Methionine (Met) is an essential amino acid for grass carp Ctenopharyngodon idella (Reference Wang1). Our previous study observed that Met deficiency caused growth retardation and feed utilisation reduction of sub-adult grass carp(Reference Wu, Tang and Jiang2). Fish growth is mainly dependent on the growth of muscle(Reference Alami-Durante, Bazin and Cluzeaud3), which is closely associated with the deposition of nutrients, the growth of muscle fibre and the formation of intramuscular connective tissue(Reference Jiang, Wu and Tang4–Reference Bugeon, Lefèvre and Fauconneau6). However, to date, there has been only sporadic reports about the effect of Met on nutritive deposition, muscle fibre growth and the formation of intramuscular connective tissue in the muscle of fish. For example, in fish muscle, it has been reported that Met increased protein and lipid deposition in juvenile grouper Epinephelus coioides (Reference Luo, Liu and Mai7) and promoted muscle fibre hypertrophy in juvenile rainbow trout Oncorhynchus mykiss (Reference Alami-Durante, Bazin and Cluzeaud3). However, these studies lack systematicity and rarely explore involved mechanisms. Hence, systematically investigating the relationship between Met and muscle growth and deeply exploring the molecule mechanisms in fish are necessary.
It is well known that muscle growth primarily included the growth of muscle fibre and surrounding intramuscular connective tissue(Reference Nishimura8). The recruitment of new muscle fibre (hyperplasia) and growth of existing fibre (hypertrophy) for muscle growth are primary contributors to the ultimate size of a fish species(Reference Johnston, Bower and Macqueen9). To our knowledge, only one study (as mentioned above) demonstrated that Met improved muscle growth by an increase in fibre hypertrophy in juvenile rainbow trout (carnivorous fish)(Reference Alami-Durante, Bazin and Cluzeaud3). However, the contribution of hyperplasia and hypertrophy to muscle growth affected by nutrients depends on fish species. For example, previous studies reported that lysine could accelerate muscle growth by promoting fibre hypertrophy for blunt snout bream Megalobrama amblycephala (herbivorous fish)(Reference Cai, Jiang and Li10), but had no impact on muscle fibre growth for Nile tilapia Oreochromis niloticus (L.) (omnivorous fish)(Reference Michelato, Vidal and Xavier11). Besides, collagen is the major component of intramuscular connective tissue influencing the functional and structural properties of muscle(Reference Christian, Kathleen and Valerie12), the synthesis of which inhibits the differentiation of myogenesis of C2C12 muscle cells in vitro (Reference Saitoh, Periasamy and Kan13). In grass carp, the main collagen in intramuscular connective tissue is type I collagen(Reference Yu, Liu and Wang14), the expression of which is regulated by the transforming growth factor-β1 (TGF-β1)/Smads signalling pathway at the transcriptional level(Reference Yu, Ma and Ji15). Type I collagen consists of α1 and α2 peptide chains (called collagen type 1 α1 (Col1α1) and collagen type 1 α2 (Col1α2)), respectively(Reference Yu, Liu and Wang14). It has been reported that Met could up-regulate the gene expression of Col1α2 in the muscle of rainbow trout(Reference Alami-Durante, Bazin and Cluzeaud3). However, whether Met affects the Col1α1 and the molecular mechanism by which Met regulates type I collagen in animal muscle are unknown. In rainbow trout, Met could decrease the gene expression of TNF-α in the muscle(Reference Alami-Durante, Bazin and Cluzeaud3). Inagaki et al.(Reference Inagaki, Truter and Tanaka16) demonstrated that TNF-α was an antagonist of TGF-β signalling in the study of primary human fetal fibroblasts. Additionally, previous studies reported that Met could stimulate the target of rapamycin complex 1 (TORC1) signalling pathway in the muscle of rainbow trout(Reference Belghit, Skiba-Cassy and Geurden17). It has been reported that mTORC1 played an important regulatory role in catalysing type I collagen synthesis in human dermal fibroblasts(Reference Shegogue and Trojanowska18). Thus, there may be some relationship between Met and muscle growth associated with muscle fibre and type I collagen as well as potential TGF-β1/Smads and TORC1 signalling in fish, which deserves research.
Taken together, based on our previous study demonstrating that Met deficiency resulted in growth retardation and feed utilisation reduction, the present research was for the first time conducted systematically with the primary objective of investigating the effect of Met on the deposition of muscle nutrients, the hyperplasia and hypertrophy of muscle fibre and type I collagen synthesis as well as related signalling pathways (such as TGF-β1/Smads and TORC1 signalling), which might provide partial theoretical evidence for the mechanisms of Met-regulated muscle growth in fish. In addition, grass carp, with rapid growth and high yield, is one of the most abundant freshwater fish species(Reference Liu, Guo and Wu19). In our previous study for on-growing grass carp, the optimal Met requirement was 10·41 g/kg of the diet only based on specific growth rate(Reference Tang, Feng and Liu20). However, amino acid requirement might vary with different indices(Reference Gan, Jiang and Wu21). Therefore, the optimal Met requirement for on-growing grass carp with different indices was also evaluated, which might possess important production significance for improving fish muscle growth.
