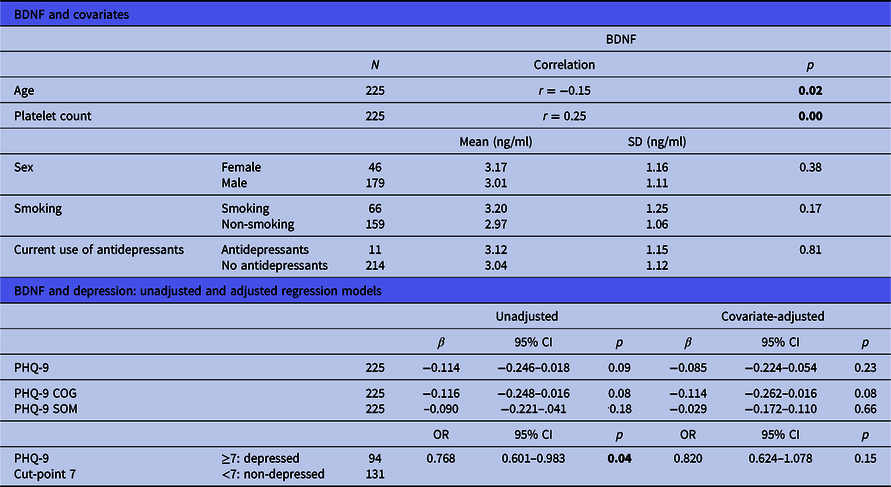
BDNF, brain-derived neurotrophic factor; SD, standard deviation; 95% CI, 95% confidence interval; PHQ-9, Patient Health Questionnaire-9; SOM, somatic depressive symptoms; COG, cognitive-affective depressive symptoms; OR, odds ratio.
Significant p values (<0.05) are highlighted in bold.
BDNF concentrations and PHQ-9 cognitive-affective and somatic subscales
There was no statistically significant correlation between the PHQ-9 somatic subscale and BDNF (β = −0.090, CI = −0.221−0.041, p = 0.18, Table 2). The correlation between the PHQ-9 cognitive-affective subscale and BDNF closely missed significance (β = −0.116, CI = −0.248−0.016, p = 0.08). A statistical trend for a negative correlation with BDNF concentrations was also found after controlling for confounders (β = −0.114, CI = −0.262−0.016, p = 0.08).
BDNF concentrations and CCI
Lower BDNF concentrations were significantly associated with a higher burden of somatic comorbidity (CCI, β = −0.149, CI = −0.280−0.018, p = 0.03, Table 3). This link narrowly missed significance after controlling for confounders (β = −0.123, CI = −0.254−0.006, p = 0.06). Furthermore, lower BDNF concentrations were associated with higher scores for non-cardiac comorbidity burden (modified CCI, β = −0.131, CI = −0.262−0.000, p = 0.05). This association was no longer statistically significant after controlling for confounders (β = −0.103, CI = −0.236−0.030, p = 0.13).
Table 3. Relationships of BDNF with somatic comorbidity, congestive heart failure and acute coronary syndrome (unadjusted and adjusted regression models)
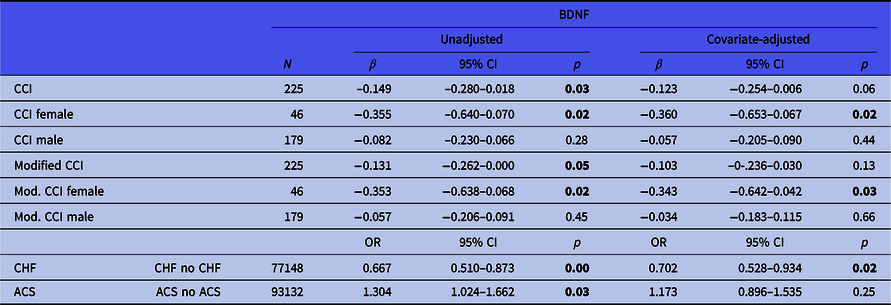
BDNF, brain-derived neurotrophic factor; SD, standard deviation; 95% CI, 95% confidence interval; ACS, acute coronary syndrome; CHF, congestive heart failure; CCI, Charlson Comorbidity Index; Mod. CCI, modified CCI (without cardiac conditions); OR, odds ratio.
