Clinical, biomedical and in vitro studies have provided evidence that conjugated 18-C fatty acids (FA) including isomers of conjugated linoleic acid (CLA) exhibit potential anti-inflammatory, immuno-modulatory, anti-obesity and anti-carcinogenic activities as well as improve biomarkers of cardiovascular health( Reference Wahle, Heys and Rotondo 1 , Reference Benjamin and Spener 2 ). Isomers of conjugated linolenic acid (CLN) containing a conjugated diene double-bond system also have similar biological effects( Reference Koba and Yanagita 3 , Reference Hennessy, Ross and Devery 4 ) with potential as therapeutics for regulating blood glucose and body composition in humans( Reference Bassaganya-Riera, Guri and Hontecillas 5 ). Recent studies in experimental animal models or in vitro have also indicated that isomers of CLN containing a conjugated triene system have anti-carcinogenic and anti-lipogenic activity and may influence immune function( Reference Koba, Belury and Sugano 6 ). A more complete understanding of the mechanisms responsible for the formation of CLN and CLA isomers from α-linolenic acid (cis-9,cis-12,cis-15-18 : 3, ALA) is essential for the synthesis of these compounds in amounts required for the prevention of chronic human diseases or establishing biological activity in a range of mammalian species.
Ruminant fat is relatively abundant in CLA and CLN isomers containing a conjugated diene arrangement of double bonds( Reference Plourde, Destaillats and Chouinard 7 – Reference Gómez-Cortés, Tyburczy and Brenna 9 ), but only trace amounts of CLN isomers containing a conjugated triene double-bond system, which are typically found in certain plant seeds including pomegranate, tung, bitter gourd, catalpa and pot marigold( Reference Hopkins and Chisholm 10 ). In vitro studies have demonstrated that isomers of CLN trienes, prepared by alkaline treatment, have higher cytotoxic activity during incubations with human tumour cells than their non-conjugated counterparts or CLA isomers( Reference Igarashi and Miyazawa 11 ).
Meat and milk from ruminants are the principal source of CLA in the human diet, with cis-9,trans-11 as the major isomer( Reference Parodi 12 , Reference Chin, Liu and Storkson 13 ). Ruminant foods also contain a wide range of CLA isomers with double bonds located from Δ7,9 to Δ12,14 and isomers of Δ9,11,15-CLN( Reference Plourde, Destaillats and Chouinard 7 – Reference Gómez-Cortés, Tyburczy and Brenna 9 ). Geometric isomers of Δ9,11-CLA and Δ10,12-CLA are formed during the initial isomerisation of linoleic acid (cis-9,cis-12-18 : 2, LA) in the rumen( Reference Palmquist, Lock and Shingfield 14 – Reference Honkanen, Griinari and Vanhatalo 16 ). However, other CLA isomers accumulate in the rumen of cattle fed diets rich in ALA( Reference Loor, Ueda and Ferlay 17 – Reference Shingfield, Lee and Humphries 19 ). Metabolism of ALA in the rumen is thought to involve an initial isomerisation to yield cis-9,trans-11,cis-15-CLN, which is sequentially reduced to trans-11,cis-15-18 : 2 and trans-11-18 : 1 with 18 : 0 as an end product( Reference Wilde and Dawson 20 – Reference Harfoot and Hazlewood 22 ). Historical studies of ruminal ALA biohydrogenation do not consider isomers of CLA as intermediates. However, alternative pathways of ALA biohydrogenation, involving the formation of cis-9,trans-11,trans-15-CLN( Reference Gómez-Cortés, Tyburczy and Brenna 9 ), trans-9,trans-11,cis-15-CLN( Reference Waşowska, Maia and Niedzwiedzka 23 ), cis-9,trans-13,cis-15-CLN( Reference Destaillats, Trottier and Galvez 24 ) and trans-10,cis-12,cis-15-CLN( Reference Griinari and Bauman 25 ), have been proposed but not proven. Incubations of 13C-labelled ALA with bovine rumen contents were reported to result in the accumulation of fourteen uncharacterised 18 : 3 intermediates( Reference Lee and Jenkins 26 ), highlighting the complexity of ruminal ALA biohydrogenation.
Recent studies have characterised a range of intermediates formed during the incubation of ALA with ovine or bovine rumen contents( Reference Waşowska, Maia and Niedzwiedzka 23 , Reference Lee and Jenkins 26 , Reference Jouany, Lassalas and Doreau 27 ), but none has provided unambigious evidence on the biochemical pathways responsible. The present study investigated the products formed and possible mechanisms involved in the initial stages of ALA biohydrogenation by rumen microbiota based on an examination of the incorporation of 2H in FA intermediates formed during incubations of ALA with bovine rumen fluid diluted with water or deuterium oxide (D2O).
Methods
Collection of rumen contents
All experimental procedures involving animals were approved by the National Animal Ethics Committee (Hämeenlinna, Finland), in accordance with the guidelines outlined in the European Community Council Directive 86/609/EEC( 28 ). Four multiparous Finnish Ayrshire cows of mean 253 (sd 11·3) d in lactation and 601 (sd 60) kg live weight, and fitted with a rumen cannula (i.d. 100 mm; Bar Diamond, Inc.), were used as donors. Each cow received a diet based on a mixture of timothy and meadow fescue grass silage and concentrates (forage:concentrate ratio 60:40, on a DM basis). Concentrates comprised rolled barley (293 g/kg), rolled oats (270 g/kg), molassed sugar beet pulp (130 g/kg), rapeseed expeller (280 g/kg) and a proprietary mineral and vitamin premix (27 g/kg; Onni-Kivennäinen). Silage and concentrates were offered four times daily at 06.45, 13.00, 16.00 and 19.00 hours for 14 d before starting in vitro incubations. Cows were housed in a barn fitted with individual stalls with continuous access to water, and were milked at 06.30 and 16.45 hours. Samples of rumen contents (500 ml) were collected into plastic bottles from each cow immediately before morning feeding, placed in a 39°C water bath and transported to the laboratory.