Materials and methods
Experimental design and diets
The ingredients and nutrient levels in the six experimental diets are given in Table 1. The main protein sources used in the present experiment included fishmeal, soyabean protein concentrate and gelatin, and soyabean oil and fish oil were chosen to formulate the main lipid sources. According to Xu et al.(Reference Xu, Wu and Jiang22), 286·8 g/kg of dietary protein level, optimum for the growth of on-growing grass carp, was chosen to fix experimental diets. Crystalline amino acid mixture without Met was used to meet essential amino acid requirements for on-growing grass carp reported by our laboratory in the last few years. Six experimental diets with graded Met concentrations of 2·5 (unsupplemented diet), 5·0, 7·5, 10·0, 12·5 and 15·0 g/kg diet were formed by adding different amounts of dl-Met to the diets. All diets were made iso-nitrogenous with graded l-glycine instead of incremental Met, which was in line with the method used in our previous studies(Reference Tang, Feng and Sun23,Reference Dong, Jiang and Liu24) . The cysteine and Met concentrations in the six experimental diets, which were determined consistent with Wu et al.(Reference Wu, Tang and Jiang2), were 1·55, 1·52, 1·58, 1·50, 1·53 and 1·53 g/kg diet and 2·54, 4·85, 7·43, 10·12, 12·40 and 15·11 g/kg diet. In line with the method described by Hong et al.(Reference Hong, Jiang and Kuang25), at room temperature, we used an electrical fan to air dry the pellets, and then they were stored at –20°C until being used.
Table 1. Composition and nutrient levels of experimental diets
(Percentages)

* Crystal amino acid mix (g/kg diet): arginine, 1·86; histidine, 4·90; isoleucine, 5·29; leucine, 1·92; lysine, 5·12; phenylalanine, 3·64; threonine, 6·53; tryptophan, 2·17; valine, 7·00; tyrosine, 4·11; glutamic acid, 54·12; glycine, 48·15, respectively.
† Per kg of vitamin premix (g/kg): retinyl acetate (344 mg/g), 0·19; cholecalciferol (12·5 mg/g), 0·20; dl-α-tocopheryl acetate (50 %), 23·23; menadione (96 %), 1·98; cyanocobalamin (1 %), 0·94; d-biotin (2 %), 0·75; folic acid (95 %), 0·17; thiamine nitrate (98 %), 0·09; ascorhyl acetate (95 %), 9·77; niacin (99 %), 3·44; meso-inositol (97 %), 28·53; calcium-d-pantothenate (90 %), 4·19; riboflavin (80 %), 0·73; pyridoxine hydrochloride (98 %), 0·45. All ingredients were diluted with maize starch to 1 kg.
‡ Per kg of mineral premix (g/kg): MnSO4·H2O (31·8 % Mn), 2·6590; MgSO4·H2O (15·0 % Mg), 256·7933; FeSO4·H2O (30·0 % Fe), 12·6083; ZnSO4·H2O (34·5 % Zn), 8·8700; CuSO4·5H2O (25·0 % Cu), 0·9560; CaI2 (3·2 % iodine), 1·5625; Na2SeO3 (44·7 % Se), 0·0611. All ingredients were diluted with maize starch to 1 kg.
§ Crude protein and lipid contents were measured values.
‖ n-3 and n-6 were referred to Zeng et al.(Reference Zeng, Jiang and Liu89) and were calculated according to the National Research Council(90).
¶ Available P was referred to Wen et al.(Reference Wen, Jiang and Feng91) and was calculated according to the National Research Council(90).
Fish management and feeding
All protocols were approved by the University of Sichuan Agricultural Animal Care Advisory Committee, Sichuan, China, under permit no. PXR-S20163729. The on-growing grass carp were obtained from fishery, which were able to feed and swim normally, and were observed no diseases and parasites on microscopic examination. First, we kept the on-growing grass carp rearing in the outdoor tanks, which experienced natural light–dark cycle for 2 weeks to make them acclimatise to the experimental environment. Then, we randomly put 540 healthy on-growing grass carp (approximately 178·47 (SD 0·36) g) into eighteen cages (140 × 140 × 140 cm3), which eventually led to three cages in each treatment and thirty fish in each cage, as described in our previous study(Reference Jiang, Wen and Liu26). In the bottom of each cage, there was a disc (diameter 100 cm) to collect the uneaten feed, as described by Jiang et al.(Reference Jiang, Wen and Liu26). During the growing trial, we fed fish with their six corresponding diets (three replicates) to apparent satiation four times per d for 60 d, primarily on the basic of the great differences in feed intake among six treatments and the study of Met in fish by our laboratory(Reference Wu, Tang and Jiang2,Reference Tang, Wang and Jiang27) . The daily feed supplied per cages was recorded, and after 30 min of feeding, the uneaten feed on the disc was collected, followed by drying using an electrical fan at room temperature and weighing to calculate the feed intake according to Cai et al.(Reference Cai, Luo and Xue28). During the period of feed trial, the dissolved oxygen was at least 6·0 mg/l, and using the measurement method mentioned by Li et al.(Reference Li, Feng and Jiang29), the water temperature and pH were 28·6 (SD 3·1)°C and 7·4 (SD 0·3), respectively. Also, fish were reared under natural light–dark cycle throughout the trial.