Significant p values (<0.05) are highlighted in bold.
Sex-stratified analyses of BDNF concentrations and CCI
Overall somatic comorbidity burden was significantly associated with BDNF concentrations in women (CCI, β = −0.355, CI = −0.640−0.079, p = 0.02), also after controlling for confounders (β = −0.360, CI = −0.653–0.067, p = 0.02). There was no association between overall somatic comorbidity and BDNF concentrations in men (β = −0.082, CI = −0.280–0.066, p = 0.28). Likewise, the modified (non-cardiac) somatic comorbidity burden was significantly associated with BDNF concentrations in women (β = −0.353, CI = −0.638–0.068, p = 0.02), also after confounder adjustment (β = −0.343, CI = −0.642–0.042, p = 0.03). No association between non-cardiac somatic comorbidity and BDNF was found in men (β = −0.034, CI = −0.183–0.115, p = 0.66).
BDNF concentrations and CHF
BDNF concentrations were lower in patients with CHF than in patients without it (2.73 ng/ml vs. 3.20 ng/ml, OR = 0.667, CI = 0.510–0.873, p = 0.00). This difference remained constant after adjusting for possible confounders (OR = 0.702, CI = 0.528–0.934, p = 0.02).
BDNF concentrations and ACS
BDNF concentrations were higher in patients with ACS, compared to CHD patients without ACS (3.23 ng/ml vs. 2.90 ng/ml, OR = 1.304, CI = 1.024–1.662, p = 0.03). However, this statistically significant difference disappeared after adjusting for confounders (OR = 1.173, CI = 0.896–1.535, p = 0.25).
Discussion
The current study investigated the relationship between depressive symptoms, somatic comorbidity and BDNF concentrations in patients with CHD.
BDNF and depression in patients with CHD
The results of this study show that the association of lower BDNF concentrations with depressive symptoms in patients with CHD no longer exists when adjustments are made for possible confounders. Likewise, a statistical trend towards a linear relationship between depressive symptoms and BDNF concentrations disappears after controlling for possible confounders. While one of two pre-existing investigation of BDNF concentrations and depression in a sample of cardiac patients also found no association, which might be due to the very small subsample (n = 4) of depressed patients (Fukushima et al., Reference Fukushima, Kinugawa, Homma, Masaki, Furihata, Yokota, Matsushima, Takada, Kadoguchi, Oba, Okita and Tsutsui2015), a very recent study found a negative association of BDNF levels and depression scores as well as depression groups (Han et al., Reference Han, Zhang, Wang, Yang, Guo, Li, Zhang, Wang, Chen, Geng and Jiang2019). However, Han et al. did not report any confounder adjustments. As Molendijk et al. (Reference Molendijk, Spinhoven, Polak, Bus, Penninx and Elzinga2014) reported studies investigating BDNF concentrations and depression show heterogeneity of outcomes and clinical characteristics, the latter being poorly reported in most studies. The majority of studies investigating BDNF and depression were only controlled for sex or age as possible confounders. Many studies did not control for any possible confounders, although some adjusted for BMI or smoking status. The present study showed particularly that the somatic depressive symptoms in the PHQ-9 depression scale did not show an association with BDNF, once the influence of somatic confounding variables was taken into account, which can be explained by the large overlap of somatic depressive symptoms and CHD symptoms. In contrast, a small link was shown between cognitive-affective depressive symptoms and BDNF concentrations that only narrowly missed statistical significance, also after adjusting for somatic confounders. This association at trend level could yield different results in a larger sample. Our data leave the position of the hypothesis about the role of BDNF in mood control found in the literature unclear (Duman & Monteggia, Reference Duman and Monteggia2006); however, they do support Molendijk et al.’s conclusion that the link between depression and BDNF concentrations is smaller than was initially thought (Molendijk et al., Reference Molendijk, Spinhoven, Polak, Bus, Penninx and Elzinga2014).
