Introduction
Epilepsy, a chronic disease of the central nervous system, is characterized by abnormal brain electrical activity leading to seizures, stereotyped behavioural alterations and occasionally loss of awareness (WHO, 2019). Epilepsy and its related consequences account for a substantial proportion of the world's burden of neurological diseases (Beghi et al., Reference Beghi, Giussani, Nichols, Abd-Allah, Abdela, Abdelalim, Abraha, Adib, Agrawal and Alahdab2019; WHO, 2019). The global burden of disease has been estimated to be 45.9 million patients (39.9–54.6 million) with all-active epilepsy, accounting for more than 13.5 million disability-adjusted life years and 126 000 deaths in 2016 (Beghi et al., Reference Beghi, Giussani, Nichols, Abd-Allah, Abdela, Abdelalim, Abraha, Adib, Agrawal and Alahdab2019). In developed countries, the overall incidence of epilepsy is 48.9 per 100 000 person-years. However, this proportion is 2–3 times higher in low- and middle-income countries (LMIC) (139 per 100 000 person-years) (WHO, 2019), where zoonotic and vector-borne infections are predominantly reported (Ngugi et al., Reference Ngugi, Bottomley, Kleinschmidt, Wagner, Kakooza-Mwesige, Ae-Ngibise, Owusu-Agyei, Masanja, Kamuyu and Odhiambo2013; Singh et al., Reference Singh, Angwafor, Njamnshi, Fraimow and Sander2020). It has been estimated that almost 80% of people with epilepsy live in LMIC, especially in sub-Saharan Africa (Beghi et al., Reference Beghi, Giussani, Nichols, Abd-Allah, Abdela, Abdelalim, Abraha, Adib, Agrawal and Alahdab2019; WHO, 2019). The high incidence of epilepsy in these areas is attributed to some neurotropic infections, including cysticercosis, toxocariasis, onchocerciasis, toxoplasmosis and malaria (Ngugi et al., Reference Ngugi, Bottomley, Kleinschmidt, Wagner, Kakooza-Mwesige, Ae-Ngibise, Owusu-Agyei, Masanja, Kamuyu and Odhiambo2013; Singh et al., Reference Singh, Angwafor, Njamnshi, Fraimow and Sander2020).
Malaria is a life-threatening infection caused by Plasmodium parasites, transmitted to humans by the bite of the infected female Anopheles mosquito (WHO, 2020). Malaria infection is endemic in Africa, Asia and South America (WHO, 2020). The World Malaria Report has estimated 241 million malaria cases and 627 000 malaria-related deaths in 2020 worldwide, of which 95% of all malaria cases and 96% of deaths were from the WHO African region (WHO, 2020). There are 5 Plasmodium species causing malaria infection in humans, of which P. vivax (dominant outside Africa) and P. falciparum (dominant in Africa) are the most common species with the greatest threats (Guerra et al., Reference Guerra, Snow and Hay2006; Battle et al., Reference Battle, Lucas, Nguyen, Howes, Nandi, Twohig, Pfeffer, Cameron, Rao and Casey2019). Cerebral malaria (CM), characterized by coma and parasitaemia, is a life-threatening consequence of malaria infection, mainly induced by P. falciparum and rarely by P. vivax (Hora et al., Reference Hora, Kapoor, Thind and Mishra2016; Luzolo and Ngoyi, Reference Luzolo and Ngoyi2019; Mukhtar et al., Reference Mukhtar, Eisawi, Amanfo, Elamin, Imam, Osman and Hamed2019). CM has a case-fatality rate of 15–20% and can cause several neurological sequelae in survivors, including language regression, cortical blindness, ataxia, gross motor deficits, behavioural abnormalities, seizure and epilepsy (Brewster et al., Reference Brewster, Kwiatkowski and White1990; Carme et al., Reference Carme, Bouquety and Plassart1993; van Hensbroek et al., Reference van Hensbroek, Palmer, Jaffar, Schneider and Kwiatkowski1997; Ngoungou et al., Reference Ngoungou, Poudiougou, Dulac, Dicko, Boncoeur, Traoré, Coulibaly, Keita, Preux and Doumbo2007; Birbeck et al., Reference Birbeck, Molyneux, Kaplan, Seydel, Chimalizeni, Kawaza and Taylor2010).
