Cruciferous vegetables are rich sources of glucosinolates. Isothiocyanates (ITC), the hydrolysis products of glucosinolates produced by plant myrosinase or by bacterial thioglucosidase, have been shown to have anticarcinogenic properties(Reference Keum, Jeong and Kong1–Reference Clarke, Dashwood and Ho4). Myrosinase in cruciferous vegetables is deactivated by cooking(Reference Howard, Jeffery and Wallig5); therefore, when cooked cruciferous vegetables are consumed, gut bacteria are mainly responsible for ITC production in the human body(Reference Shapiro, Fahey and Wade6, Reference Getahun and Chung7).
Inter-individual differences in glucosinolate metabolism have been observed in previous feeding studies. The amounts of ITC excreted in urine varied substantially after participants consumed the same amount of cruciferous vegetables or glucosinolates(Reference Shapiro, Fahey and Wade6–Reference Shapiro, Fahey and Wade11), but much less variation was observed if ITC were consumed directly(Reference Shapiro, Fahey and Wade11). It has been proposed that part of the inter-individual difference in glucosinolate metabolism is due to differences in gut bacterial composition. The importance of gut bacteria in glucosinolate metabolism was also confirmed in a feeding study, which has shown that urinary ITC excretion after cooked cruciferous vegetable consumption decreased significantly when participants were pre-treated with oral antibiotics and bowel cleansing(Reference Shapiro, Fahey and Wade6). To date, in vitro experiments incubating mixed or pure cultures of bacteria with glucosinolates have confirmed that several bacterial species residing in the human gut, such as Escherichia coli, Bacteroides thetaiotaomicron, Enterococcus faecalis, Enterococcus faecium, Lactobacillus agilis, certain Peptostreptococcus spp. and Bifidobacterium spp., have the ability to metabolise glucosinolates in culture(Reference Brabban and Edwards12–Reference Llanos Palop, Smiths and Brink16). However, to our knowledge, no studies have evaluated glucosinolate conversion ex vivo by human gut bacteria with in vivo data of glucosinolate metabolism (i.e. the ITC excretion level in urine) from multiple individuals.
We hypothesised that differences in gut microbiota composition are partially responsible for differences in glucosinolate metabolism. Thus, not only the amount and processing of cruciferous vegetables consumed but also the gut microbiota composition contributes to the host exposure to bioactive ITC. Our aim was to explore the relationship between gut microbiota and glucosinolate metabolism, using terminal restriction fragment length polymorphism (tRFLP) analysis to describe human gut bacterial communities(Reference Li, Hullar and Schwarz17). tRFLP takes advantage of the sequence variation of the 16S ribosomal RNA (rRNA) gene to generate sequence fragments. The tRFLP pattern, which includes both the number and size of the tRFLP sequence fragments, is used to characterise the compositional differences in gut bacterial communities.
Specifically, we examined (1) whether there were differences in faecal microbiota composition between selected high- and low-ITC excreters (based on the urinary ITC excretion level after a standardised broccoli meal) and (2) whether faecal bacteria from the high-ITC excreters, compared with those from the low-ITC excreters, were able to degrade more glucosinolate ex vivo. Additionally, we also explored the associations between habitual dietary cruciferous vegetable intake and faecal microbiota composition and between habitual dietary cruciferous vegetable intake and urinary ITC excretion status after a prescribed broccoli dose.
Methods
Participant recruitment and intervention
We recruited twenty-three individuals for the present pilot study. Participants were healthy adults living in the Seattle area. Exclusion criteria included the following: (1) medical history of gastrointestinal, hepatic or renal disorders; (2) pregnancy or lactation; (3) weight loss or gain >4·5 kg within the past year; (4) major changes in eating habits within the past 3 months; (5) antibiotic use within the past 3 months; (6) current use of prescription medication (including oral contraceptives). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center (FHCRC). Written informed consent was obtained from all participants.
Dietary intake, health and demographic data
All participants completed a self-administered demographic and health questionnaire and two FFQ: a general FFQ developed at the FHCRC containing 114 food items and eight beverage items with portion size estimation and a specific cruciferous vegetable intake FFQ containing seventy-nine cruciferous vegetable items with portion size estimation and cooking method description (Arizona Cruciferous Vegetable FFQ; Arizona Diet and Behavioral Assessment Center, Tucson, AZ, USA). The intakes of major nutrients were estimated from the general FHCRC FFQ based on Nutrition Data System for Research software (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN, USA). Raw, cooked and total cruciferous vegetable intakes were estimated from the Arizona cruciferous vegetable intake FFQ(Reference Thomson, Newton and Graver18). Demographic and health data included parameters such as age, sex, race, weight, height and intestinal health status (diarrhoea and constipation).
