INTRODUCTION
In the UK over the past 5 years, the annual number of new HIV diagnoses has stabilized at a high level [1, 2]. In many other European countries, new HIV diagnoses continue to rise [3, 4]. In 2007, the highest rate across the three World Health Organisation (WHO) European subregions [3] (see Appendix for subregions) was observed in the East (165 per million population), followed by the West (77 per million) and Centre (10 per million) [4]. In Eastern Europe (EE), injecting drug use (IDU) is the main transmission route although infections acquired heterosexually, mostly in sexual partners of IDU populations, have also risen [3, 4]. Prevalence estimates of HIV in Georgia, Belarus, Ukraine, Latvia and Estonia in injecting drug users exceed 10% [3]. In Central Europe (CE), HIV prevalence remains low and stable with an increasing number of HIV cases acquired through sexual transmission [4].
There is evidence that population movement facilitates the spread of HIV [Reference Hamers5–Reference Gyarmathy and Neaigus9]. In 2007, an estimated 831 026 Central and Eastern Europe (C&EE)-born adults were living in England, Wales and Northern Ireland (E,W&NI) whereas, in 2000 the figure was 303 957 [10]. Two thirds of C&EE migrants in 2007 (65%, 539 017/831 026) were from the eight accession countries (A8) that joined the EU in 2004 (Poland, Czech Republic, Slovakia, Hungary, Slovenia, Estonia, Latvia, Lithuania) [10–12]. This recent rise in migration to the UK has generated some concern and media speculation of potential adverse health and economic impact [Reference Jones and Brogan13–Reference Roberts17]. In this study, we assess the impact of HIV epidemics in C&EE on the HIV epidemic in the UK.
METHODS
We analysed HIV data in adults (⩾15 years) in E,W&NI (data for Scotland was omitted as country of birth was not available) from three HIV surveillance systems held at the Health Protection Agency, Centre for Infections, including: (1) new HIV diagnoses, which collects detailed demographic, epidemiological information and CD4 count at diagnosis; (2) unlinked anonymous genitourinary medicine (GUM) clinic survey, which measures the prevalence of previously undiagnosed HIV by using residual serum leftover syphilis blood samples from individuals attending 15 sentinel GUM clinics; (3) unlinked anonymous HIV seroprevalence survey of neonatal dried blood spots (conducted in England only), which measures overall HIV prevalence in woman giving birth [2]. Data are reported for the period 2000–2007 or 2007 alone.
The WHO European subregion definitions for CE and EE were used [3]. To calculate rates of new HIV diagnoses, migration data from the quarterly household-based Labour Force Survey (July–September 2000–2007) were used [10]. Population estimates of people living in E,W&NI were obtained from the Office for National Statistics [18]. Proportions were calculated among all individuals for whom the relevant information was available. Numbers may rise as further reports are received, particularly for recent years.
RESULTS
New HIV diagnoses
Between 2000 and 2007, 48 400 adults were newly diagnosed with HIV in E,W&NI, of whom 33 223 (69%) had probable country of birth reported. The percentage of newly diagnosed individuals born in C&EE was 1·2% (404/33 223), increasing from 0·8% (19/2521) in 2000 to 2·9% (104/3602) in 2007 (Fig. 1). In 2007, the rate of new HIV diagnoses in C&EE-born adults was 125 per million population (104/831 026), compared to an overall rate of 142 (5988/42 092 800) in E,W&NI. In adults born in CE, the rate was 106 per million (74/697 706) and 225 (30/133 320) in those born in EE. In 2007, A8 countries accounted for 81% (84/104) of the C&EE-born adults and 2·3% (84/3602) of all new diagnoses reported in E,W&NI (Fig. 1), presenting a rate of 156 (84/539 017) new diagnosis per million population.