Sample collection and analysis
At the termination of the 60 d growth trial, we weighed and counted the fish in each cage. Six hours after the last feeding, six fish in each treatment were selected, anaesthetised in a benzocaine (50 mg/l) bath with the introduction mentioned by Geraylou et al.(Reference Geraylou, Souffreau and Rurangwa30), after that, to determine the plasma ammonia content, we collected the blood samples from fish caudal vein, removed and stored the plasma, which was same as the method described in previous study(Reference Chen, Feng and Kuang31). Meanwhile, twelve fish conducted similar to above from each treatment were quickly caught and dissected, and the whole hepatopancreas and the left dorsal muscle above the lateral line and behind the head of body were quickly removed on ice, snap-frozen in liquid N2 and stored at –80°C for later assay of enzyme activity, RNA extraction and quantification as well as Western blot analysis. The muscle located on the same position in the right side of the same fish was also sampled for other biochemical analysis. As described in the previous study(Reference Khan, Jafri and Chadha32), we analysed the muscle crude protein, crude lipid and ash. To determine collagen content, we measured the amout of hydroxyproline (Hyp) using the method as mentioned by Periago et al.(Reference Periago, Ayala and López-Albors33). The collagen content was calculated by multiplying the Hyp content by eight as provided by AOAC method 990.26(Reference Horwitz34), due to that content of Hyp in collagen was 12·5 % if the nitrogen-to-protein factor was 6·25. Using the method in line with Yu et al.(Reference Yu, Xu and Regenstein35), we measured the free amino acid contents by employing an automatic amino acid analyser. Using the gas chromatographic method determines the fatty acid constitutes(Reference Xu, Dong and Zuo36). Muscle and hepatopancreas samples were homogenised in ice-cold physiological saline solution (ten volumes, w/v) and centrifuged at 6000 g and 4°C for 20 min, and then, the supernatant was collected for enzyme activity analysis. According to the description of Jiang et al.(Reference Jiang, Feng and Tang37) and Yang et al.(Reference Yang, Ding and Tan38), we measured plasma ammonia content, the activity of glutamate-oxaloacetate transaminase and glutamate-pyruvate transaminase in the hepatopancreas and muscle and fatty acid activity (fatty acid synthase; FAS) in the muscle of fish, respectively.
Morphometric analysis
The muscle tissue was fixed in 4 % paraformaldehyde, dehydrated in ethanol/methanol and embedded in paraffin. Then tissue was sectioned to 5–7 µm. Sections were stained using standard haematoxylin–eosin and examined by a Nikon TS100 light microscope according to Kokou et al.(Reference Kokou, Sarropoulou and Cotou39). Morphometric analysis was performed using Image Pro Plus® 4·5 image analysis software (Media Cybernetics). According to the description by Dubovitz et al.(Reference Dubovitz, Brooke and Neville40), the smallest diameter of 200 muscle fibres of per sample of three grass carp from each treatment was measured and distributed into three diameter classes (<20, 20–50 or >50 µm), as described by de Almeida et al.(Reference de Almeida, Carvalho and Pinhal41).
Real-time PCR reaction analysis
We used the RNAiso Plus Kit (TaKaRa) to extract total RNA from muscle samples to quantitatively analyse the differentiation of related target genes of collagen-related signalling molecules affected by different Met concentrations. We employed spectrophotometric analysis to verify the purity and concentration of total RNA, ensuring that the OD260:OD280 ratio was from 1·9 to 2·0 and the amounts of total RNA for inverse transcription were at most 1 µg as requested by the manufacturer’s instructions of reverse transcription kit (TaKaRa Biotechnology Co. Ltd), respectively. We used agarose gel electrophoresis (1 %) to determine the quality of total RNA (28S:18S rRNA bands were approximately 2:1). The cDNA was synthesised from RNA using PrimeScript™ RT Reagent Kit (TaKaRa), which was conducted under the guidance of the manufacturer’s instructions. Online Supplementary Table S1 shows the primer sequences used in the present study. To normalise cDNA loading, according to the evaluation of reference gene conducted previously by our laboratory (data not shown), our selected reference gene was β-actin. On the basic of the specific gene standard curves, which generated from the 10-fold serial dilutions, we calculated the amplification efficiency involved with all genes (n 6). After confirming that the amplification efficiency of the primer was about 100 %, we used the 2−ΔΔCT method to calculate the transcript expression data according to Livak et al.(Reference Livak and Schmittgen42).
Western blotting
The procedures used to conduct Western blotting analysis were the same as those mentioned in our previous reports(Reference Hu, Zhang and Feng43,Reference Jiang, Liu and Jiang44) . We used the BCA protein assay kit (Beyotime Biotechnology Inc.) to determine the protein concentration in supernatant. Subsequently, we loaded equal amounts of protein samples (40 µg per lane) obtained from respective treatments to each lane and electrophoresed on an SDS-glycine polyacrylamide gel, and then, the protein samples were transferred to a polyvinylidene difluoride membrane for the Western blot analysis. The membrane was blocked for 1 h with 0·5 % BSA at room temperature and then incubated with primary antibody overnight at 4°C. The appropriate antibodies used in the current experiment are as follows: anti-t-TOR, p-TORSer2448, t-S6K1, p-S6K1Ser389, t-4E-BP1, p-4E-BP1Thr37/46, t-Smad4, t-Smad2, p-Smad2Ser467 and β-actin. In our previous study, we have proved that the antibodies of β-actin (AF7018, 1:3000 dilution), anti-total TOR (AF6308, 1:1000 dilution) and p-TORSer2448 (AF3308, 1:1000 dilution) (Affinity BioReagents) chosen in the present research could successfully react with grass carp proteins of interest. The antibodies of anti-Smad4 (AF5247, 1:1000 dilution), t-Smad2 (AF6449, 1:1000 dilution) and p-Smad2Ser467 (AF3449, 1:1000 dilution) were also purchased from the same company mentioned above (Affinity BioReagents), and t-S6K1 (9202, 1:1000 dilution), p-S6K1Ser389 (9234, 1:1000 dilution), t-4E-BP1 (9452, 1:1000, dilution) and p-4E-BP1Thr37/46 (9459, 1:1000 dilution) were purchased from Cell Signaling Technology and used in grass carp according to Shi et al.(Reference Shi, Jin and Sun45). Immediately after, the polyvinylidene difluoride membranes were washed three times with tris-buffered saline with Tween, followed by an incubation with horseradish peroxidase-conjugated secondary antibody for 1·5 h. We visualised and quantified the bands using an ECL kit (Millipore) and an NIH Image 1.63 software, respectively. The protein levels for Met-supplemented groups were expressed as a relative value to that in Met-unsupplemented group. The experiment was conducted at least three times, and similar results were obtained each time.