Broccoli feeding
We instructed participants to avoid any foods or supplements containing cruciferous vegetable components from 3 d before until 24 h after the broccoli feeding. A detailed list of cruciferous vegetables was provided to participants for reference. In this way, all glucosinolate metabolites in urine were expected to originate from broccoli that we provided. All participants ate a standardised mid-day meal that included 200 g of cooked broccoli (Food Services of America, Seattle, WA, USA) and 283 g of prepared macaroni and cheese (Lean Cuisine; Nestlé USA, Glendale, CA, USA) at the FHCRC Human Nutrition Laboratory. The broccoli used in the present study was purchased as a single lot and stored at − 20°C before cooking. Individual frozen broccoli aliquots (200 g) were weighed, steamed for 5 min to deactivate myrosinase and stored at − 20°C again until the serving day. The frozen steamed broccoli was microwaved for 2 min before being served. A subset of ten participants (five with the highest and five with the lowest urinary ITC excretion after this broccoli feeding, defined as high- and low-ITC excreters) were selected to repeat the broccoli feeding 1–2 months after the first feeding.
Biological sample collection and processing
Participants collected a faecal sample within 24 h before the broccoli feeding. Participants were provided with a collection guide and appropriate faecal collection supplies including a commode (Fisher Scientific, Fair Lawn, NJ, USA) and a small collection vial with scooping spoon as well an outer transport vial (Sarstedt, Nümbrecht, Germany). Participants were asked to put a pea-sized aliquot of faeces into a collection tube containing RNAlater solution (Ambion, Austin, TX, USA) and 3 mm glass beads (Fisher Scientific), which helped disperse the faeces in RNAlater. RNAlater protects the integrity of nucleic acids in faecal samples at room temperature (Nechvatal et al.(Reference Nechvatal, Ram and Basson19); F Li, unpublished results). If participants completed the collection at home, we asked them to store the sample in the home refrigerator (approximately 4°C) until delivery to the research clinic. For the 24 h following the broccoli meal, participants collected their urine continuously. For the ten participants who were selected to receive the second broccoli meal, an additional faecal sample and 24 h urine sample were collected from each individual. All procedures remained the same except that participants were required to defecate at the FHCRC for the second collection so that the faecal samples could be delivered immediately to the laboratory for bacterial cultivation. All faecal samples placed in RNAlater were stored at − 80°C until DNA extraction. In addition, 24 h urine volumes were measured and urine aliquots were stored at − 80°C until ITC analysis.
Bacterial cultivation and analysis
Faecal bacterial cultivation with glucoraphanin
We used a special cultivation medium designed to provide compounds that bacteria usually utilise in the human gut – a medium used previously for a simulation of the human intestinal microbial ecosystem (SHIME)(Reference Molly, Vande Woestyne and Verstraete20). It contained yeast extract (3 g/l; Fisher Scientific), starch (3 g/l; Sigma-Aldrich, St Louis, MO, USA), cysteine (0·5 g/l; Sigma-Aldrich), arabinogalactan (1 g/l; Sigma-Aldrich), glucose (0·4 g/l; Sigma-Aldrich), xylan (1 g/l; MP Biomedicals, Solon, OH, USA), gastric mucin (1 g/l; MP Biomedicals), proteose peptone (3 g/l; Oxoid, Basingstoke, Hants, UK) and pectin (2 g/l; Acros Organics, Geel, Belgium). The medium pH was adjusted to 7·0 before incubation. Faeces (1 g) were weighed, series diluted in the SHIME medium into 105 dilution, and then inoculated in 1 ml SHIME for ex vivo bacterial cultivation. Samples were incubated either with or without 50 μm-glucoraphanin (C2 Bioengineering, Hovedgaden, Denmark) anaerobically in duplicate using a GasPak 150 System (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) for 24 or 48 h at 37°C. Glucoraphanin was chosen particularly in the present study, because it is the major glucosinolate found in broccoli. We also added trace mineral supplement and vitamin supplement (ATCC, Manassas, VA, USA) into the medium to promote bacterial growth (1:100 (v/v) dilution). The SHIME medium with 50 μm-glucoraphanin but without any bacteria served as an abiotic control. After incubation was terminated, the faecal cultures were centrifuged at 20 000 g for 10 min to separate the bacteria from the medium. The supernatants were saved for ITC analysis. The bacterial pellets were stored in RNAlater at − 80°C until DNA extraction.