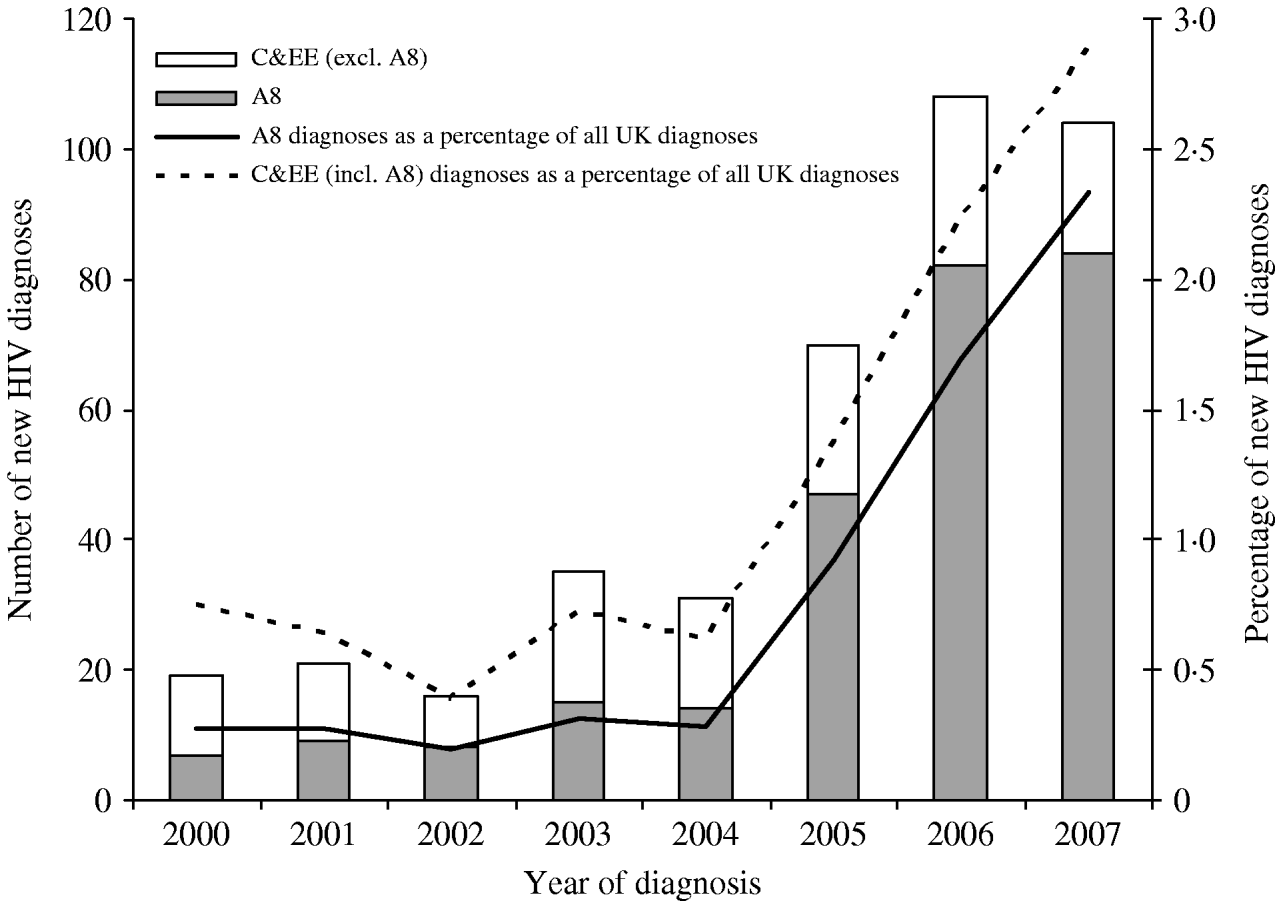
Fig. 1. New HIV diagnoses among individuals born in Central and Eastern Europe (C&EE): England, Wales and Northern Ireland, 2000–2007. A8, The eight accession countries that joined the EU in 2004.