Incubations with rumen contents
Batch culture incubations were performed in 100 ml glass flasks( Reference Honkanen, Griinari and Vanhatalo 16 ). Strained rumen contents were diluted 1:2 (v/v) with modified McDougall buffer( Reference Honkanen, Griinari and Vanhatalo 16 ) prepared using de-ionised water or 99 % 2H-enriched water (D2O; Cambridge Isotope Laboratories, Inc.). A sample of 50 ml of diluted rumen fluid, 400 mg of ground dry hay, 5 mg of ALA (10-1803-30; Larodan Fine Chemicals AB) prepared as a suspension in aqueous Tween 80( Reference Honkanen, Griinari and Vanhatalo 16 ) and 5 mg of 19:0 (10-1900-13; Larodan Fine Chemicals AB) dissolved in ethanol (5 mg/ml) was incubated under carbon dioxide at 39°C for 0, 1·5, 3·0 and 12 h. Control incubations containing hay, Tween 80 and rumen fluid diluted with de-ionised water were also established over the same time course. At each designated time point, flasks were placed immediately in ice-cold water, and the contents were stored at −20°C. Incubations were performed in triplicate with samples of rumen contents from each cow.
Lipid extraction and fatty acid analysis
Flask contents were freeze-dried for 24 h at −90°C under a partial pressure of 103 Pa (Brown Christ Gamma 2; Melsungen AG). In total, 200 mg of freeze-dried incubation contents were mixed with 0·5 ml of de-ionised water and the pH was adjusted to 2·0 with 2 m-hydrochloric acid. Lipids were extracted in 4 ml of heptane–isopropanol (3:2, v/v). Extraction was repeated and both organic phases recovered were combined, washed with de-ionised water, dried over 200 mg of sodium sulphate and evaporated to dryness under a constant stream of N2 at 30°C. Fatty acid methyl esters (FAME) were prepared from total lipids using a two-step base–acid catalysed procedure( Reference Kairenius, Toivonen and Shingfield 29 ). Samples of FAME were also converted to 4,4-dimethyloxazoline (DMOX) derivatives by incubation overnight with 2-amino-2-methyl-1-propanol under an N2 atmosphere at 170°C( Reference Kairenius, Toivonen and Shingfield 29 ).
FAME were quantified using a GC (model 6890; Hewlett-Packard) equipped with a flame-ionisation detector and a 100-m fused silica capillary column (i.d. 0·25 mm) coated with a 0·2-µm film of cyanopropyl polysiloxane (CP-SIL 88; Agilent Technologies Inc.). The total FAME profile in a 2-μl sample at a split ratio of 1:50 was determined using a temperature gradient programme( Reference Shingfield, Ahvenjärvi and Toivonen 30 ), and H2 as a carrier gas operated at 206·8 kPa for 50 min, which was increased at a rate of 34·5 kPa/min to a final pressure of 310·3 kPa, that was maintained for a further 7 min. The nominal initial flow rate was 2·1 ml/min. FAME and DMOX derivatives were analysed by GC-MS using a GC equipped with a quadrupole selective mass detector (model 5973N; Agilent Technologies Inc.), operated at 230°C in the positive electron ionisation mode using an ionisation voltage of 70 eV. Chromatography was performed using the same temperature gradient and column type used for GC analysis of FAME and helium as the carrier gas( Reference Kairenius, Toivonen and Shingfield 29 ). The analysis was repeated to separate methyl esters of trans-10,cis-15-18 : 2 and trans-11,cis-15-18 : 2 using an alternative 100-m column coated with a highly polar ionic liquid SLB-IL111 column (100 m×0·25 mm i.d., 0·2-µm film thickness; Sigma-Aldrich)( Reference Delmonte, Fardin-Kia and Kramer 31 , Reference Alves and Bessa 32 ). Helium was used as the carrier gas, operated at a nominal initial flow rate of 1·0 ml/min at a constant pressure of 264·8 kPa with a temperature programme as follows: initial oven temperature was maintained at 168°C for 30 min, increased at 1°C/min to 200°C and maintained at 200°C temperature for 10 min. The distribution of CLA and CLN isomers was determined by HPLC (model 1090; Hewlett-Packard) using four silver-impregnated silica columns (ChromSpher 5 Lipids, 250×4·6 mm; 5-µm particle size; Agilent Technologies Inc.) coupled in series. Methyl esters of CLA or CLN were separated under isocratic conditions at 22°C using 0·1 % or 0·2 % (v/v) acetonitrile in heptane, respectively, at a flow rate of 1 ml/min and monitoring column effluent at 233 and 268 nm( Reference Shingfield, Ahvenjärvi and Toivonen 30 ). Isomers were identified based on retention time comparisons with methyl ester standards containing a mixture of CLA isomers (Sigma-Aldrich), or geometric isomers of 8,10,12-CLN and 9,11,13-CLN (Larodan Fine Chemicals AB).
FA were identified based on GC-MS analysis of FAME and DMOX derivatives and interpretation of mass spectra according to published guidelines( Reference Spitzer 33 , Reference Christie 34 ). For most products, the deduced FA structure was verified by comparison with an online reference spectra library( 35 ). Double-bond geometry was deduced based on relative retention times and known elution order for a mixture of geometric ∆9,12-18 : 2 and ∆9,12,15-18 : 3 methyl esters (Sigma-Aldrich) during GC analysis( Reference Kramer, Blackadar and Zhou 36 ). The double-bond geometry of 9,11,15-18 : 3 isomers was inferred based on the elution order reported in the literature( Reference Lerch, Shingfield and Ferlay 8 , Reference Gómez-Cortés, Tyburczy and Brenna 9 , Reference Waşowska, Maia and Niedzwiedzka 23 ).
Enrichment of m/z n+1, n+2 and n+3 isotopomers (molecular ion +1, +2 and +3, respectively) was determined by GC-MS analysis of FAME. Enrichment in water was determined by gas isotope ratio MS using a VG SIRA 10 (VG Isotech) gas isotope ratio MS fitted with a split flight tube and H/2H collector( Reference Wallace, McKain and Shingfield 15 ). Corrections for H3+ were made using dedicated software at the time of measurement. The position of 2H labelling of incubation products was determined by GC-MS analysis of DMOX derivatives.