Data analysis
Growth performance parameters were calculated as shown in Table 2.
Table 2. Index formula for percentage weight gain (PWG), specific growth rate (SGR), feed efficiency (FE) and feed intake (FI) of on-growing grass carp (Ctenopharyngodon idella)
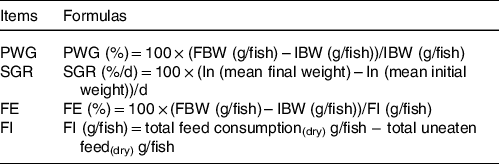
IBW, initial body weight.
We confirmed the normality and homoscedasticity assumptions prior to the statistical analysis for all data. Results are presented as mean values and standard deviations. Statistical analysis of all data was conducted using one-way ANOVA followed by the Duncan’s multiple-range test to evaluate significant differences between the treatment groups at the level of P < 0·05 with SPSS 18.0 (SPSS Inc.). On the basis of the means and standard deviations of growth and muscle growth-related parameters, the minimum effect size was calculated to be 0·65 according to the method of Searcy-Bernal(Reference Searcy-Bernal46). With the effect size of 0·65, a significance level of 0·05 and the six replicates in each treatment, the statistical power was calculated to be 0·80 using the R pwr package according to Grey et al.(Reference Grey, Chiasson and Williams47). Quadratic regression mode was employed to determine the optimal dietary Met levels of on-growing grass carp according to different indices.
Results
Growth performance, feed utilisation and amino acid metabolism-related parameters of on-growing grass carp
All experimental diets were well accepted by the fish, and the mortality rates were zero for all grass carp during and after the 60- d trial. As shown in Table 3, there was no significant difference on initial body weight of fish (P > 0·05). With Met levels from 2·54 to 7·43 g/kg of the diet, the growth performance and feed utilisation parameters (specific growth rate, feed efficiency and feed intake) were elevated significantly individually (P < 0·05) and then decreased significantly for feed intake and specific growth rate (P < 0·05) and gradually for feed efficiency with higher Met levels in the diet. From Table 3, we found that the Met requirement for on-growing grass carp suggested to be 7·43 g/kg of the diet as growth was maximised at this point and showed a decline with further increase in Met supplementation. However, when we used quadratic regression analysis to determine the optimal dietary Met level for on-growing grass carp based on percentage weight gain (PWG), it was 9·54 g/kg diet, corresponding to 33·19 g/kg protein of diet, as shown in Fig. 1. When Met compositions increased from 2·54 to 10·12 g/kg of the diet, the activities of glutamate-oxaloacetate transaminase in the hepatopancreas and muscle increased gradually and then decreased gradually. The activities of glutamate-pyruvate transaminase in the hepatopancreas and muscle prominently advanced with Met compositions range 2·54 to 10·12 g/kg of the diet (P < 0·05) and then reduced significantly (P < 0·05). With Met compositions not higher than 7·43 g/kg of the diet, the plasma ammonia content was markly decreased (P < 0·05) and then gradually increased when Met was at higher concentrations.
Table 3. Growth performance, feed utilisation, amino acid metabolism-related parameters and regular nutritional compositions in the muscle of on-growing grass carp (Ctenopharyngodon idella) fed diets with graded levels of methionine (Met) (g/kg) for 60 d
(Mean values and standard deviations)

IBW, initial body weight (g/fish); FBW, final body weight (g/fish); FI, feed intake (g/fish); FE, feed efficiency; PWG, percentage weight gain (%); SGR, specific growth rate (%/d); GOT, glutamate-oxaloacetate transaminase (U/g tissue); GPT, glutamate-pyruvate transaminase (U/g tissue); PAC, plasma ammonia contents (μmol/l).
a,b,c,d,e,f Mean values in a row with unlike superscript letters are significantly different (P < 0·05; ANOVA and Duncan’s multiple-range tests).
* Mean values and standard deviations for three replicate groups, with thirty fish in each group.
† Mean values and standard deviations for six replicate groups.
‡ Mean values and standard deviations for six replicate groups. Moisture (%); crude protein (%); crude lipid (%); ash (%); Hyp, hydroxyproline (μg/mg tissue); collagen (μg/mg tissue).
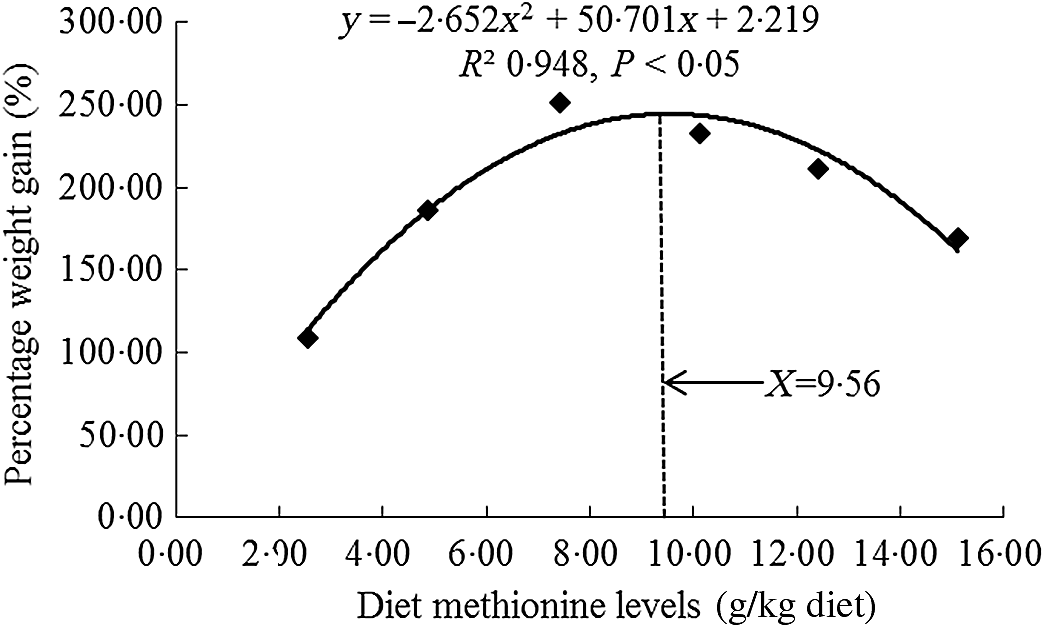
Fig. 1. Quadratic regression analysis of percentage weight gain for on-growing grass carp (Ctenopharyngodon idella) fed diets with graded levels of methionine (g/kg) for 60 d.