Seizures and other status epilepticus are common neurological manifestations in children with malaria, especially those with CM. However, a significant proportion of these manifestations may be simple febrile seizures (Kariuki et al., Reference Kariuki, Rockett, Clark, Reyburn, Agbenyega, Taylor, Birbeck, Williams and Newton2013; Angwafor et al., Reference Angwafor, Bell, Njamnshi, Singh and Sander2019). Most seizures are prolonged with focal characteristics occurring when body temperature is less than 38°C implying that other mechanisms besides fever, probably directly related to the parasite, are involved (Waruiru et al., Reference Waruiru, Newton, Forster, New, Winstanley, Mwangi, Marsh, Winstanley, Snow and Marsh1996; Angwafor et al., Reference Angwafor, Bell, Njamnshi, Singh and Sander2019). Despite this evidence, there are several gaps in our understanding of the relationship between malaria infection and development of epilepsy. A systematic review by Christensen and Eslick (Reference Christensen and Eslick2015) demonstrated a significant positive association between CM and epilepsy. However, the review only included studies on severe CM, necessitating a more focused study on all malaria infections. Moreover, several recent studies have been published in this area, indicating the need for an updated comprehensive meta-analysis. Therefore, to address this gap, the present study assesses the relationship between malaria infection and epilepsy development using a comprehensive meta-analysis to summarize and include updated findings.
Materials and methods
This study follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement as guidance to design the study and report and interpret the findings (Moher et al., Reference Moher, Shamseer, Clarke, Ghersi, Liberati, Petticrew, Shekelle and Stewart2015).
Search strategy and study selection
Four literature databases were systematically searched for relevant publications, including PubMed/Medline, Scopus, Embase and Web of Science collection, from inception to 10 May 2022. The inclusive keywords composed of malaria, severe malaria, cerebral malaria, brain malaria, Plasmodium, Plasmodium falciparum, Plasmodium vivax, epilepsy, seizure, neurological complications, neurological sequelae, relationship and association. The search process was performed by combining these keywords using ‘OR’ and/or ‘AND’ logical operators. The detailed search strategy in databases is presented in Fig. S1. To avoid potential missing relevant publications and grey literature, other sources such as Google Scholar, OpenGrey, ProQuest and references list of all studies were searched and included in the meta-analysis. No language restrictions were applied, and articles in languages other than English were translated to English using the online tool ‘Google Translate’ (https://translate.google.com/). All retrieved publications were exported to EndNote Reference Manager X8 (Clarivate Analytics, Philadelphia, PA, USA) where duplicates were removed.
Inclusion and exclusion criteria
After duplicate removal, 2 reviewers (A. A. K. and A. R.) independently screened the titles and abstracts of identified references, followed by full-text screening based on predefined inclusion and exclusion criteria. All the discrepancies were resolved by consulting with the lead investigator (A. R.). The main criteria for inclusion were: (1) peer-reviewed observational studies with cross-sectional, cohort or case–control design; (2) studies that included patients with confirmed malaria infection or patients with epilepsy as the cases, and the controls were individuals without malaria, or without the epilepsy/neurological complications; (3) studies that used internationally recognized diagnostic assays or guidelines to diagnose malaria infection or epilepsy; (4) studies that the risk point estimate was reported as an odds ratio (OR) and confidence intervals (CIs), or the information was presented such that an OR and 95% CI could be calculated. The exclusion criteria for this study included (1) studies that failed to quantitatively assess the relationship between malaria and epilepsy; (2) studies that an OR and 95% CI could not be calculated; (3) studies without original data (e.g. systematic reviews and letters); (4) case reports and case-series studies; (5) full-text of the article was not available (e.g. abstract or conference articles).