Data analysis
Amounts of FA in incubation flasks were analysed by ANOVA for repeated measures with a statistical model that included the fixed effect of incubation time, treatments (control and test incubations containing added ALA with de-ionised water or D2O) and their interaction and random effect of replicate nested within cow assuming a compound symmetry covariance structure using the MIXED procedure of SAS (version 9.2; SAS Institute, Inc.). Denominator df were calculated using the Kenward-Rogers method. The same statistical model was used to compare the abundance of n+1, n+2 and n+3 isotopomers of products formed during incubations of ALA with D2O or de-ionised water (natural enrichment). Enrichment of n+1, n+2 and n+3 isotopomers was calculated from the m/z ratios at n, n+1, n+2, n+3 and n+4 by deconvolution( Reference Wallace, McKain and Shingfield 15 , Reference Campbell 37 ). Changes in the enrichment of n+1, n+2 and n+3 isotopomers of products formed during 0, 1·5, 3·0 and 12 h of incubation of ALA with D2O were analysed by ANOVA for repeated measures with a statistical model that included the fixed effect of incubation time and random effect of replicate nested within cow assuming a compound symmetry covariance structure and calculation of denominator df by the Kenward-Rogers method. Fixed effects were considered significant at P<0·05. Least square means with pooled standard errors are reported.
Results
Metabolism of α-linolenic acid during incubations with strained rumen contents
Strained ruminal digesta samples collected from four lactating cows were diluted with buffer containing de-ionised water or D2O and incubated with ALA under anaerobic conditions for up to 12 h. Concentrations of added ALA declined rapidly (Fig. 1), resulting in the appearance (P<0·05) of 18 : 3 and 18 : 2 intermediates relative to control incubations (Fig. 2). The relative disappearance of ALA (Fig. 1) and formation of intermediates were similar (Fig. 2), but not identical during incubations of ALA with rumen fluid diluted with water or D2O.

Fig. 1 Disappearance of α-linolenic acid (ALA) during 0 to 12 h incubations of ground hay with strained rumen fluid diluted in de-ionised water (![]() ), rumen fluid diluted in de-ionised water and 5 mg of added ALA (
), rumen fluid diluted in de-ionised water and 5 mg of added ALA (![]() ) or rumen fluid diluted in 56·6 (sem 1·33) % moles per cent excess (MPE) deuterium oxide and 5 mg of added ALA (
) or rumen fluid diluted in 56·6 (sem 1·33) % moles per cent excess (MPE) deuterium oxide and 5 mg of added ALA (![]() ). Rumen contents were collected from four cows and incubated at 39°C under carbon dioxide. Each point represents the least square mean of n 12 measurements (pooled sem 0·083 mg/flask). a,b,c Mean values for each incubation time with unlike letters were significantly different (P<0·05).
). Rumen contents were collected from four cows and incubated at 39°C under carbon dioxide. Each point represents the least square mean of n 12 measurements (pooled sem 0·083 mg/flask). a,b,c Mean values for each incubation time with unlike letters were significantly different (P<0·05).
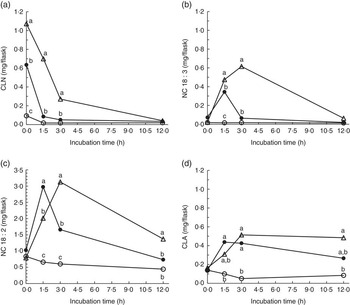
Fig. 2 Formation of (a) total conjugated linolenic acid (CLN), (b) non-conjugated 18 : 3 (NC 18 : 3), (c) non-conjugated 18 : 2 (NC 18 : 2) and (d) conjugated linoleic acid (CLA) during 0 to 12 h incubations of ground hay with strained rumen fluid diluted in de-ionised water (![]() ), rumen fluid diluted in de-ionised water and 5 mg of added α
-linolenic acid (ALA) (
), rumen fluid diluted in de-ionised water and 5 mg of added α
-linolenic acid (ALA) (![]() ) or rumen fluid diluted in 56·6±1·33 % moles per cent excess deuterium oxide and 5 mg of added ALA (
) or rumen fluid diluted in 56·6±1·33 % moles per cent excess deuterium oxide and 5 mg of added ALA (![]() ). Rumen contents were collected from four cows and incubated at 39°C under carbon dioxide. Each point represents the mean of n 12 measurements (sem 0·026, 0·020, 0·098 and 0·043 mg/flask for total CLN, NC 18 : 3, NC 18 : 2 and CLA, respectively). a,b,c Mean values for each incubation time with unlike letters were significantly different (P<0·05).
). Rumen contents were collected from four cows and incubated at 39°C under carbon dioxide. Each point represents the mean of n 12 measurements (sem 0·026, 0·020, 0·098 and 0·043 mg/flask for total CLN, NC 18 : 3, NC 18 : 2 and CLA, respectively). a,b,c Mean values for each incubation time with unlike letters were significantly different (P<0·05).
Formation of octadecatrienoic acids during incubation of α-linolenic acid with strained rumen contents
Addition of ALA increased (P<0·05) the formation of CLN isomers compared with hay and rumen fluid alone (Table 1). Cis-9,trans-11,cis-15-18 : 3 was the most abundant CLN isomer in all incubations, but smaller amounts of trans-9,trans-11,cis-15-CLN were also detected (Table 1). Cis-9,trans-11,Cis-15-CLN was formed in the greatest quantity immediately after the addition of ALA to strained rumen contents, but disappeared from flask contents over the course of 12 h incubations. Formation of trans-9,trans-11,cis-15-CLN from ALA was greatest during the first 1·5–3 h of incubation, but the amounts declined thereafter. Small amounts of trans-9,trans-11,cis-13-CLN were also formed (Table 1). Addition of ALA with rumen contents increased (P<0·05) the appearance of cis-7,cis-12,cis-15-18 : 3, cis-8,cis-12,cis-15-18 : 3 and trans-8,cis-12,cis-15-18 : 3 in flask contents, with the highest amounts detected after 1·5 and 3 h of incubation.
Table 1 Amounts of 18 : 3 intermediates formed during 0 to 12 h incubations of α-linolenic acid with rumen contents diluted in buffer prepared using de-ionised H2O or deuterium oxide (D2O)Footnote * (Mean values with their pooled standard errors)

a,b,c Mean values within a row for each incubation time with unlike superscript letters were significantly different (P<0·05).
* Incubations established in 100-ml flasks containing 400 ml ground dried hay and 50 ml of diluted rumen fluid maintained at 39°C under carbon dioxide containing no additional α-linolenic acid (CON) or 5 mg of added α-linolenic acid and rumen fluid diluted with de-ionised H2O or D2O.