Regular nutritive compositions in the muscle of on-growing grass carp
As shown in Table 3, with Met compositions in the diet not higher than 7·43 g/kg of the diet, the crude protein, crude lipid, Hyp and collagen contents were significantly heightened (P < 0·05) and then diminished smoothly. When the increase in Met compositions in the diet was from 2·54 to 7·43 g/kg of the diet, we also found that the moisture content gradually decreased, and with Met compositions from 7·43 to 15·11 g/kg of the diet, that increased sustainably. The ash decreased as Met levels increased, with plateauing at 7·43 g/kg diet (P > 0·05). With using quadratic regression analysis to estimate the optimal Met requirement for on-growing grass carp according to the muscle collagen content, we found that it was 9·28 g/kg of the diet, corresponding to 32·29 g/kg protein of diet (Fig. 2).
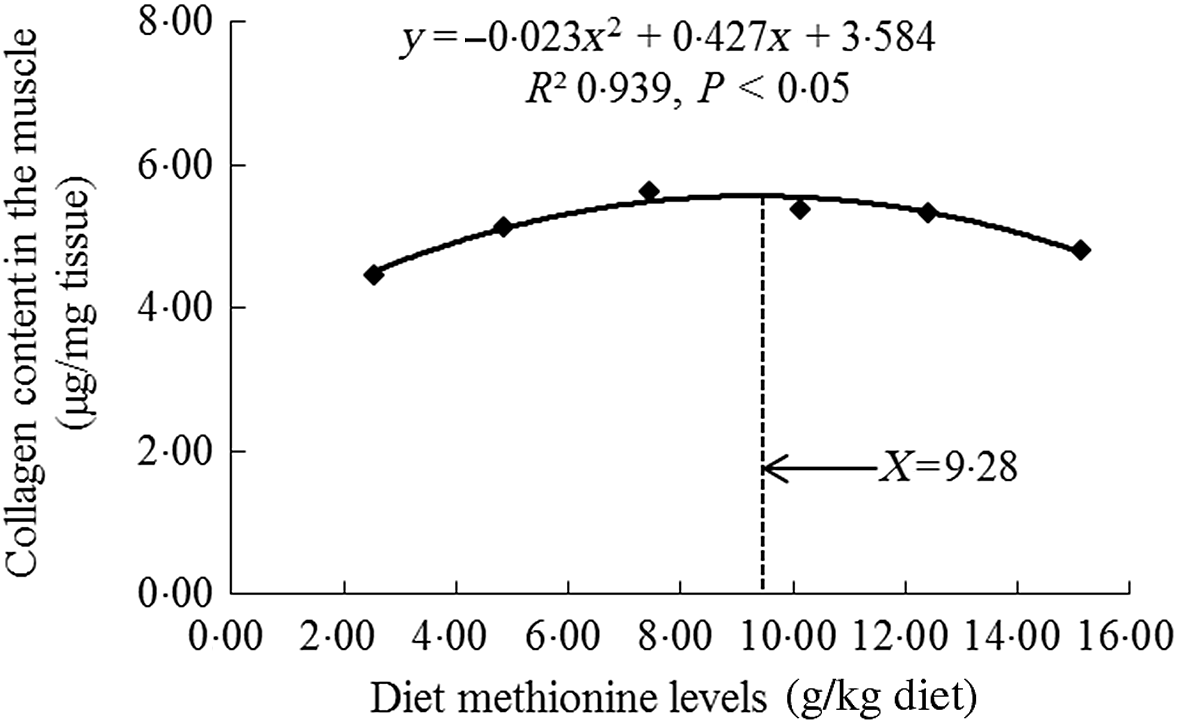
Fig. 2. Quadratic regression analysis of collagen in the muscle for on-growing grass carp (Ctenopharyngodon idella) fed diets with graded levels of methionine (g/kg) for 60 d.
Free amino acid compositions in the muscle of on-growing grass carp
As shown in Table 4, the contents of total essential amino acid, total amino acid, Asp and Ser were all maximum at point of Met concentration of 10·12 g/kg diet, and in the 7·43 g Met/kg diet group, the contents of Ile, Lys, Gly, Glu, Leu and Arg were highest. The Met content was lowest in the group fed with Met-unsupplemented diet, while there were no significant change when dietary Met levels were higher than 2·54 g/kg of the diet. We found that the Thr content did not significantly change in Met-supplemented groups, but all were slightly higher than that in 2·54 g Met/kg of the diet group. In comparison with Met-unsupplemented group, the content of Phe obviously elevated with Met compositions reached to 10·12 g/kg diet (P < 0·05) and then showed flat (P > 0·05). The cysteine content gradually increased as increasing Met levels even though there were no significant difference within the range of 7·43–15·11 g Met/kg diet groups (P > 0·05). We also found that the differences of the contents of Ala,Val, His, Tyr and Pro were not significant among all treats (P > 0·05).