Terminal restriction fragment length polymorphism analysis
We used the tRFLP method based on the bacterial 16S rRNA gene to compare the bacterial community fingerprinting patterns in both faecal samples and ex vivo cultivation samples(Reference Liu, Marsh and Cheng21). RNAlater was removed from the samples by dilution with PBS, centrifugation at 20 000 g for 10 min and removal of the supernatant. Bacterial genomic DNA was extracted following the methods described previously(Reference Li, Hullar and Lampe22) using the Qiagen stool DNA minikit (Qiagen, Irvine, CA, USA) and physical disruption. Extractions were conducted in triplicate for each faecal sample and singly for each cultivation sample. PCR was performed using bacterial 16S rRNA gene universal primers 27f (6-carboxyfluorescein-labelled) and 519r(Reference Li, Hullar and Schwarz17, Reference Lane23), and PCR conditions and post-PCR treatments were as described previously(Reference Li, Hullar and Schwarz17). An aliquot of 20 ng digested DNA from each sample was mixed with formamide and the internal size standard GeneScan ROX 500 (Applied Biosystems, Foster City, CA, USA). The samples were run at the FHCRC Genetic Analysis Laboratory on an ABI Prism 3100 Genetic Analyser in GeneScan mode for tRFLP analysis (Applied Biosystems).
Quantitative PCR
Because the initial bacterial cell numbers and bacterial growth in cultivation culture samples varied, we considered the difference in bacterial cell number in these samples when we compared the bacterial glucoraphanin degradation rates. We used quantitative PCR to estimate the total bacterial 16S rRNA gene copy number in each ex vivo cultivation sample and adjusted the glucoraphanin degradation rate by these numbers. Quantitative PCR was performed on these twenty samples using bacterial 16S rRNA gene universal primers 330f (5′-ACTCCT ACGGGA GGCAGC AGT-3′) and 530r (5′-GTATTA CCGCGG CTGCTG GCAC-3′) (Invitrogen, Carlsbad, CA, USA). Bacterial genomic DNA from culture samples was amplified using the SYBR Green qPCR SuperMix-UDG kit (Invitrogen) on an ABI 7900HT real-time PCR system (Applied Biosystems). The estimation of bacterial 16S rRNA gene copy numbers in these samples was based on the standard curve with different concentrations of bacterial genomic DNA with known 16S rRNA gene copy numbers(Reference Ahmed, Macfarlane and Fite24).
Glucosinolate and isothiocyanate analysis
ITC and derivatives (‘total ITC’) in 24 h urine samples were converted to 1,3-benzodithione-2-thiole via a cyclocondensation reaction and measured by HPLC using an internal calibration of sulforaphane solution(Reference Chung, Jiao and Conaway25–Reference Kristensen, Krogholm and Frederiksen27). Briefly, 100 μl of the urine sample were incubated with 500 μl of 100 mm-K2HPO4 (pH 8·5) and 600 μl benzenedithiol (1·42 g/l in 2-propanol; Sigma-Aldrich) in a shaking water-bath at 65°C for 1 h. A set of sulforaphane (MP Biomedicals) calibration standards (5–500 μm), a blank and a quality-control sample, were treated in the same manner. Chromatographic separation of the cyclocondensation product occurred under gradient conditions on a C18 μBondapak 150 × 3·9 mm column (Waters Corporation, Milford, MA, USA), which was attached to an 1100 UV-HPLC (Agilent Technologies, Santa Clara, CA, USA). The mobile phase was methanol–water (70:30, v/v). UV setting was 365 nm.
To measure the remaining glucoraphanin in the culture medium after 24 or 48 h incubation, 100 μl of the culture supernatant were treated with 0·1 mg thioglucosidase (Sigma-Aldrich) at 37°C, pH 7, for 1 h. Time-series experiments showed that 1 h is enough to convert the remaining glucoraphanin in the SHIME medium into sulforaphane (F Li, unpublished results). After the thioglucosidase treatment, the cyclocondensation reaction was performed as described earlier. Because ITC is much less stable than glucosinolates in the culture medium (Llanos Palop et al.(Reference Llanos Palop, Smiths and Brink16); F Li, unpublished results), the ITC produced by bacteria (i.e. sulforaphane from glucoraphanin) during the incubation did not accumulate so that the final ITC concentration was negligible. The present pilot study showed that sulforaphane degraded quickly in the SHIME medium and was hardly detectable after 24 or 48 h incubation. The cyclocondensation products were extracted twice with 2 ml hexane to remove other components in the medium and blown dry under N2. The residue was reconstituted in 70 % methanol and analysed by HPLC as described earlier. Freshly prepared glucoraphanin solutions in the SHIME medium (5–100 μm) were used to generate the calibration curve. They were treated in the same manner as culture supernatant samples.