† Significance due to incubation time, incubation treatment and their interaction.
‡ Pooled sem for n 36 measurements.
All incubation flasks contained ∆10,12,15-CLN. Addition of ALA increased (P<0·05) ∆10,12,15-CLN formation during 1·5 h of incubation with rumen contents diluted in water (Table 1). However, formation of 10,12,15-CLN between 0 and 3 h did not differ (P>0·05) between incubations of ALA with D2O and the control. After 12 h, the amounts of ∆10,12,15-CLN were lower (P<0·05) for incubations containing D2O (Table 1).
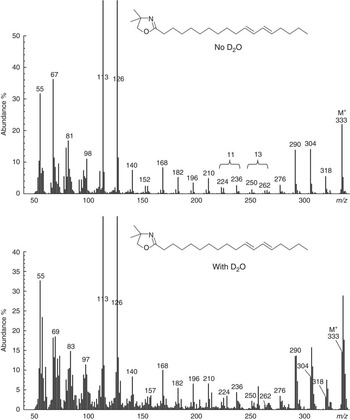
Mass spectra of methyl esters prepared from flask contents over the course of 0 to 12 h incubations of ALA with D2O indicated that geometric isomers of ∆9,11,15-CLN contained a single 2H label. Independent analysis of the enrichment in water allowed for the comparison of the ratio of moles per cent excess (MPE) in FAME with the MPE of water. All samples of water had a similar enrichment of 56·6 (SE 1·33) %. Calculated labelling (MPE intermediate/MPE water) averaged 0·79 and 0·83 for cis-9,trans-11,cis-15-18 : 3 and trans-9,trans-11,cis-15-CLN, respectively (Table 2). The mass spectrum of the DMOX derivative of cis-9,trans-11,cis-15-CLN formed during incubations of ALA with D2O containing buffer indicated enrichment of ion fragments from the molecular ion to m/z 262 (Fig. 3). The occurrence of ion fragment isotopomers with m/z<262 was comparable with the natural abundance of 22·2 %. Analysis of ion fragments located the incorporation of 2H on C-13 of the FA moiety. The mass spectrum of trans-9,trans-11,cis-15-CLN indicated a similar pattern of enrichment with labelling on C-13 (data not presented).

Fig. 3 Mass spectrum of the 4,4-dimethyloxazoline derivative of cis-9,trans-11,cis-15-18 : 3 formed from α-linolenic acid during incubations with strained rumen contents diluted with buffer prepared using de-ionised water or deuterium oxide. Gaps of 12 atomic mass units between m/z 196 and 208, 222 and 234 and 276 and 288 located double bonds at Δ9, 11 and 15, respectively. Enrichment of ion fragments at m/z 262 and 263 indicate labelling on C-13 of the fatty acid moiety during incubations with 2H-containing buffer.
Table 2 Enrichment of n+1, n+2 and n+3 isotopomers of 18 : 3 intermediates formed during incubations of α-linolenic acid (ALA) with strained rumen contents diluted in 2H-containing bufferFootnote * (Mean values with their pooled standard errors)

a,b,c Mean values within a row for each incubation time with unlike superscript letters were significantly different (P<0·05).
* Enrichment calculated from the ratio of moles per cent excess (MPE) in the incubation product/MPE in water. Mean 56·6 (se 1·33) % MPE enrichment in deuterated water. Natural abundance of n+1 and n+2 isotopomers was determined for the same products formed during incubations of ALA with strained rumen contents diluted in de-ionised water.
† Significance due to incubation time.
‡ Pooled sem for n 12 measurements.
Labelling (MPE sample/MPE water) of n+1 isotopomers of trans-9,trans-11,cis-13-CLN increased (P<0·001) during 12 h incubations with ALA (Table 2). Abundance of n+1 isotopomers of trans-9,trans-11,cis-13-CLN formed during 1·5, 3 and 12 h of incubation of ALA with D2O was higher (P<0·001) compared with natural abundance (average 35·7 and 21·8 %, respectively). Enrichment of n+1 isotopomers of cis-7,cis-12,cis-15-18 : 3, cis-8,cis-12,cis-15-18 : 3 and trans-8,cis-12,cis-15-18 : 3 formed after 1·5 and 3 h of incubation with ALA averaged 0·01, −0·19 and 0·01, respectively (Table 2). Relative abundance of n+1 isotopomers for Δ7,12,15-18 : 3 and Δ8,12,15-18 : 3 formed in the presence of D2O did not differ (P>0·05) from natural enrichment. Furthermore, the mass spectra of the DMOX derivatives of cis-7,cis-12,cis-15-18 : 3 (online Supplementary Fig. S1) and cis-8,cis-12,cis-15-18 : 3 (online Supplementary Fig. S2) provided no evidence of 2H labelling.
The ∆10,12,15-CLN isomer eluted immediately after cis-9,trans-11,cis-15-CLN during GC-MS analysis. It was not possible to obtain reliable mass spectra for the methyl ester or DMOX derivative of ∆10,12,15-CLN formed during 0 to 12 h incubations of ALA with D2O. Calculated labelling at n+1 of ∆10,12,15-CLN formed after 1·5 and 3 h incubations averaged 0·28 (Table 2). Relative abundance of n+1 isotopomers of ∆10,12,15-CLN was higher compared with natural enrichment (P<0·05), but the position of the 2H label could not be located. Concentrations of 10,12,15-CLN were too low to allow the MPE at n+2 to be estimated accurately.
Formation of octadecadienoic acids during incubation of α-linolenic acid with strained rumen contents
Cis-12,cis-15-18 : 2 and trans-11,cis-15-18 : 2 represented the major non-conjugated 18 : 2 products formed during incubations with ALA, with smaller amounts of trans-10,cis-15-18 : 2 also detected (Table 3). Incubation of ALA with rumen contents diluted with water increased (P<0·05) the amount of cis-12,trans-14-CLA, trans-11,cis-13-CLA, trans-11,trans-13-CLA, trans-12,cis-14-CLA, trans-12,trans-14-CLA and trans-13,trans-15-CLA in flask contents (Table 3). However, the amounts of CLA products formed differed during incubations of ALA with D2O or water. Trans-10,cis-12-CLA and trans-10,trans-12-CLA were detected in all flask contents but the amount of trans-10,trans-12-CLA did not change (P=0·47) over the course of all incubations (Table 3). The amount of trans-10,cis-12-CLA in samples containing added ALA with water or D2O decreased (P<0·05) during incubation from 3 to 12 h.