Regarding the adjustment for cardiovascular conditions (e.g. ACS or CHF), it is important to point to the fact that the causal direction of the association between cardiovascular conditions and BDNF, especially concerning different disease stages, still remains unclear (Ejiri et al., Reference Ejiri, Inoue, Kobayashi, Shiraki, Otsui, Honjo, Takahasi, Ohashi, Ichikawa, Terashima, Mori, Awano, Shinke, Shite, Hirata, Yokozaki, Kawashima and Yokoyama2005; Hashimoto, Reference Hashimoto2013; Bahls et al., Reference Bahls, Könemann, Markus, Wenzel, Friedrich, Nauck, Völzke, Steveling, Janowitz, Grabe and Felix2019). Our models investigating this association cannot account for this uncertainty. Furthermore, including a confounder that is a descendant of the outcome can introduce a statistical bias (Shrier & Platt, Reference Shrier and Platt2008). If we assume a unidirectional causal link from BDNF to ACS and CHF (ACS and CHF descendants of BDNF), then controlling for ACS and CHF in the link between BDNF and depression would mean a risk of introducing such a statistical bias. However, an exclusion of these confounders based on this concern did not alter the significance levels of our results.
BDNF and its link to somatic comorbidity, CHF and ACS:
The present study did not provide clear results in our overall sample about a possible association between somatic comorbidity and BDNF concentrations. However, in our sample of patients with CHD, this association appeared to be stronger than the link between depression and BDNF concentrations. However, sex-stratified analyses revealed statistically significant associations for overall somatic comorbidity as well as non-cardiac somatic comorbidity in women, also after adjustment for confounders. Although our female sample only consisted of 46 participants, this finding is in line with associations of BDNF and cardiovascular outcomes only in female participants of a large study investigating both sexes (Schmalhofer et al., Reference Schmalhofer, Markus, Gras, Kopp, Janowitz, Grabe, Groß, Ewert, Gläser, Albrecht, Eiffler, Völzke, Friedrich, Nauck, Steveling, Könemann, Wenzel, Felix, Dörr and Bahls2019). Sex-dependent effects of BDNF are commonly explained by mechanisms involving sex steroids (Carbone & Handa, Reference Carbone and Handa2012), but the sex-specific association on BDNF and cardiorespiratory fitness found by Schmalhofer et al. was independent of menopause status (Schmalhofer et al., Reference Schmalhofer, Markus, Gras, Kopp, Janowitz, Grabe, Groß, Ewert, Gläser, Albrecht, Eiffler, Völzke, Friedrich, Nauck, Steveling, Könemann, Wenzel, Felix, Dörr and Bahls2019). Therefore, sex-specific effects of BDNF might involve more than sex steroids. Taken together, our results add to the literature that reports mechanisms involving BDNF beyond neurological and also beyond cardiovascular pathophysiology (Chen et al., Reference Chen, Liang, He, An, Zhao and Wu2016; Wang et al., Reference Wang, Freeman, Sathish, Thompson, Pabelick and Prakash2016) and that also identified sex-dependent processes (Wang et al., Reference Wang, Freeman, Sathish, Thompson, Pabelick and Prakash2016; Schmalhofer et al., Reference Schmalhofer, Markus, Gras, Kopp, Janowitz, Grabe, Groß, Ewert, Gläser, Albrecht, Eiffler, Völzke, Friedrich, Nauck, Steveling, Könemann, Wenzel, Felix, Dörr and Bahls2019). Nevertheless, these results from our small female subsample must be interpreted cautiously and further research is warranted.