Data extraction and quality assessment
Two reviewers (A. T. and A. A. K.) independently extracted the following information from each eligible study using a standardized data extraction form: first author, publication year, type of participants (malaria-based or epilepsy-based), study design (cross-sectional, cohort or case–control), type of population (adult, children or both), country, diagnostic assay for malaria infection (blood smear, serology or medical history), the number of cases and control subjects, the prevalence of epilepsy or malaria in individuals of each of the subject groups. The quality of cohort and case–control studies was assessed by the Newcastle–Ottawa Scale (NOS) (Text S1), as endorsed by the Cochrane network (Stang, Reference Stang2010; Higgins et al., Reference Higgins, Thomas, Chandler, Cumpston, Li, Page and Welch2019). Moreover, an adapted NOS was used for the quality assessment of cross-sectional studies (Text S2) (Herzog et al., Reference Herzog, Álvarez-Pasquin, Díaz, Del Barrio, Estrada and Gil2013). In both scoring scales, the quality of each eligible study was rated as high (7–9 scores), moderate (4–6 scores) or poor (0–3 scores).
Data synthesis and statistical analysis
Meta-analyses were performed using Stata software ver. 17 (Stata Corporation, College Station, TX, USA). The pooled prevalence of epilepsy or malaria infection for each case and control group was estimated at a 95% CI using the DerSimonian–Laird random-effects model (REM) (DerSimonian and Laird, Reference DerSimonian and Laird2015). The variances in the meta-analysis were stabilized by transforming the raw prevalence estimates using the Freeman–Tukey double arcsine transformation (Hamza et al., Reference Hamza, van Houwelingen and Stijnen2008). The ORs from individual studies were calculated to assess the association between malaria infection and epilepsy in each study. Then, the ORs from individual studies were combined to produce a pooled OR, employing the REM with a restricted maximum-likelihood estimator. Between-study heterogeneity was evaluated by the Cochrane Q test and I 2 statistics. The I 2 value greater than 75% was considered as considerable heterogeneity (Higgins et al., Reference Higgins, Thompson, Deeks and Altman2003). The results were stratified into subgroups by diagnostic methods, study design, type of participants and type of studies to better explore the potential effect modification by the study characteristics on study outcome as well as potential sources of heterogeneity. The robustness of the results was evaluated by iteratively removing 1 study to assess each study's influence on the pooled estimate for both outcomes. Potential publication bias was determined using a contour-enhanced funnel plot and Egger's test, as up to 10 eligible studies were included in the meta-analysis (Egger et al., Reference Egger, Smith, Schneider and Minder1997). A cumulative meta-analysis was conducted to explore the trend of evidence accumulation. Results with P < 0.05 (2-sided) were considered statistically significant.