Men accounted for almost two-thirds (64%, 258/404) of C&EE-born adults diagnosed between 2000 and 2007, of whom 59% (149/252) reported sex between men (SBM). Median age at diagnosis was slightly higher for men than women (30 vs. 26 years respectively). Sixty-one per cent (220/362) of C&EE-born adults probably acquired their infection in C&EE (212 in their country of birth), of whom half (110/218) were infected heterosexually, a quarter (56/218) through SBM and one-fifth (48/218) through IDU (Table 1). One hundred and twelve (31%) C&EE-born adults probably acquired their HIV infection in the UK over the period. Of the men, 86% (65/76) were probably infected through SBM and all with the exception of one of the women (35/36) through heterosexual contact (Table 1). In UK-born adults, only 0·2% (15/8522) probably acquired their HIV infection in C&EE over the 8-year period.
Table 1. New HIV diagnoses in individuals born in Central and Eastern Europe (C&EE), by route of infection and probable world region of infection: England, Wales and Northern Ireland, 2000–2007
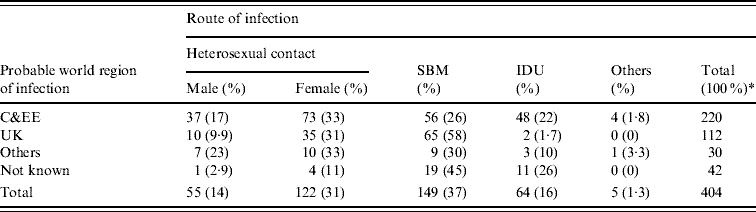
SBM, Sex between men; IDU, injecting drug use.
* Percentages are as a proportion of probable world region of infection subtotals. Total includes persons for whom route of infection were not reported.
Of C&EE-born adults diagnosed in 2000–2007 and who were probably infected heterosexually in the UK, 78% (31/40) reported a partner infected in sub-Saharan Africa. In UK-born adults probably infected heterosexually in the UK, 0·5% (6/1205) reported a sexual partner infected in C&EE, 24% (295) a partner infected in the UK and 44% (530) a partner infected in sub-Saharan Africa.
CD4 count at diagnosis is an important determinant of HIV-related morbidity and mortality in the UK. Of the 404 C&EE-born adults, 72% (292) had a CD4 count at diagnosis, of whom 25% (74/292) were diagnosed with a CD4 count <200 cells/mm3 (within 3 months of diagnosis), at a point after which therapy should have begun. This proportion was similar to UK-born adults diagnosed in 2000–2007 (26%, 2144/8283) and lower than sub-Saharan African-born adults (38%, 5305/13 798).
Undiagnosed HIV infection in GUM attendees
In 2007, C&EE-born adults accounted for 4·3% (4612/107 664) of attendees at sentinel GUM clinics across E,W&NI, an increase from 2·6% (1722/65 979) in 2000. The prevalence of previously undiagnosed HIV in C&EE-born adults was 0·46% (95% CI 0·30–0·70, 23/4612) in 2007, similar to that in UK-born adults (0·47%; 95% CI 0·42–0·52, 358/75 379). In 2007, the proportion of C&EE-born GUM attendees who reported having ever injected drugs was 0·63% (95% CI 0·40–0·86, 29/4612), the same as UK-born attendees (0·63%, 95% CI 0·57–0·69; 478/75 737).
HIV prevalence in women giving birth
In 2007, HIV prevalence in C&EE-born women giving birth in England was 0·08% (11/13 621), compared to a prevalence of 0·05% (76/165 654) in UK-born women. There have been no important trends since 2000.
DISCUSSION
Our study indicates that to date there has been little impact of the C&EE HIV epidemics on the UK epidemic despite recent European political expansion. Although a rise in reports of new diagnoses (from 19 in 2000 to 104 in 2007) was noted, C&EE-born adults represented only 2·9% of new diagnoses in 2007 and 1·2% of all HIV-diagnosed adults over the 8-year period. About one third of newly diagnosed C&EE-born individuals probably acquired their infection in the UK. The rate of new HIV diagnoses in C&EE-born adults was lower than the overall rate of HIV diagnoses in adults in E,W&NI. The prevalence of HIV in GUM clinic attendees and pregnant women remains very low in this group. Late presentation (CD4 count <200 cells/mm3 within 3 months of diagnosis) of HIV remains a concern in C&EE-born adults newly diagnosed in the UK. Individuals diagnosed late are at greater risk of early death [Reference Chadborn19, Reference Chadborn20].