The patients with CHD and comorbid CHF showed lower concentrations of BDNF, compared to patients with CHD but without CHF. This finding is in line with the results published by Takashio et al. (Reference Takashio, Sugiyama, Yamamuro, Takahama, Hayashi, Sugano, Izumiya, Hokimoto, Minamino, Yasuda, Anzai and Ogawa2015) and Kadowaki et al. (Reference Kadowaki, Shishido, Honda, Narumi, Otaki, Kinoshita, Nishiyama, Takahashi, Arimoto, Miyamoto, Watanabe and Kubota2016), who found lower BDNF concentrations in CHF patients, compared to the controls. Since we compared patients with CHD and comorbid CHF and patients with CHD but without comorbid CHF, our results suggest a negative dose–response relationship for cardiovascular dysfunction and BDNF concentrations which occurs independently of depressive symptoms. Recent findings about the link between CHF and BDNF have suggested that an impairment in skeletal muscle BDNF secretion and the skeletal muscle energy metabolism may be the mechanisms explaining lower BDNF levels in CHF patients (Fukushima et al., Reference Fukushima, Kinugawa, Homma, Masaki, Furihata, Yokota, Matsushima, Takada, Kadoguchi, Oba, Okita and Tsutsui2015; Takashio et al., Reference Takashio, Sugiyama, Yamamuro, Takahama, Hayashi, Sugano, Izumiya, Hokimoto, Minamino, Yasuda, Anzai and Ogawa2015; Kadowaki et al., Reference Kadowaki, Shishido, Honda, Narumi, Otaki, Kinoshita, Nishiyama, Takahashi, Arimoto, Miyamoto, Watanabe and Kubota2016). On the other hand, Rasmussen et al. (Reference Rasmussen, Brassard, Adser, Pedersen, Leick, Hart, Secher, Pedersen and Pilgaard2009) showed that three-quarter of BDNF concentrations were produced in the brain and only a minor part of BDNF synthetisation was localised in skeletal muscles. Manni et al. (Reference Manni, Nikolova, Vyagova, Chaldakov and Aloe2005) found decreased BDNF concentrations in ACS patients, compared to healthy controls. In contrast, our study showed that of the patients with CHD, those with ACS, initially had higher BDNF concentrations than those patients with CHD but without ACS, a finding which did not remain statistically significant after adjusting for confounders. A possible increase in BDNF expression after cardiac injury has been suggested by Okada et al. (Reference Okada, Yokoyama, Toko, Tateno, Moriya, Shimizu, Nojima, Ito, Yoshida, Kobayashi, Katagiri, Minamino and Komuro2012) and Donovan et al. (Reference Donovan, Miranda, Kraemer, McCaffrey, Tessarollo, Mahadeo, Sharif, Kaplan, Tsoulfas, Parada, Toran-Allerand, Hajjar and Hempstead1995). However, this ACS-associated increase in BDNF was no longer apparent in our sample after controlling for possible confounders.
Based on the literature, we hypothesise a causal relationship from BDNF to depression; therefore, we interpret depression as a descendant of BDNF. As described above, adjusting for a descendant of the outcome can introduce a statistical bias (Shrier & Platt, Reference Shrier and Platt2008). Since the causal role of BDNF is not conclusively answered and since our main goal was to investigate the relationships of BDNF with three somatic conditions (ACS, CHF and overall somatic comorbidity) independently from depression, we decided to include depression as a confounder nonetheless. However, an exclusion of depression from the set of confounders did not change the results for CHF and ACS. Only for overall comorbidity (CCI), the relationship to BDNF stayed statistically significant also after controlling for confounders (β = −0.138, CI = −0.266–0.009, p = 0.04) when depression was removed from the set of confounders, which hints to a link between BDNF and somatic comorbidity which involves a role of depression.
Limitations
The limitations of the present study are a small sample size that was not based on a power analysis considering the high prevalence of CHD and lack of opportunity to not only adjust for platelet count but also for platelet activation, as the potential mechanism between depression and BDNF levels (Serra-Millàs, Reference Serra-Millàs2016). Furthermore, the cross-sectional study design and a limited generalisability to patients with CHD must be noted as limitations. Since BDNF levels show a wide range depending on the specific measurement protocols used in different laboratories (Polacchini et al., Reference Polacchini, Metelli, Francavilla, Baj, Florean, Mascaretti and Tongiorgi2015), no reference values for serum BDNF exist to allow comparisons of the values generated in different laboratories.