To measure the glucosinolate and ITC content in the broccoli used for feeding, 200 g broccoli cooked in the same manner were homogenised with 100 ml of water for 2 h, and then 1 g slurry was treated with 1 mg thioglucosidase (Sigma-Aldrich) at 37°C, pH 7, for 1 h. Control samples without thioglucosidase were treated in the same manner. After the thioglucosidase treatment, the cyclocondensation reaction was performed as described earlier. The samples were centrifuged at 20 000 g for 1 min, and the supernatants were then subjected to chromatographic analysis as described earlier.
Statistical analysis
Student's t tests were performed to compare age, BMI, 24 h urinary ITC after broccoli feeding, total energy intake, fibre intake (log-transformed), cooked cruciferous vegetable intake (log-transformed) and total cruciferous vegetable intake (log-transformed) between the high (n 5)- and low (n 5)-ITC excreters. Fisher's exact tests were performed to compare raw cruciferous vegetable intake (binary coded as 0 = no intake, 1 = some intake) between the two groups.
The proportion of glucoraphanin degraded by bacteria in faecal culture samples (n 10, adjusted by the abiotic control samples, i.e. medium without faecal inoculation) was calculated, divided by the 16S rRNA gene copy number of the sample (determined by quantitative PCR as described earlier), and then plotted against incubation time. Student's t tests were used to compare the adjusted glucoraphanin degradation percentage between the five faecal culture samples from the high-ITC excreters and the five faecal culture samples from the low-ITC excreters after 24 and 48 h incubation. Stata 9.0 (StataCorp LP, College Station, TX, USA) was used for these statistical analyses. A P value < 0·05 was considered to be statistically significant. All tests were two-sided.
The tRFLP profiles were analysed as described elsewhere(Reference Li, Hullar and Schwarz17). Non-metric multidimensional scaling ordination (NMS) analysis, multi-response permutation procedure (MRPP), blocked multi-response permutation procedure (MRBP) and cluster analysis were performed based on the transformed P i values using PC-ORD (MjM Software Design, Gleneden Beach, OR, USA)(Reference Li, Hullar and Schwarz17). Both NMS and cluster analyses were used to assess the bacterial community composition of twenty faecal samples (from ten participants, two collections per participant) and twenty faecal culture samples (from ten participants, after 24 and 48 h incubation), respectively. MRPP was used to test (1) whether faecal bacterial communities differed between the high- and low-ITC excreters and (2) whether faecal culture bacterial communities after 48 h incubation differed between the high- and low-ITC excreters. MRBP was used to test (1) whether faecal culture bacterial communities inoculated with and without glucoraphanin differed after 48 h incubation and (2) whether faecal culture bacterial communities incubated with glucoraphanin for 24 h differed from those incubated for 48 h.
In addition, we used linear regression models to assess the relationships between faecal bacterial community structure and habitual cruciferous vegetable intake and between faecal bacterial community structure and urinary ITC excretion. The dependent variable was the NMS axis value of each faecal sample. The independent variable was the cruciferous vegetable intake (log-transformed) or the 24 h urinary ITC excretion after the first broccoli feeding. All models were adjusted for daily energy intake and dietary fibre intake. If any associations between the NMS axis and cruciferous vegetable intake or urinary ITC were found, additional linear regressions were performed. The independent variable was cruciferous vegetable intake or urinary ITC, and the dependent variable was the arcsine-transformed value of the specific tRFLP peak(s) that was significantly associated with the corresponding axis in the NMS analysis. To determine the putative taxonomic affiliation the identified tRFLP fragment(s) belonging to, the fragment size was compared with the in silico digestion results of the SILVA 100 16S rRNA gene database(Reference Pruesse, Quast and Knittel28) using Fragment Finder(Reference Kaplan, Hullar and Sappelsa29).