Table 4. Free amino acid compositions (mg/100 g tissue) in the muscle of on-growing grass carp (Ctenopharyngodon idella) fed diets with graded levels of methione (Met) (g/kg) for 60 d
(Mean values and standard deviations; six replicate groups)

TAA, total amino acids; TEAA, total essential amino acids.
a,b,c,d Mean values in a row with unlike superscript letters are significantly different (P < 0·05; ANOVA and Duncan’s multiple-range tests).
Fatty acid profile and fatty acid synthase activity in the muscle of on-growing grass carp
The fatty acid profile and FAS of on-growing grass carp muscle affected by graded Met levels are shown in Table 5. With Met compositions in the diet increased up to 7·43 g/kg of the diet, the content of C14:0 gradually decreased and then increased gradually. The contents of C16 : 1, C20 : 2, C20 : 3n-3, C18 : 3n-6, C18 : 1n-9t, C18 : 1n-9c, C20 : 5n-3, ∑MUFA, ∑PUFA and ∑n-3 (PUFA) were all highest at point of Met concentrations of 10·12, 12·40, 12·40, 7·43, 15·11, 7·43, 10·12, 7·43, 12·40 and 10·12 g/kg diet, respectively. The contents of C17:1 in the 2·54, 4·83 and 15·11 g/kg of the diet groups were obviously lower than that in groups with 7·43, 10·12 and 12·40 g Met/kg diet (P < 0·05). We found in the diet with 7·43 g/kg of Met that the C16:0 content was lowest (P < 0·05), and the differences among remaining groups for that were not significant (P > 0·05). With Met concentrations reached to 12·40 and 7·43 g/kg of the diet, the contents of C17 : 0 and C20 : 3n-6 showed significantly higher in comparison with control group (P < 0·05), respectively, and then evidently reduced for C17 : 0 (P < 0·05) and plateaued for C20 : 3n-6 (P > 0·05). In the diet with Met level of 15·11 g/kg diet, the content of C18:0, which showed no significant difference with 12·40 g Met/kg diet group (P > 0·05), was highest, and that was not statistically significant in remaining groups (P > 0·05). In the muscle of fish fed a diet containing 12·40 g/kg of Met, the C20 : 0 content was highest, but showed no significant difference with 4·85 g Met/kg diet group (P > 0·05), while in groups with 2·54 and 10·12 g Met/kg diet, the C14 : 1 content was highest. The C23 : 0 content decreased as Met levels increased to 7·43 g/kg diet, and there was no significant difference in remaining groups (P > 0·05). The content of ∑UFA was highest in 7·43 g Met/kg diet group (P < 0·05), followed by 10·12 g Met/kg diet group, and in remaining groups, that showed no significant difference (P > 0·05). For fish fed a diet with 7·43 g/kg of Met, the content of C22 : 1n-9 was lowest (P < 0·05). The ∑SFA content decreased with methionine compositions in the diet reached to 7·43 g/kg diet, and when Met compositions were above 7·43 g/kg of the diet, that increased gradually. However, we found that Met compositions in the diet had no impact on the contents of C15 : 0, C21 : 0, C22 : 0, C20 : 1n-9, C24 : 1n-9, C22 : 6n-3, C18 : 3n-3, C18 : 2n-6t, C18 : 2n-6c, C22 : 2 and ∑n-6 (PUFA) as well as the ratio of ∑n-3:∑n-6 (P > 0·05). The activity of FAS gradually increased when Met concentrations reached to 10·12 g/kg of the diet and then smoothly reduced.
Table 5. Fatty acid (FA) profile (% of total FA methyl esters) and fatty acid synthase (FAS) activity in the muscle of on-growing grass carp (Ctenopharyngodon idella) fed diets with graded levels of methionine (Met) (g/kg) for 60 d
(Mean values and standard deviations; six replicate groups)

UFA, unsaturated fatty acids.
a,b,c,d Mean values in a row with unlike superscript letters are significantly different (P < 0·05; ANOVA and Duncan’s multiple-range tests).
Histological analysis of muscle of on-growing grass carp
Figure 3 shows the muscle morphology of on-growing grass carp affected by graded Met compositions in the diet, and the statistical results related to frequency of distribution (%) of muscle fibres in diameter classes are shown in Table 6. Haematoxylin–eosin stain showed that muscle fibres of all groups were characterised by mosaic appearance with different diameters. With increasing Met in the diet up to 7·43 g/kg diet, the frequency of <20 μm diameter gradually decreased and then increased gradually. The frequency distribution of >50 μm diameter in 7·43 and 10·12 g Met/kg diet groups showed higher than that in remaining four groups (P < 0·05). However, the frequency distribution of 20–50 μm diameters showed no significance among all treats (P > 0·05).