Table 3 Amounts of 18 : 2 intermediates formed during 0 to 12 h incubations of α-linolenic acid with rumen contents diluted in buffer prepared using de-ionised H2O or deuterium oxide (D2O)Footnote * (Mean values with their standard errors)

a,b,c Mean values within a row for each incubation time with unlike superscript letters were significantly different (P<0·05).
* Incubations established in 100-ml flasks containing 400 ml ground dried hay and 50 ml of diluted rumen fluid maintained at 39°C under carbon dioxide containing no additional α-linolenic acid (CON) or 5 mg of added α-linolenic acid and rumen fluid with de-ionised H2O or D2O.
† Significance due to incubation time, incubation treatment and their interaction.
‡ sem for n 36 measurements.
Mass spectra of methyl esters were used to calculate the MPE ratios of n+1, n+2 and n+3 isotopomers of 18 : 2 products formed during incubations with added ALA (Table 4). Enrichment of n+1 isotopomers of cis-12,cis-15-18 : 2, trans-10,cis-15-18 : 2, trans-11,cis-15-18 : 2, trans-11,cis-13-CLA, trans-11,trans-13-CLA and cis-9,trans-11-CLA from 1·5 to 12 h incubations averaged 1·13, 1·03, 1·01, 0·89, 0·83 and 0·18, respectively (Table 4). Labelling at n+2 for the same intermediates averaged 0·99, 0·75, 0·74, 0·63, 0·46 and −0·01, respectively. Average enrichment of n+3 isotopomers was 0·43, 0·17, 0·13, 0·05, 0·16 and <0·01, respectively. For 3 to 12 h incubations, enrichment of n+1, n+2 and n+3 isotopomers of trans-12,trans-14-CLA averaged 1·0, 0·91 and 0·52, respectively.
Table 4 Enrichment of n+1, n+2 and n+3 isotopomers of 18 : 2 intermediates formed during incubations of α-linolenic acid (ALA) with strained rumen contents and 2H-containing bufferFootnote * (Mean values with their pooled standard errors)

a,b,c Mean values within a row for each incubation time with unlike superscript letters were significantly different (P<0·05).
* Enrichment calculated from the ratio of moles per cent excess (MPE) in the incubation product/MPE in water. Mean 56·6 (se 1·33) % MPE enrichment in deuterated water. Natural abundance of n+1, n+2 and n+3 isotopomers was determined for the same products formed during incubations of ALA with strained rumen contents diluted in de-ionised water.
† Significance due to incubation time.
‡ Pooled sem for n 12 measurements.
The molecular ion of the DMOX derivative of trans-11,cis-15-18 : 2 formed during incubations of ALA with D2O at m/z 335 (n+2) confirmed the incorporation of two 2H labels in the FA moiety (Fig. 4). Relative abundances of ion fragments at m/z 210 and 211 and at m/z 264, 265 and 266 located the incorporation of a single 2H on C-9 and another on C-13. The mass spectrum of the DMOX derivative of cis-12,cis-15-18 : 2 formed in the presence of D2O indicated a molecular ion at m/z 336 (n+3), confirming an octadecadienoic acid structure and incorporation of three 2H atoms, but the position of each label could not be determined accurately (online Supplementary Fig. S3). Furthermore, the amounts of trans-10,cis-15-18 : 2 in flask contents were too low to obtain reliable MS spectra. Mass spectra of the methyl esters of trans-11,cis-13-CLA and trans-11,trans-13-CLA revealed a molecular ion at m/z 296 (n+2), indicating that both intermediates formed from ALA during incubations with D2O contained two 2H labels. The mass spectrum of the DMOX derivative of trans-11,trans-13-CLA (Fig. 5) indicated enrichment in ion fragments at m/z 334 and 335 (n+1 and n+2, respectively), confirming the incorporation of two or more 2H atoms. An increase in ion fragment isotopes at m/z 211 (n+1) and m/z 306 (n+2) suggested labelling at C-9 and C-16, respectively. However, during the course of incubations with D2O, the abundance of the ion fragment at m/z 336 (n+3) increased, which along with enrichment of n+2 isotopomers with m/z<306 suggested that another 2H label was incorporated between C-9 and C-16. The mass spectrum of the DMOX derivative of trans-12,trans-14-CLA revealed a molecular ion at m/z 336 (data not presented), indicating incorporation of three 2H atoms, but the locations could not be established.

Fig. 4 Mass spectrum of the 4,4-dimethyloxazoline derivative of trans-11,cis-15-18 : 2 formed from α-linolenic acid during incubations with strained rumen contents diluted with buffer prepared using de-ionised water or deuterium oxide. An abundant ion at m/z 264 along with gaps of 12 atomic mass units between m/z 224 and 236 and 278 and 290 confirmed a Δ11,15 double-bond arrangement. Enrichment of ion fragments at m/z 210 and 211 (n+1) and 264 and 266 (n+2) indicated labelling on C-9 and C-13 of the fatty acid moiety during incubations with 2H-containing buffer.

Fig. 5 Mass spectrum of the 4,4-dimethyloxazoline derivative of trans-11,trans-13-18 : 2 from α-linolenic acid during incubations with strained rumen contents diluted with buffer prepared using de-ionised water or deuterium oxide. Gaps of 12 atomic mass units between m/z 224 and 236 and 250 and 262 located double bonds at ∆11 and 13, respectively. Enrichment of ion fragments at m/z 210 and 211 (n+1) and 304 and 306 (n+2) provide tentative evidence of labelling on C-9 and C-16 of the fatty acid moiety during incubations with 2H-containing buffer.
Formation of octadecenoic acids, oxygenated fatty acids and stearic acid during incubation of α-linolenic acid with strained rumen contents
A mixture of trans-4 to trans-16-18 : 1 and cis-9 to cis-16-18 : 1 accumulated during incubations with ALA. Trans-11,-13,-14,-15 and -16-18 : 1 and cis-15-18 : 1 represented the most abundant octadecenoic intermediates. Owing to extensive labelling during incubations with D2O, none of the 18 : 1 isomers could be resolved during GC analysis, preventing the amounts synthesised and enrichment patterns to be accurately determined. Nevertheless, the mass spectrum of the DMOX derivative of trans-11-18 : 1 indicated the incorporation of 2H atoms on C-9, C-13 and C-15 of the FA moiety (data not presented).