Results
Dietary intake, health and demographic data
In the present study, twenty-three healthy individuals, aged 20–70 years, participated. All, but one, were non-smokers. In the ten participants selected for further evaluation (including the only smoker), t tests showed that there were no significant differences in age, BMI, total energy intake, dietary fibre intake, cooked and total cruciferous vegetable intake between the low- and high-ITC excreters. The mean raw cruciferous vegetable intake appears different between the two groups but was not statistically significantly different (P = 0·23, Fisher's exact test), probably due to the high within-group variation (Table 1).
Table 1 Demographic, health and dietary intake information of the high- and low-isothiocyanate (ITC) excreters selected based on a standardised broccoli feeding
(Mean values and standard deviations)
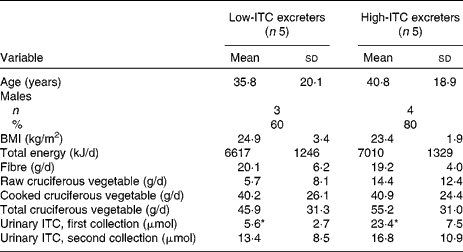
* Mean values were significantly different (P < 0·01).
Broccoli glucosinolates and isothiocyanate
After thioglucosidase treatment, the ITC content in the examined broccoli sample was measured as 0·6 μmol/g, which was translated into approximately 120 μmol of total glucosinolates in the fed broccoli. In contrast, in broccoli samples that were not treated with thioglucosidase, the ITC content was only 0·0015 μmol/g. This confirmed that there was little conversion of glucosinolate to ITC during the homogenising process of the cooked broccoli, indicating that plant myrosinase had been deactivated by cooking.
Urinary isothiocyanate
The inter-batch coefficient of variance for the quality-control sample was approximately 6 %, indicating a good method reproducibility. Total urinary ITC excretion after the first broccoli meal was 12·7 (sd 8·0) μmol/24 h for all twenty-three participants. There was substantial individual variation in total ITC excretion (range: 1·2–34·7 μmol/24 h, approximately 1–29 % of the ingested glucosinolate dose). The five high excreters had 23·4 (sd 7·5) μmol total ITC excretion/24 h, while the five low excreters had 5·6 (sd 2·7) μmol excretion/24 h (P < 0·01). When these selected ten participants were fed the second broccoli meal, there was no significant difference in 24 h urinary ITC excretion between the two groups (Table 1). This finding is contrary to our expectation.
Glucoraphanin degradation in faecal bacterial culture samples
The faecal bacteria from the low-ITC excreters (n 5) degraded 1·5 (sd 1·7) and 5·6 (sd 4·8) % of glucoraphanin after the 24 and 48 h incubation after correction for degradation in the abiotic control samples, while those of the high-ITC excreters (n 5) degraded 5·0 (sd 3·6) and 13·3 (sd 5·9) % of glucoraphanin after the 24 and 48 h incubation, respectively. Student's t tests showed that, after total bacterial 16S rRNA gene copy number adjustment, there was a significant difference in the proportion of glucoraphanin degraded by bacteria from the high-ITC excreters compared with the low-ITC excreters after 48 h (P = 0·05), but not after 24 h (P = 0·09) (Fig. 1).

Fig. 1 Bacterial degradation of glucoraphanin in the simulation of the human intestinal microbial ecosystem medium. Each line represents a faecal culture sample from one participant. ‘L’ and ‘H’ indicate low- and high-isothiocyanates excreters after the first broccoli feeding. Glucoraphanin in the medium was measured indirectly after hydrolysis to ITC. The proportion of glucoraphanin remaining in the medium was corrected by the abiotic control samples (corrected percentage of glucoraphanin left in the medium = observed percentage left in the medium/percentage left in the abiotic control sample) and adjusted by the 16S ribosomal RNA gene copy number (108 gene copies). ◆, 1H; ![]() , 4L;
, 4L; ![]() , 6L; ■, 8H; ▲, 11H; ●, 12H;
, 6L; ■, 8H; ▲, 11H; ●, 12H; ![]() , 13L;
, 13L; ![]() , 14H;
, 14H; ![]() , 15L;
, 15L; ![]() , 18L.
, 18L.