Results
Literature search and characteristics of studies included
The primary databases screening resulted in 3419 potentially relevant publications, of which 3390 were excluded after duplicate removal and the titles and abstracts screenings. After the in-depth screening of 29 full-text articles for their eligibility, 17 studies containing 24 datasets remained for the meta-analysis (Versteeg et al., Reference Versteeg, Carter, Dzombo, Neville and Newton2003; Carter et al., Reference Carter, Neville, White, Ross, Otieno, Mturi, Musumba and Newton2004; Ngoungou et al., Reference Ngoungou, Dulac, Poudiougou, Druet-Cabanac, Dicko, Mamadou Traore, Coulibaly, Farnarier, Tuillas and Keita2006a, Reference Ngoungou, Koko, Druet-Cabanac, Assengone-Zeh-Nguema, Launay, Engohang, Moubeka-Mounguengui, Kouna-Ndouongo, Loembe and Preux2006b; Idro et al., Reference Idro, Gwer, Kahindi, Gatakaa, Kazungu, Ndiritu, Maitland, Neville, Kager and Newton2008; Opoka et al., Reference Opoka, Bangirana, Boivin, John and Byarugaba2009; Birbeck et al., Reference Birbeck, Molyneux, Kaplan, Seydel, Chimalizeni, Kawaza and Taylor2010; Postels et al., Reference Postels, Taylor, Molyneux, Mannor, Kaplan, Seydel, Chimalizeni, Kawaza and Birbeck2012; Ngugi et al., Reference Ngugi, Bottomley, Kleinschmidt, Wagner, Kakooza-Mwesige, Ae-Ngibise, Owusu-Agyei, Masanja, Kamuyu and Odhiambo2013; Kamuyu et al., Reference Kamuyu, Bottomley, Mageto, Lowe, Wilkins, Noh, Nutman, Ngugi, Odhiambo and Wagner2014; Wagner et al., Reference Wagner, Ngugi, Twine, Bottomley, Kamuyu, Gómez-Olivé, Connor, Collinson, Kahn and Tollman2014; Ae-Ngibise et al., Reference Ae-Ngibise, Akpalu, Ngugi, Akpalu, Agbokey, Adjei, Punguyire, Bottomley, Newton and Owusu-Agyei2015; Bistervels et al., Reference Bistervels, Kariuki and Newton2016; Kakooza-Mwesige et al., Reference Kakooza-Mwesige, Ndyomugyenyi, Pariyo, Peterson, Waiswa, Galiwango, Chengo, Odhiambo, Ssewanyana and Bottomley2017; Thierry et al., Reference Thierry, Falilatou, Covalic, Elodie, Mendinatou, Didier, Alphonse and Joseph2020; Gumisiriza et al., Reference Gumisiriza, Kugler, Brusselaers, Mubiru, Anguzu, Ningwa, Ogwang, Akun, Mwaka and Abbo2021; Dolo et al., Reference Dolo, Sow, Kane, Sangare, Daou, Sangare, Sangho, Koné, Coulibaly and Coulibaly2022) (Fig. 1). Studies were published between 2003 and 2022. Overall, these studies included 6285 cases and 13 909 healthy controls. Eligible studies were performed in 7 countries and all in Africa (Kenya, Malawi, Mali, Tanzania, South Africa, Uganda and Ghana). Eighteen datasets, defined as epilepsy-based studies, recruited epileptic patients (2191 participants) as cases and individuals without epilepsy (2753 participants) as controls and assessed malaria infection in these subjects; while 6 datasets, defined as malaria-based studies, evaluated the prevalence or incidence of epilepsy in participants with (655 participants) and without (1075 participants) malaria infection. Epilepsy-based datasets had cross-sectional (n = 10) and case–control (n = 8) study designs, while all malaria-based studies had cohort study designs (4 prospective and 2 retrospective). Participants in 12 and 3 datasets were only children and only adults, respectively, while in 9 datasets, both children and adults participated. Considering diagnostic methods, 11 datasets used serological methods, 11 used blood smear parasitology methods and 2 used medical history for severe malaria. According to NOS, the overall quality of datasets was high for half of the datasets (n = 12) and moderate for the other half (Table 1). The main characteristics of the included studies are presented in Table 1.