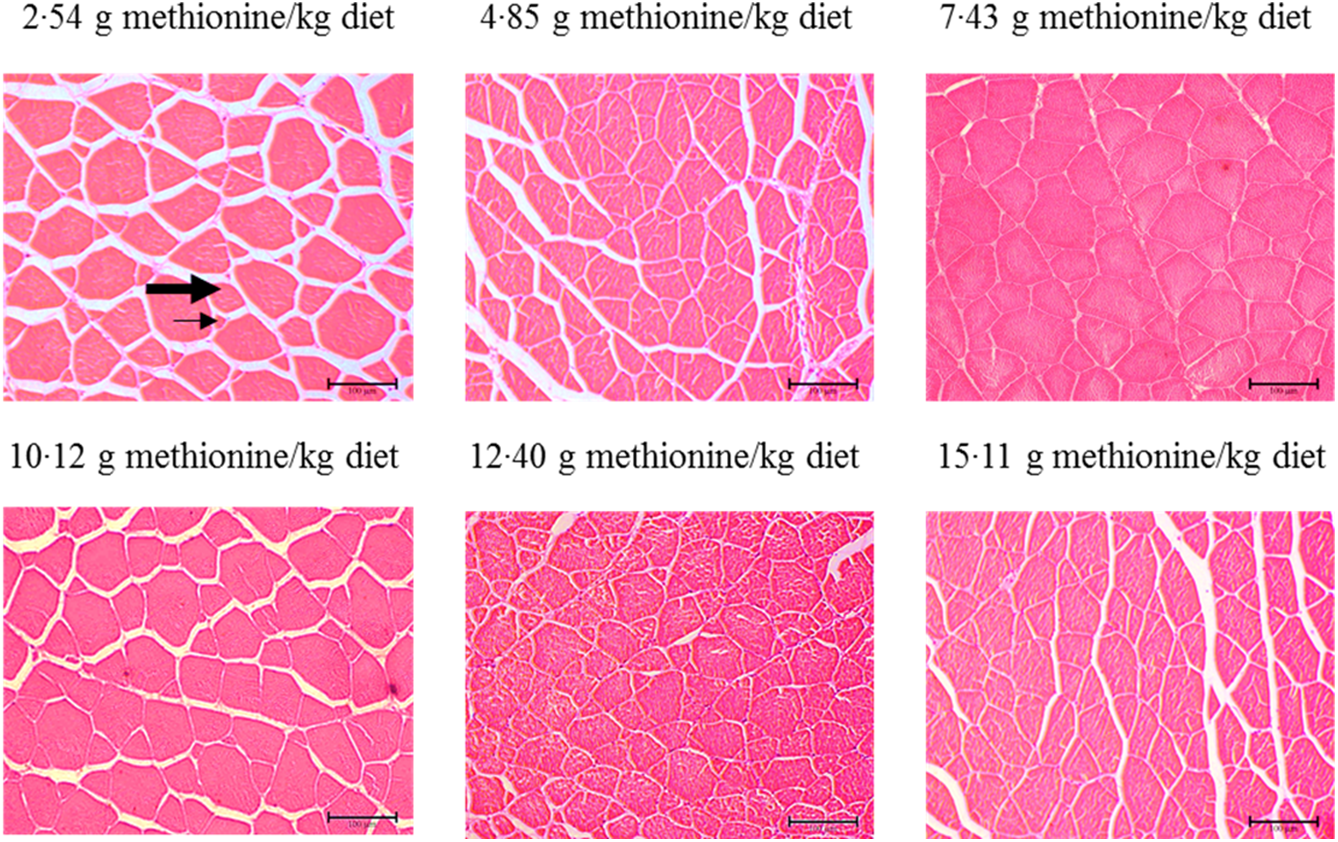
Fig. 3. Cross section of muscle of on-growing grass carp (Ctenopharyngodon idella) fed diets with graded levels of methionine (g/kg) for 60 d using haematoxylin–eosin stain. Different muscle fibre diameters are composed of small (arrows) to large (thick arrowhead) fibres.
Table 6. Frequency of distribution (%) of muscle fibres in diameter classes of on-growing grass carp (Ctenopharyngodon idella) fed diets with graded levels of methionine (Met) (g/kg) for 60 d
(Mean values and standard deviations; three replicates)

a,b,c,d Mean values in a row with unlike superscript letters are significantly different (P < 0·05; ANOVA and Duncan’s multiple-range tests).
Protin and type I collagen synthesis-related mRNA and protein levels in the muscle of on-growing grass carp
As shown in Fig. 4, with Met supplements in the diet added to 10·12 and 7·43 g/kg diet, the mRNA abundances of TOR and CK2α were advanced individually, and in further dietary Met concentrations, all were gradually plateaued (P < 0·05), respectively. When dietary Met compositions changed from 2·54 to 7·43 g/kg diet, the mRNA abundance of CK2β was significantly up-regulated (P < 0·05), and in higher Met compositions in the diet, there showed gradually downward trend for CK2β.
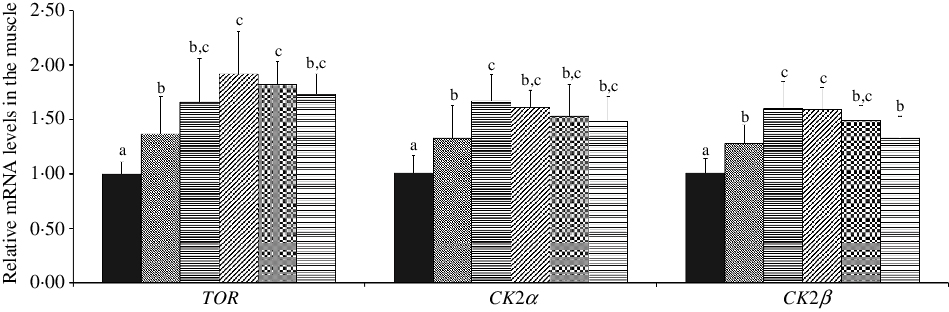
Fig. 4. Effects of dietary methionine levels on TOR, CK2α and CK2β gene expressions in the muscle of on-growing grass carp (Ctenopharyngodon idella). Data represent means of six fish in each group, error bars indicate standard deviations. a,b,c Mean values with unlike letters are significantly different (P < 0·05; ANOVA and Duncan’s multiple-range tests). Dietary methionine levels (g/kg diet): ![]() , 2·54;
, 2·54; ![]() , 4·85;
, 4·85; ![]() , 7·43;
, 7·43; ![]() , 10·12;
, 10·12; ![]() , 12·40;
, 12·40; ![]() , 15·11.