Faecal and culture bacterial community analysis
The final NMS analysis of twenty faecal bacterial communities showed that three axes explained a cumulative variation in the dataset of 88 %, with 32·4, 12 and 43·6 % explained by each of the axes. The faecal bacterial community structure was usually similar within the two collection points of the same participants, i.e. the intra-individual faecal bacterial community remained relatively stable within a period of 1–2 months between the two faecal collections. Cluster analysis showed that the two faecal bacterial communities of the same person clustered together for seven out of the ten participants, with six participants showing a similarity of >90 % between the two faecal bacterial communities (Fig. 2(a)). However, there was no indication of the faecal bacterial community clustering by ITC excreter status. MRPP confirmed that there was no significant difference between the faecal bacterial community structure of the high- and low-ITC excreters, at either the first collection point (P = 0·51) or the second collection point (P = 0·81).

Fig. 2 Cluster analysis of the bacterial community terminal restriction fragment length polymorphism profiles. The Wishart objective function was used to measure the bacterial community difference in the hierarchical dendrogram and was rescaled as percentage of similarity. (a) Faecal bacterial samples. The first number of the sample name indicates the participant number and the second number indicates the collection point (e.g. 1–1 represents sample of participant no. 1, first faecal collection). (b) Ex vivo faecal bacterial culture samples. The first number of the sample name indicates participant number and the second number indicates the day of incubation (e.g. 1–1 represents sample of participant no. 1, 1 d incubation). ▲, High-isothiocyanate excreters; △, low-isothiocyanate excreters.
NMS analysis of the twenty faecal ex vivo culture bacterial communities showed that two axes explained a cumulative variation in the dataset of 84·8, with 62·6 and 22·2 % explained by each of the axes. Cluster analysis showed that the two culture samples of each person clustered more loosely compared with the faecal samples (Fig. 2(b)), and that the faecal culture bacterial community did not cluster by ITC excreter status (Fig. 2(a)). MRPP confirmed that there was no significant difference between the faecal culture bacterial community structure of the high- and low-ITC excreters (P = 0·16). MRBP analysis showed that there was no difference between the faecal culture bacterial communities inoculated with glucoraphanin and those without glucoraphanin after 48 h incubation (P = 0·53). MRBP also showed that there was no difference between the faecal culture bacterial communities incubated with glucoraphanin for 24 h and those incubated for 48 h (P = 0·16).
Regression models showed that NMS axis 3 of the faecal bacterial communities was significantly inversely associated with habitual raw cruciferous vegetable intake (P = 0·01, R 2 0·37) but not with cooked or total cruciferous vegetable intake. There was also a trend towards an association between the tRFLP peak at 244 bp (which was inversely associated with NMS axis 3) and raw cruciferous vegetable intake (P = 0·06, R 2 0·26). Through the in silico digestion of the previously sequenced bacterial 16S rRNA genes in the SILVA database, we identified that several bacteria, including Alistipes putredinis (one of the most abundant bacterium found in the human gut), certain bacteria in the Lactococcus genus (e.g. Lactococcus lactis and Lactococcus piscium) and certain bacteria in the Desulfovibrio genus (e.g. Desulfovibrio desulfuricans and Desulfovibrio gigas), may represent this tRFLP fragment. No correlation was found between the NMS axes of the faecal bacterial communities and urinary ITC excretion.
Discussion
In the present pilot study, we examined the association between glucosinolate metabolism and gut bacterial community composition, both in vivo and ex vivo. We showed that (1) glucoraphanin degradation by faecal bacteria ex vivo differed significantly between the faecal inoculated bacterial culture samples of the high- and low-ITC excreters after 48 h incubation and (2) overall bacterial community structure did not differ significantly between the selected high- and low-ITC excreters, either in faecal samples or in ex vivo faecal culture samples.
After 48 h incubation, faecal bacteria from the high-ITC excreters degraded more glucoraphanin than those from the low-ITC excreters. We confirmed that the faecal bacteria from the high-ITC excreters compared with the low-ITC excreters were more effective at metabolising glucoraphanin, at least for those bacteria that were cultivated ex vivo. Other studies have shown that metabolites other than ITC can be formed from glucosinolates by bacteria(Reference Cheng, Hashimoto and Uda15, Reference Llanos Palop, Smiths and Brink16, Reference Krul, Humblot and Philippe30, Reference Combourieu, Elfoul and Delort31). Therefore, in theory, higher bacterial glucosinolate degradation rates do not necessarily always translate into a higher ITC yield and higher ITC exposure for the host. Nonetheless, the present results suggest that the capacity to hydrolyse glucoraphanin ex vivo may be related to overall ITC exposure in vivo. Due to the apparent instability of ITC in the culture medium, we were unable to establish directly the relationship between the ITC production in vivo and the bacterial conversion of glucosinolate to ITC ex vivo.