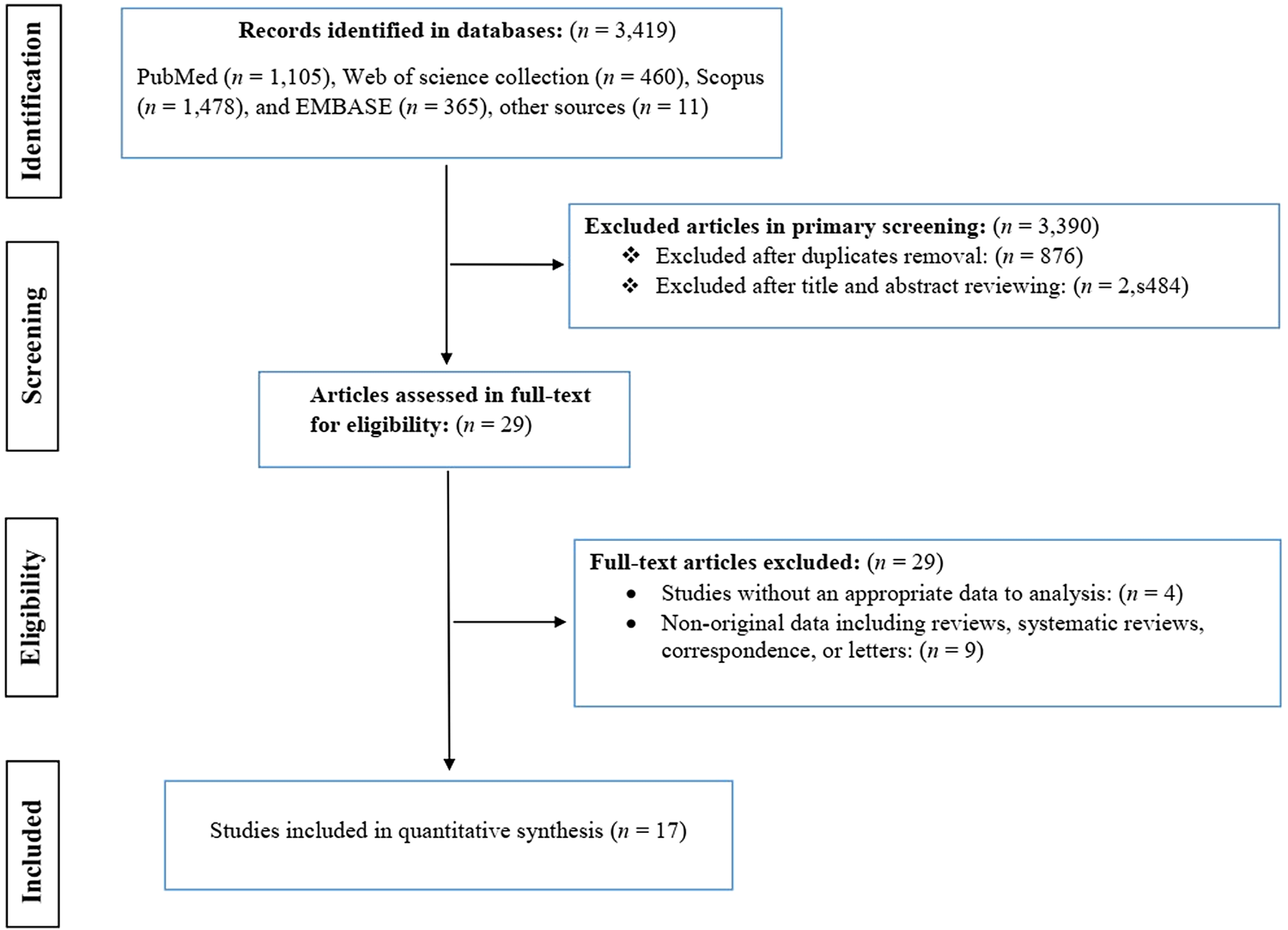
Fig. 1. PRISMA flow chart showing study selection process.
Table 1. Main characteristics of included studies evaluating the relationship between malaria infection and epilepsy

a Malaria-based studies.
b Epilepsy-based studies.