Incubations with ALA also resulted in the formation of 9-O-18 : 0, 10-O-18 : 0 and 13-O-18 : 0, none of which was labelled after 12 h (data not presented). For all incubations, 18 : 0 was the end product containing multiple 2H labels. The molecular ion of the DMOX derivative of 18 : 0 at m/z 342 (337+5) indicated that up to five 2H atoms were incorporated during the reduction of ALA to 18 : 0, but the locations could not be determined.
Discussion
Most reports based on incubations with ruminal digesta( Reference Waşowska, Maia and Niedzwiedzka 23 ) or pure cultures of rumen bacteria( Reference Kepler and Tove 38 ) indicate that biohydrogenation of ALA is initiated by isomerisation of the cis-12 double bond to yield a cis-9,trans-11,cis-15-CLN intermediate catalysed by a 12-cis,11-trans isomerase( Reference Lee and Jenkins 26 ). Alternative pathways of ALA biohydrogenation have been proposed( Reference Gómez-Cortés, Tyburczy and Brenna 9 , Reference Waşowska, Maia and Niedzwiedzka 23 – Reference Griinari and Bauman 25 ), but the mechanisms and products formed are not known. In the present investigation, incubations of ALA with ruminal contents were made with or without D2O to better understand the fate of ALA in the rumen. Control incubations were also performed, allowing identification of products formed from ALA during incubations with strained rumen contents. The non-ionic surfactant Tween 80 was used to disperse ALA that has been shown to result in more extensive biohydrogenation during incubations with rumen contents compared with sonication or dissolving substrates in ethanol( Reference Khiaosa-ard, Leiber and Soliva 39 ). Incubations with rumen contents diluted with water or D2O resulted in the appearance of similar products, but the rate of added ALA disappearance and reaction kinetics were slower in the presence of the 2H isotope.
Cis-9,trans-11,cis-15-CLN was the major product synthesised from ALA, but trans-9,trans-11,cis-15-CLN was also detected. Both Δ9,11,15-CLN products formed during incubations of ALA with D2O were labelled on C-13. Enrichment of the n+1 isotopomer of cis-9,trans-11,cis-15-CLN was greatest immediately after the addition of ALA. Incorporation of the 2H label in trans-9,trans-11,cis-15-CLN was consistent across all incubation times. Labelling of geometric 9,11,15-CLN isomers is analogous to the incorporation of 2H in 9,11-CLA formed during incubations of LA with D2O and pure cultures of Butyrivibrio fibrisolvens ( Reference Wallace, McKain and Shingfield 15 , Reference Kepler and Tove 38 , Reference Kepler, Tucker and Tove 40 ), Bifidobacterium breve and Propionibacterium freudenreichii subsp. shermanii DSM 4902T( Reference McIntosh, Shingfield and Devillard 41 ), ovine rumen contents( Reference Wallace, McKain and Shingfield 15 ) or human mixed faecal bacteria( Reference McIntosh, Shingfield and Devillard 41 ). The appearance of a single label on C-13 of 9,11-CLA isomers was explained by H abstraction on C-11, which for reasons of thermodynamic stability was followed by re-arrangement of the double bond and the assimilation of a proton from water( Reference Wallace, McKain and Shingfield 15 , Reference Kepler and Tove 38 , Reference McIntosh, Shingfield and Devillard 41 ). Abstraction of a single H at C-11 and formation of a radical intermediate would also explain an increase in n+1 isotopomers and the labelling pattern of 9,11,15-CLN isomers formed from ALA. The cis-9,trans-11,cis-15-CLN isomer was formed in much higher amounts than trans-9,trans-11,cis-15-CLN, which may be related to possible differences in the thermodynamics of these reactions.
A common mechanism for the synthesis of Δ9,11-CLA from LA and Δ9,11,15-CLN from ALA during incubations with mixed rumen microbiota is not unexpected, given that the same 12-cis,11-trans isomerase isolated from B. fibrisolvens is capable of both reactions( Reference Kepler, Tucker and Tove 40 ). Functional studies of the Δ12-cis-Δ11-trans-isomerase isolated from B. fibrisolvens ( Reference Kepler and Tove 38 ) suggest that the reaction is initiated by π electrons of C-9 double bond interacting with the hydrophobic binding site of the enzyme( Reference Kepler, Tucker and Tove 40 ). Other bacteria are capable of converting ALA to CLN isomers in vitro. Lactobacillus plantarum AKU 1009a catalyses the synthesis of cis-9,trans-11,cis-15-CLN and trans-9,trans-11,cis-15-CLN from ALA( Reference Kishino, Ogawa and Ando 42 , Reference Kishino, Ogawa and Yokozeki 43 ). Geometric isomers of 9,11,15-CLN are also formed during incubations of ALA with strains of Bifidobacterium ( Reference Gorissen, Raes and Weckx 44 – Reference Hennessy, Barrett and Paul Ross 46 ) and Propionibacterium ( Reference Hennessy, Barrett and Paul Ross 46 ). It remains unclear as to whether the mechanisms of 9,11,15-CLN synthesis from ALA are common to both food-producing bacterial strains and ruminal bacteria.
Incubations of ALA with rumen contents resulted in the formation of trans-9,trans-11,cis-13-CLN. Enrichment of the n+1 isotopomer of trans-9,trans-11,cis-13-CLN increased over the course of 12-h incubations, but was consistently lower compared with incorporation of 2H in Δ9,11,15-CLN products (average 0·20 and 0·81, respectively). However, the labelling pattern of trans-9,trans-11,cis-13-CLN was inconclusive. Nevertheless, an increase in the abundance of the n+1 isotopomer above natural enrichment suggests that the conversion of trans-9,trans-11,cis-13-CLN from ALA involves an exchange of H with water, by a mechanism that apparently differs from Δ9,11,15-CLN formation. The isomerase isolated from Propionibacterium acnes is capable of converting ALA to CLN isomers with trans-11,trans-13,cis-15-CLN as the main product with trace amounts of trans-10,cis-12,cis-15-CLN also being formed( Reference Hornung, Krueger and Pernstich 47 ). Incubation of ALA with rumen contents resulted in the formation of ∆10,12,15-CLN but in amounts too low to conclude about the possible mechanisms responsible.