We did not observe any significant difference in overall faecal or culture bacterial community structure between the high- and low-ITC excreters, although we hypothesised that differences in gut microbiota may contribute to the inter-individual variation in glucosinolate to ITC conversion. Several reasons may explain this discordance. Most importantly, there may be functional redundancy in glucosinolate metabolism for gut bacteria, i.e. bacterial species that have the same metabolic function may not be related closely phylogenetically. Previous in vitro studies have observed that bacteria that are able to hydrolyse glucosinolates belong to several different phylogenetic families, including Actinobacteria, Firmicutes and Bacteroidetes(Reference Brabban and Edwards12–Reference Llanos Palop, Smiths and Brink16). Phylogenetic analysis of various glucosidase genes also showed that they are not solely attached to one bacterial phylogeny(Reference Mian32). Bacteria belonging to different phylogenetic groups may have relevant genes to code enzymes involved in releasing glucose from complex molecules in the gut environment for energy utilisation. Lateral gene transfer may also happen between different species. Thus, if different individuals harbour different types of distantly related bacteria having glucosinolate-metabolising activity, it would be difficult to identify an overall difference in gut microbiota between the high- and low-ITC excreters. Moreover, the tRFLP analysis was based on the bacterial 16S rRNA gene, but not on functional genes involved in glucosinolate metabolism. This may explain in part why we did not find an association between the urinary ITC amount and the faecal microbiota NMS axis values or a specific tRFLP fragment. Although the 16S rRNA gene is the best phylogenetic biomarker to examine community composition, it may not directly reflect the difference in gut bacterial glucosinolate metabolism among individuals.
The natural inter-individual difference in gut microbiota may also conceal the difference between the two groups of low- and high-ITC excreters. The tRFLP fingerprinting method applied in the present study offered us a rapid and efficient snapshot of gut microbiota, yet it may not provide sufficiently fine resolution of this complex community. The relatively subtle differences of a few species out of hundreds of members in the community among individuals may need to be resolved by more comprehensive and thorough techniques, such as sequencing. Finally, besides the gut bacterial composition, other factors may have influenced glucosinolate metabolism(Reference Shapiro, Fahey and Wade11, Reference Holst and Williamson33). The bioavailability of glucosinolate could be different when different processing and cooking methods are adapted(Reference Song and Thornalley34). ITC may be further degraded by bacteria(Reference Llanos Palop, Smiths and Brink16, Reference Tang, Bhothipaksa and Frank35). Polymorphisms in glutathione S-transferase genes influence the disposition of ITC in the body and thus ITC excretion in the urine(Reference Steck, Gammon and Hebert36–Reference Lampe and Peterson39). Despite our efforts to minimise the variation of some of these factors in the study, we also observed variation in total ITC excretion within the same participant after the two broccoli feedings. Interestingly, of the four participants who had the greatest difference in urinary ITC excretion between the two broccoli feedings (participant no. 1, 11, 13 and 15), three (no. 1, 11 and 13) also showed the substantial changes in faecal bacterial community during the period between the two feedings (Fig. 2(a)). These results suggest that the variation in ITC excretion may not be explained just by random variation, but that fluctuations in gut microbiota composition may contribute to the variation of in vivo glucosinolate metabolism. This supports the hypothesis that gut microbiota composition influences glucosinolate metabolism.
In the present study, we observed an association between the habitual raw cruciferous vegetable intake and one NMS axis (axis 3), more specifically, bacteria that represented the tRFLP fragment of 244 bp. However, no studies have examined the role of Lactococcus bacteria, Desulfovibrio bacteria or A. putredinis, the putative species representing this tRFLP fragment, in glucosinolate metabolism. One possible explanation is that gut bacteria might be exposed to a higher level of ITC when raw cruciferous vegetables are consumed by the host compared with the cooked cruciferous vegetables, because partial degradation of glucosinolate by plant myrosinase may happen before it reaches the colon. Whether these species have a role in further utilising ITC compounds in the human gut needs to be further examined. Desulfovibrio bacteria are sulphate-reducing bacteria, which utilise sulphate as their terminal electron acceptor for energy production(Reference Gibson40). Sulphate formed during the metabolism of sulphur-containing compounds (e.g. glucosinolates and ITC) in cruciferous vegetables may serve as the substrates for these bacteria and promote their growth in the human gut. It is also possible that other constituents in cruciferous vegetables (e.g. carbohydrates that can be utilised by Lactococcus and A. putredinis) or even other dietary behaviour associated with raw cruciferous vegetable consumption lead to this observed association between this tRFLP fragment and the habitual raw cruciferous vegetable intake.