Overall analysis and subgroup analyses
As displayed in Table 1 and Fig. 2, the overall meta-analysis indicated a significant positive association between malaria infection and epilepsy development (OR 2.36; 95% CI 1.44–3.88) (Table 2 and Fig. 2). In subgroup analysis, malaria-based studies showed a strong positive association (OR 7.10; 95% CI 3.50–14.38), while this association was marginally significant in epilepsy-based studies (OR 1.70; 95% CI 0.99–2.91). Considering the study design, both retrospective (OR 5.2; 95% CI 2.17–12.46) and prospective (OR 10.87; 95% CI 3.06–38.58) cohorts indicated significant positive associations, while cross-sectional (OR 1.74; 95% CI 0.84–3.62) and case–control (OR 1.64; 95% CI 0.71–3.79) studies showed non-significant positive associations (Table 2). Considering the diagnostic methods, studies that had used the blood smear method yielded a significant positive association (OR 4.80; 95% CI 2.36–9.77), while other methods showed non-significant positive associations. Regarding the type of participants, quality of studies and year of publications, studies that used only children (OR 3.92; 95% CI 1.81–8.50), those with high qualities (OR 3.61; 95% CI 1.62–8.04) and those published before 2010 (OR 6.39; 95% CI 4.25–9.62) showed significant positive associations. More details on subgroup analyses are presented in Table 2. The funnel plot demonstrated no publication bias in the studies included in this meta-analysis (Fig. S2).

Fig. 2. Forest plot, pooled with random effects, regarding the association between malaria infection and epilepsy.
Table 2. Sub-group analysis of the pooled prevalence and odds ratios for the association between malaria and epilepsy

Although the overall heterogeneity was high (I 2 = 95.02%, Q = 613.95, P < 0.001), it was low or moderate in some sub-groups, especially for those indicating significant positive association, including malaria-based studies (I 2 = 20.2%, Q = 6.53, P = 0.258), retrospective (I 2 = 0.0%, Q = 0.19, P = 0.666), prospective cohorts (I 2 = 48.1%, Q = 5.77, P = 0.123) and studies published before 2010 (I 2 = 33.3%, Q = 8.82, P = 0.184) (Table 2). Multivariate meta-regression was performed to further explore the sources of heterogeneity based on the study design, type of participants, publication year and diagnostic methods. Multivariate meta-regression analysis indicated that only the diagnostic method (C = −0.0001; P < 0.001) could be the source of heterogeneity and accounted for 64.5% of between-study heterogeneity.
Sensitivity and cumulative analysis
The sensitivity analysis was conducted to evaluate the possible influence of any individual study on the main results. The analysis assessed whether omitting 1 study substantially altered the main outcome or magnitude of the summary estimates of the remainders. This analysis indicated that the exclusion of any individual study did not significantly alter the overall results of the meta-analysis (Fig. 3), implying high stability of the results.

Fig. 3. Sensitivity analysis after each study was removed.
A cumulative meta-analysis was also performed to evaluate the consistency of the evidence over the years as recent studies were added. The cumulative analysis indicated that between 2003 and 2022, there was a positive association between malaria infection and epilepsy development but with a swinging effect size and narrowing CIs (Fig. 4).

Fig. 4. Cumulative meta-analysis regarding the association between malaria infection and epilepsy.
Discussion
Epilepsy is a highly prevalent neurological disorder in LMIC, especially in malaria-endemic areas; however, a definitive causative relationship is yet to be established. The results of this systematic review and meta-analysis are consistent with a previous study (Christensen and Eslick, Reference Christensen and Eslick2015), indicating a significant positive association between malaria infection and epilepsy, particularly for patients who survived CM. All subgroup analyses also showed a positive association, although the results were more significant in cohort studies that followed patients with CM. Moreover, the epilepsy-based studies indicated a marginally significant association, suggesting future well-designed studies are required to establish a definitive association between malaria infection and epilepsy development.