Incubations of ALA with rumen contents also resulted in the formation of cis-7,cis-12,cis-15-18 : 3 and Δ8,12,15-18 : 3 isomers. Assignment of double-bond geometry of these products based on GC retention times would require further validation based on NMR or reductive ozonolysis. In earlier investigations, complementary Ag+-TLC and GC-MS analysis identified cis-5,cis-12-18 : 2, cis-6,cis-12-18 : 2, cis-7,cis-12-18 : 2, cis-8,cis-12-18 : 2 and trans-8,cis-12-18 : 2 as intermediates of LA metabolism during incubations with ruminal digesta( Reference Honkanen, Griinari and Vanhatalo 16 ). The appearance of the 18 : 3 intermediates containing a cis-12 and cis-15 double-bond arrangement provides the first evidence that transformation of ALA may also involve migration of the cis-9 double bond. Enrichment in n+1 isotopomers of Δ7,12,15-18 : 3 and Δ8,12,15-18 : 3 during incubations of ALA with D2O did not differ from natural enrichment, indicating that formation of these products does not involve H exchange with water. Cis-trans isomerisation of doubly deuterated cis-9-18 : 1 to trans-9-18 : 1 does not result in the loss of 2H( Reference von Wallbrunn, Richnow and Neumann 48 ). Thus, an absence of labelling confirms that formation of Δ7,12,15-18 : 3 and Δ8,12,15-18 : 3 from ALA does not involve prior formation of Δ9,11,13-CLN or Δ9,11,15-CLN. It is not possible to conclude on whether double-bond migration is catalysed by a cis-trans isomerase or via an alternative series of reactions, however. Hydrogenation of LA with iridium, palladium and ruthenium catalysts is known to generate trans-8,cis-12-18 : 2 and cis-9,trans-13-18 : 2 with cis-8,cis-12-18 : 2 and cis-9,cis-13-18 : 2 predicted as minor products( Reference Kitayama, Muraoka and Takahashi 49 ). Under these circumstances, double-bond migration has been explained by the release of the H atom from the adjacent methylene group at Δ8 or Δ14 of the semi-hydrogenated C–C bond and subsequent rotation of the C–C bond during abstraction of the H atom( Reference Kitayama, Muraoka and Takahashi 49 ). Further investigations involving incubations of ALA with living and irradiated rumen contents would be required to establish whether a cis-trans isomerase or non-enzymatic reaction catalyses re-arrangement of the cis-9 double bond.
Trans-11,cis-15-18 : 2 was the major 18 : 2 product formed from ALA, consistent with the established pathway of ALA biohydrogenation( Reference Harfoot and Hazlewood 22 – Reference Griinari and Bauman 25 , Reference Jouany, Lassalas and Doreau 27 ). The n+2 isotopomer of trans-11,cis-15-18 : 2 during incubations of ALA with D2O were highly enriched after 1·5 h of incubation with single 2H labels incorporated on C-9 and C-13. The labelling pattern is consistent with Δ11,15-18 : 2 intermediates being formed from Δ9,11,15-CLN labelled on C-13, with reduction of the cis-9 double bond involving H abstraction on C-10 and incorporation of a single H from water on C-9. Previous experiments have shown that the reduction of cis-9,trans-11-18 : 2 to trans-11-18 : 1 results in labelling on C-9 during incubations of LA and D2O with ruminal digesta( Reference Wallace, McKain and Shingfield 15 ) or human intestinal bacteria( Reference McIntosh, Shingfield and Devillard 41 ).
Under routine GC analysis with a polar 100-m capillary column, trans-10,cis-15-18 : 2 and trans-11,cis-15-18 : 2 elute as a single peak that can only be separated using a 100-m GC column with an ionic liquid stationary phase( Reference Alves and Bessa 32 ) or Ag+ solid-phase extraction and semi-preparative HPLC( Reference Turner, Meadus and Mapiye 50 ). Re-analysis of FAME in the present study using the SLB-IL111 column provided confirmation that trans-10,cis-15-18 : 2 is formed from ALA. An increase in the n+2 isotopomer indicates that transformation of ALA to trans-10,cis-15-18 : 2 by rumen microbiota involves exchange of two H ions from water. It has been suggested that trans-10,cis-15-18 : 2 is a product formed from the reduction of trans-10,cis-12,cis-15-CLN in the rumen( Reference Griinari and Bauman 25 , Reference Alves and Bessa 32 ). Even though enrichment of n+1 isotopomers of ∆10,12,15-CLN was detected during incubations of ALA with D2O, the labelling pattern of the trans-10,cis-15-18 : 2 product was inconclusive. L. plantarum AKU 1009a is capable of converting ALA to trans-10,cis-15-18 : 2 via the formation of cis-9,trans-11,cis-15-18 : 3 and trans-9,trans-11,cis-15-18 : 3 as intermediates( Reference Kishino, Ogawa and Yokozeki 43 ). It is not clear whether the same transformation also occurs in the rumen.
Incubations of ALA with rumen contents also resulted in the formation of cis-12,cis-15-18 : 2. The n+1, n+2 and n+3 isotopomers of cis-12,cis-15-18 : 2 were progressively enriched during incubations of ALA with D2O, but the position of 2H labels could not be located. It is possible that cis-12,cis-15-18 : 2 originates from the reduction of cis-7,cis-12,cis-15-18 : 3 or Δ8,12,15-18 : 3 or from the direct reduction of ALA. However, the increase in the n+3 isotopomer of cis-12,cis-15-18 : 2 suggests that an alternative mechanism may be responsible. All incubations contained LA, but the amounts did not increase following ALA addition. No enrichment in n+1, n+2 or n+3 isotopomers was detected over the course of incubations with D2O, indicating that ALA is not converted to LA by rumen microbiota.