The present study has several strengths. To our knowledge, this is the first human study that examines the relationships between gut microbiota composition, ITC production and cruciferous vegetable intake within the same group of individuals. Habitual cruciferous vegetable intakes were estimated more precisely using a validated cruciferous vegetable-specific FFQ(Reference Thomson, Newton and Graver18). Potential confounding factors such as total energy and fibre intakes were adjusted in the regression models. The amount of broccoli fed and cooking procedures were well controlled. Participants consumed all the broccoli provided under supervision of the study staff and reported no other cruciferous vegetable consumption 72 h before and during the urine collection; this helped to normalise glucosinolate intake across all participants. Glucoraphanin, the glucosinolate we used in the ex vivo incubation, is the major glucosinolate found in broccoli. Unlike some of the previous in vitro bacterial incubation studies which used glucosinolate concentrations in the range of 1–15 mm(Reference Llanos Palop, Smiths and Brink16, Reference Krul, Humblot and Philippe30, Reference Combourieu, Elfoul and Delort31), the concentration that we used, 50 μm, could more accurately reflect luminal concentrations expected after the consumption of 120 μmol glucosinolates. We used tRFLP, a high-throughput molecular fingerprinting approach, and geometric analysis methods to characterise the faecal and culture bacterial communities. These analyses offered rapid yet reliable data for a picture of overall community composition. Additionally, we were also able to link putative bacterial species to specific tRFLP fragments based on the available bacterial 16S sequences.
We recognise that energy intake and cruciferous vegetable intake collected through FFQ may be subject to recall bias, which might lead to either over- or underestimation of the strength of association between dietary intake and the biological measurements. Using the SHIME medium for bacterial cultivation might be considered both as a strength and a limitation of the present study. The SHIME medium was formulated to mimic substrate available to bacteria in the human gut and therefore maximise gut bacterial growth(Reference Molly, Vande Woestyne and Verstraete20). In this regard, addition of glucoraphanin may not have been a sufficient enough perturbation in the substrate pool to select for glucosinolate-metabolising bacteria. This may partially explain why we did not observe overall differences in gut bacterial communities between the faecal culture samples incubated with and without glucoraphanin. There are several other limitations to the present study. The small sample size probably limits our ability to find existing differences in gut microbiota composition between the two ITC excreter groups. Nonetheless, the present study further demonstrates the complexity of the human gut microbiota, in terms of both community composition and metabolic capacity. There are also large differences between the in vivo bacterial metabolism and in vitro bacterial incubation model. Because ≤ 30 % of gut bacterial species can be cultivated and due to the lack of food–food interactions(Reference Hayashi, Sakamoto and Benno41), observations in vitro need to be carefully evaluated before links can be established to the in vivo results. Also, faecal samples differ in gut bacterial composition compared with luminal contents. Bacterial community composition changes along the digestive tract and between different environments such as intestinal mucosa and lumen. Nonetheless, because the variation in gut bacterial community within a person is much less than the variation between individuals, faecal samples remain the best available samples for studies in healthy, intact humans(Reference Eckburg, Bik and Bernstein42).
In summary, we observed significant differences in the proportion of glucoraphanin degraded ex vivo by faecal bacteria obtained from the high- and low-ITC excreters who were identified based on a standardised broccoli meal. However, no difference was observed in bacterial community structure between the two groups, either in faecal samples or in ex vivo faecal bacterial cultivation samples. We also identified putative gut bacterial species that may be associated with raw cruciferous vegetable intake. More intensive bacterial sequencing may help further elucidate the community composition and possible differences that cannot be revealed by tRFLP fingerprinting. Identification of gut bacterial species associated with the conversion of glucosinolates to ITC may inform prebiotic and probiotic strategies to alter the production of ITC in vivo, allowing individuals to derive more health benefits from cruciferous vegetable consumption.
Acknowledgements
F. L., M. A. J. H. and J. W. L. designed the study; F. L. conducted the study; F. L., M. A. J. H., S. A. A. B. and J. W. L. analysed the data; F. L., M. A. J. H. and J. W. L. wrote the manuscript; J. W. L. had the primary responsibility for the final content. All authors read and approved the final manuscript. The authors have no conflicts of interest. The present study was funded by the National Cancer Institute grants R01 CA070913 and R56 CA070913.