The association between malaria and epilepsy development is likely explained by more than 1 mechanism, possibly due to CM and its related inflammation (Singh et al., Reference Singh, Angwafor, Njamnshi, Fraimow and Sander2020). However, the pathophysiological mechanisms leading to CM and its related neurological complications are not yet fully understood (Christensen and Eslick, Reference Christensen and Eslick2015). The current knowledge is largely based on a few reports of human autopsy and findings from models of Plasmodium berghei infection in C57BL/6J mice (Oca et al., Reference Oca, Engwerda and Haque2013; Singh et al., Reference Singh, Angwafor, Njamnshi, Fraimow and Sander2020). The key processes that were elucidated in these studies were cytoadherence of the parasitized erythrocytes and inflammation. It is demonstrated that upregulation of infections-induced inflammatory markers such as tumour necrosis factor, intracellular adhesion mediator 1 and angiopoietin 2 can induce endothelial damage in the cerebral blood vessels (Conroy et al., Reference Conroy, Glover, Hawkes, Erdman, Seydel, Taylor, Molyneux and Kain2012; Storm and Craig, Reference Storm and Craig2014; Cruz et al., Reference Cruz, Wu, Ulrich, Craig and Garcia2016; O'Regan et al., Reference O'Regan, Gegenbauer, O'Sullivan, Maleki, Brophy, Dalton, Chion, Fallon, Grau and Budde2016; Shabani et al., Reference Shabani, Ouma, Idro, Bangirana, Opoka, Park, Conroy and John2017; Storm et al., Reference Storm, Jespersen, Seydel, Szestak, Mbewe, Chisala, Phula, Wang, Taylor and Moxon2019) and maybe effective in the initiation of seizures and other status epilepticus. Moreover, it is suggested that vascular-ischaemic lesions resulting from the sequestration of parasitized erythrocytes and the Durck's malarial granuloma that comprises of reactive astrocytes during the acute attack of CM could lead to structural damage to the brain and induce epileptogenic lesions (Aleem, Reference Aleem2005; Ngoungou and Preux, Reference Ngoungou and Preux2008). Finally, neurotoxins such as quinolinic acid and autoantibodies to voltage-gated calcium channels that are induced in CM might have a role in the developing of seizures in children with severe malaria (Dobbie et al., Reference Dobbie, Crawley, Waruiru, Marsh and Surtees2000; Lang et al., Reference Lang, Newbold, Williams, Peshu, Marsh and Newton2005; Ngoungou and Preux, Reference Ngoungou and Preux2008), which requires further investigations.
Although the present study comprehensively evaluated the associations between malaria infection and epilepsy, some limitations should be acknowledged in the interpretation of our findings. First, some studies used medical history or serological methods to define malaria, which could be subject to diagnosis biases in pooled OR; however, subgroup analysis was performed in this study according to diagnostic criteria to overcome this limitation. Second, there were insufficient data considering sex, age or other comorbidities, and notably, information about other infections was underrepresented in some included studies. Despite these drawbacks, the findings and interpretations presented here provided useful insights concerning the association between malaria infection and epilepsy.
In conclusion, our findings indicated that patients with malaria, especially those with CM, are at a higher risk of epilepsy development; however, several gaps remain in our understanding of pathophysiological mechanisms of this event. Therefore, further human or experimental studies are needed to focus on long-term sequelae of CM such as epilepsy; neuropathological and physiological changes leading to these sequelae; the impact of therapeutic agents that are used during CM episodes; and the impact of other epileptogenic intracranial infections on the development of epilepsy and other neurological sequelae.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182022001780.
Data availability
Data supporting results are provided within the article and in the Supplementary materials.
Acknowledgements
The corresponding author would like to thank the administrators and the staffs of Babol University of Medical Sciences.
Author's contributions
A. A. K., Y. D. and A. R. conceived the study; A. A. K., Y. D. and S. A. initially searched the literature and collected all data from the included articles; A. R. and M. S. analysed and interpreted the data; A. A. K., M. S. and A. R. drafted the manuscript; and all authors commented on the drafts of the manuscript and approved the final draft of the paper.
Financial support
This research is supported by the Health Research Institute of Babol University of Medical Sciences, Iran (IR.MUBABOL.REC.1399.295).
Conflict of interest
None.
Ethical standards
Not applicable.