The major pathways of ALA biohydrogenation do not consider isomers of CLA as intermediates. In the present investigation, small amounts of trans-11,cis-13-CLA, trans-11,trans-13-CLA and trans-12,trans-14-CLA were formed from ALA. Enrichment of n+2 isotopomers indicates that formation of Δ11,13-CLA involves an exchange of H with water. The mass spectrum of the DMOX derivative was difficult to interpret but provided some indications of labelling on C-9 and C-16. Over the course of 12-h incubations, an increase in the enrichment of n+3 isotopomers was detected, indicating that the formation of trans-11,trans-13-CLA involved the incorporation of another 2H label, but the location could not be established with a high degree of certainty. Earlier studies have shown that formation of cis-9,trans-11-CLA from LA results in labelling on C-13( Reference Wallace, McKain and Shingfield 15 ). Further reduction of cis-9,trans-11-CLA to trans-11-18 : 1 results in the incorporation of 2H on C-9( Reference McIntosh, Shingfield and Devillard 41 ). Assuming that the same mechanisms are also involved in transforming CLN products to CLA, then labelling on C-9 would be expected if Δ11,13-CLA is formed by the reduction of Δ9,11,13-CLN. Given the uncertainties in the labelling pattern, it was not possible to confirm Δ9,11,15-CLN as a precursor for Δ11,13-CLA formation or recent reports that strains of B. fibrisolvens convert trans-11,cis-15-18 : 2 to trans-11,cis-13-CLA( Reference Fukuda, Nakanishi and Chikayama 51 ). Much earlier investigations reported that cis-9,trans-11,cis-13-CLN is hydrogenated to trans-11-18 : 1, which in the presence of D2O resulted in labelling at C-9, C-10, C-13 and C-14, whereas cis-9,trans-11,trans-13-CLN was not hydrogenated( Reference Rosenfled and Tove 52 ).
Mechanisms explaining the transformation of ALA to trans-12,trans-14-CLA are not resolved from the present investigation. An increase in the n+3 isotopomer of trans-12,trans-14-CLA was detected after 12 h of incubation with ALA, but the labelling pattern was not informative. A possible explanation is that trans-12,trans-14-CLA is formed from the isomerisation of trans-11,cis-15-18 : 2 or geometric isomers of 11,13-CLA by a mechanism that involves an exchange of H with water. Earlier investigations with 13C-labelled ALA reported significant 13C enrichment in Δ8,10-CLA, Δ9,11-CLA, Δ10,12-CLA and Δ11,13-CLA intermediates, suggesting that multiple CLA isomers are formed from ALA during incubations with rumen contents( Reference Lee and Jenkins 26 ). Earlier studies have reported formation of trans-11,trans-13-CLA and cis-11,trans-13-CLA from ALA( Reference Jouany, Lassalas and Doreau 27 ). In the present study, addition of ALA did not increase Δ8,10-CLA, Δ9,11-CLA or Δ10,12-CLA formation and or enrich cis-9,trans-11-18 : 2 at n+2. This would exclude ALA being transformed to CLA products other than geometric isomers of Δ11,13 and Δ12,14-CLA in the rumen.
Multiple 18 : 1 isomers accumulated during incubations of ALA with rumen contents. It was not possible to resolve all 18 : 1 isomers during GC and GC-MS analysis because of the broad peak shapes of isomers containing multiple 2H labels. However, cis-12-18 : 1, cis-15-18 : 1, trans-11-18 : 1, trans-12-18 : 1, trans-13-18 : 1, trans-14-18 : 1, trans-15-18 : 1 and trans-16-18 : 1 were detected in flask contents during incubations of ALA with and without D2O. The mass spectrum of the DMOX derivative of trans-11-18 : 1 indicated the incorporation of 2H atoms on C-9, C-13 and C-15, consistent with this isomer originating from the sequential reduction of cis-9,trans-11,cis-15-CLN (labelled on C-13) and trans-11,cis-15-18 : 2 (labelled on C-9 and C-13), with the reduction of the cis-15 double bond being associated with the assimilation of 2H on C-15. In all incubations, 18 : 0 was detected as the major end product of ALA biohydrogenation, which was found to contain up to five 2H labels.
Overall, incubations of physiological amounts of ALA with strained rumen contents offer an explanation for the appearance of cis-9,trans-11,trans-15-CLN, Δ9,11,13-CLN, trans-11,trans-13-18 : 2, trans-12,trans-14-18 : 2, trans-10,cis-15-18 : 2, trans-11,cis-15-18 : 2 and cis-12,cis-15-18 : 2 in bovine muscle( Reference Plourde, Destaillats and Chouinard 7 , Reference Nassu, Dugan and He 53 ), adipose( Reference Turner, Meadus and Mapiye 50 , Reference Nassu, Dugan and He 53 ) and milk fat( Reference Lerch, Shingfield and Ferlay 8 , Reference Kairenius, Ärölä and Leskinen 54 ). Data from this and earlier investigations indicate that the abundance of specific FA containing one or more trans double bonds can be expected to be higher in meat or milk from ruminants fed diets rich in ALA, but the implications on the health of human consumers are, however, uncertain.
Conclusions
Incubations of ALA with rumen contents with or without D2O indicated that biohydrogenation proceeds via several distinct mechanisms leading to the formation of a diverse range of intermediates, many of which have not been characterised previously. Products formed by alternative metabolic pathways were quantitatively less important than the established intermediates of ALA biohydrogenation in the rumen. The complexity of the ruminal microbiota may account for a large part of the diversity of such reactions. Other than for the main routes of ALA metabolism, we have little knowledge of which microbial species might catalyse the different reactions.
Acknowledgements
The authors gratefully acknowledge and appreciate the technical assistance of Minna Aalto (Natural Resources Institute Finland) during lipid analysis.
This study was supported in part by core funding from the Finnish Ministry of Agriculture and Forestry, the Scottish Government Rural and Environment Science and Analytical Services Division and a PhD studentship from the Raisio Science Foundation and the August Johannes and Aino Tiura Agricultural Science Foundations (awarded to A. M. H.). None of the funders had any role in the experimental design, data analysis or writing of this article.
The authors’ contributions are as follows: A. M. H. and K. J. S. designed the study; A. M. H. completed the in vitro incubations; A. M. H., H. L., R. J. W., N. M., V. T. and K. J. S. contributed to the analysis of lipids, determination of 2H enrichment and FA identification; A. M. H. analysed the data under the supervision of R. J. W. and K. J. S.; A. M. H. and K. J. S. wrote the manuscript; R. J. W., N. M. and H. L. provided advice and critically reviewed the manuscript. All the authors have read and approved the manuscript content.
There are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516001446