Several factors help explain the limited impact of the C&EE HIV epidemics on the UK. First, migration data indicate that most C&EE migrants to the UK in recent years have been predominately from low HIV prevalence areas (Poland, Slovakia, Lithuania) [10, 21], where some prevalence rates of HIV are lower than in the UK [4]. Second, HIV epidemics in many C&EE countries are driven by a very high prevalence in injecting drug users [3, 4], and there is evidence that these individuals may have less opportunities or motivation to migrate to other countries given often difficult socioeconomic, legal and medical circumstance [Reference Gyarmathy and Neaigus9, Reference Bobrova22–Reference Renton24]. Furthermore, better access to free needle-exchange services in the UK [Reference Aceijas23, Reference Hickman25, Reference Trace, Riley and Stimson26] may have reduced the likelihood of C&EE-born individuals becoming infected through IDU after their arrival. Offering voluntary and confidential HIV testing to injecting drug users and scaling up measures to reduce onward transmission in this group and their sexual partners remains a priority in C&EE countries [3].
Stigma, discrimination and the lack of confidentiality (in some countries) are also major barriers to HIV testing and access to care in men who have sex with men (MSM) [Reference Danziger27–30]. MSM were over-represented (58%) in C&EE-born men diagnosed in the UK. This is in contrast to national HIV figures from CE and EE countries where MSM accounted for 17% and 0·5%, respectively, of new diagnoses in men in recent years [31]. Our findings are likely to reflect under-reporting of MSM in these countries and/or selective migration of MSM to the UK as a result of stigma and discrimination.
The results show a small number of UK-born individuals having acquired their infection in C&EE. This highlights the ongoing need for awareness of safer sex in travellers to high-prevalence areas.
The study has several limitations. Data for Scotland had to be omitted as country of birth is not available. Probable route of infection and place of infection were missing for a quarter of new diagnoses and CD4 count for a third of cases. However, there is no evidence to suggest a bias in data collection or follow-up. Misreported or missing information may also influence prevalence estimates based on unlinked anonymous surveillance. Previously undiagnosed HIV infection reported in GUM clinic attendees is likely to be higher than the general population as these individuals are known to have substantially higher risk than the general population [2]. Rates of diagnoses rely on migration estimates from the Labour Force Survey which are subject to both sampling and non-sampling errors [32].
CONCLUSION
National HIV surveillance systems indicate little impact of the C&EE HIV epidemics on the UK epidemic. C&EE-born adults accounted for only 1·2% of newly diagnosed adults in E,W&NI for 2000–2007 and the HIV prevalence in GUM clinic attendees and pregnant woman born in C&EE is very low. In addition to targeted interventions for IDU, HIV prevention efforts should particularly focus on C&EE-born MSM, both in their country of origin and within the UK.
APPENDIX. WHO European subregions
The West (23 countries): Andorra, Austria, Belgium, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Malta, Monaco, Netherlands, Norway, Portugal, San Marino, Spain, Sweden, Switzerland, United Kingdom. The Centre (15 countries): Albania, Bosnia and Herzegovina, Bulgaria, Croatia, Cyprus, Czech Republic, Hungary, the Former Yugoslav Republic of Macedonia, Montenegro, Poland, Romania, Serbia, Slovakia, Slovenia, Turkey. The East (15 countries): Armenia, Azerbaijan, Belarus, Estonia, Georgia, Kazakhstan, Kyrgyzstan, Latvia, Lithuania, Republic of Moldova, Russian Federation, Tajikistan, Turkmenistan, Ukraine, Uzbekistan.
ACKNOWLEDGEMENTS
We thank NHS HIV related services in the UK and the many individuals who contribute to HIV surveillance. The help, advice and support of Dr Ruth Gilbert is gratefully acknowledged. HIV and AIDS reporting in the UK, Unlinked Anonymous GUM Surveillance and Unlinked Anonymous HIV Seroprevalence Survey of Neonatal Dried Blood Spots are funded by the Health Protection Agency.
DECLARATION OF INTEREST
None.