A storage time of more than 12 months can reduce BDNF concentrations in serum samples (Trajkovska et al., Reference Trajkovska, Marcussen, Vinberg, Hartvig, Aznar and Knudsen2007). None of our 225 samples were stored for more than 13 months. However, 11 samples were stored for more than 12 months. An exclusion of these 11 samples would alter our result for ACS and the modified CCI (see Table 3): unadjusted regression models showed no association between BDNF and ACS (OR = 1.236, CI = 0.963–1.586, p = 0.10) or BDNF and modified CCI (β = −0.126, CI = −0.260–0.009, p = 0.07).
When confounding factors are adjusted for, the specification of covariates always implies causal assumptions that are rarely made explicit. The present study aims to disassemble the associations of BDNF, depression and cardiovascular conditions; therefore, we needed to imply causal assumptions for complex biological mechanisms, although the current literature does not conclusively answer questions about causalities in this field. To further improve the investigation of causalities and the specification of important confounders, the use of directed acyclic graphs (DAGs) and DAG-specific reviews for a certain field of research (e.g. Lewis & Kuerbis, Reference Lewis and Kuerbis2016; Williams et al., Reference Williams, Bach, Matthiesen, Henriksen and Gagliardi2018) appear recommendable in future research.
Conclusion
This data show that severe cardiac disease (as indicated by CHF) is associated with lower BDNF concentrations, independent of potential confounders and depressive symptoms. In our sample of patients with CHD, a link between lower BDNF concentrations and depression groups did not withstand a consideration of possible confounders.
Taken together, the present study found an association between cardiovascular dysfunction and serum BDNF concentrations, while no covariate-adjusted links between depressive symptoms or somatic comorbidity and BDNF concentrations were found.
The findings of the present study support the necessity of considering relevant confounders, especially variables associated with cardiac dysfunction and illness when investigating the link between BDNF and depression. Overall, carefully reporting of clinical characteristics and the development of study designs that aim to minimise the effect of confounding factors might help to clarify the role of BDNF in the pathogenesis of depression.
Acknowledgements
We gratefully acknowledge technical assistance from Silvia Saft. Furthermore, we gratefully thank Victoria Engelmann, Lena Kuner, Julia Brandt and Katharina Warnke for their assistance in recruiting patients and collecting samples.
Authors Contributions
Mira Tschorn: study conception and design, coordination of data acquisition, analysis and interpretation of data, drafting of manuscript, and final approval
Stella Linnea Kuhlmann: acquisition of data, analysis and interpretation of data, critical revision and final approval of the article
Nina Rieckmann: study conception and design, coordination of data acquisition, analysis and interpretation of data, critical revision and final approval of the article
Katja Beer: study conception and design, coordination of data acquisition, analysis data, critical revision and final approval
Laura Grosse: coordination of data acquisition, analysis of data, critical revision and final approval of the article
Volker Arolt: study conception and design, coordination of data acquisition, critical revision and final approval of the article
Johannes Waltenberger: acquisition of data, study conception and design, critical revision and final approval of the article
Wilhelm Haverkamp: acquisition of data, study conception and design, critical revision and final approval of the article
Jacqueline Müller-Nordhorn: study conception and design, critical revision and final approval of the article
Rainer Hellweg: study conception and design, acquisition of data (lab analyses), analysis and interpretation of data, critical revision and final approval of the article
Andreas Ströhle: study conception and design, interpretation of data, critical revision and final approval of the article
Financial support
The current study was an add-on study to the study Depression Care for Hospitalized Coronary Heart Disease Patients (CDCare). CDCare was supported by the German Federal Ministry of Education and Research (grant number: 01GY1154). The funder had no role in the study design, data collection and analysis, interpretation of data, preparation of the manuscript, or decision to publish.
Conflict of interest
Volker Arolt received support from the German Federal Ministry of Education and Research, European Union and the Medical Faculty Münster for the submitted work and personal fees from Allergan, Janssen-Cilag, Lundbeck, Otsuka, Servier and Trommsdorff, unconnected with the submitted work.
Johannes Waltenberger reports personal fees and non-financial support from Bayer, Boehringer Ingelheim, Daiichi-Sankyo and Biotronik, as well as personal fees from MSD, Berlin Chemie, Sanofi Aventis and Vifor, unconnected with the submitted work.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.