, 15·11.
As shown in Fig. 5, when Met compositions in the diet increased in the range 2·54 to 10·12 g/kg diet, we found that the protein abundance of p-TORSer2448 increased and then decreased gradually. Also, we found that the protein level of t-TOR increased gradually with methioinine supplements added to 10·12 g/kg of the diet and then decreased gradually. The ratio of p-TORSer2448: t-TOR was lowest in 4·85, 7·43 and 10·12 g Met/kg diet groups and showed no significant change in remaining groups (P > 0·05). The protein level of p-S6K1Ser389 and the ratio of p-S6K1Ser389: t-S6K1 were highest in 7·43, 10·12 and 12·40 g Met/kg diet groups (P < 0·05), and the changes among remaining groups were not significant (P > 0·05). The levels of dietary Met had no impact on the protein abundances of t-S6K1, p-4E-BP1Thr37/46 and t-4E-BP1 as well as the ratio of p-4E-BP1Thr37/46: t-4E-BP1 (P > 0·05).
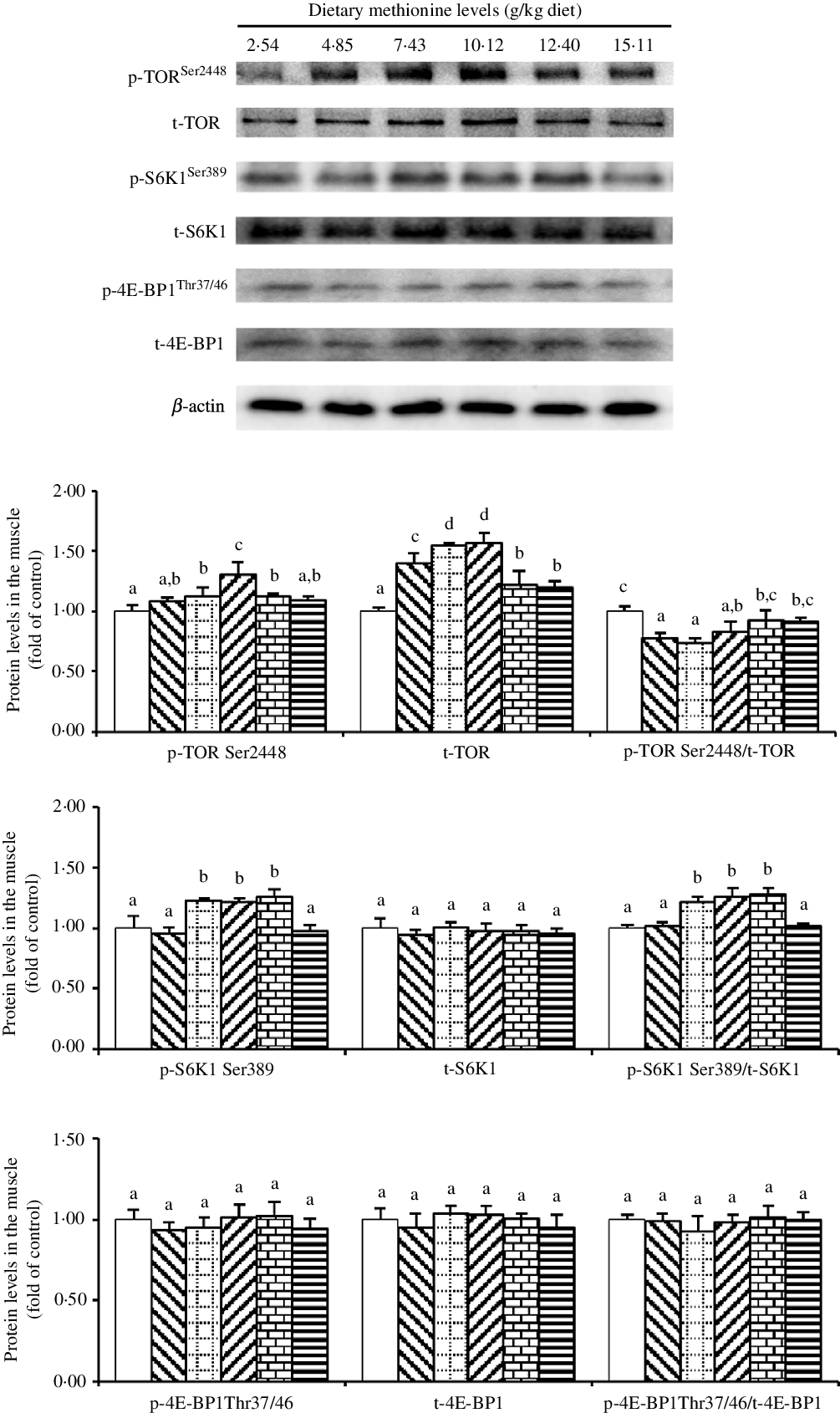
Fig. 5. Western blot analysis of protein expression of genes involved in protein metabolism in the muscle of on-growing grass carp (Ctenopharyngodon idella) fed diets with graded levels of methionine (g/kg) for 60 d. Data represent means of three fish in each group, error bars indicate standard deviations. a,b,c,d Mean values with unlike letters are significantly different (P < 0·05; ANOVA and Duncan’s multiple-range tests). Dietary methionine levels (g/kg diet): ![]() , 2·54;
, 2·54; ![]() , 4·85;
, 4·85; ![]() , 7·43;
, 7·43; ![]() , 10·12;
, 10·12; ![]() , 12·40;
, 12·40; ![]() , 15·11.
, 15·11.
As shown in Fig. 6, in the muscle, as Met supplements added to 7·43, 10·12, 7·43 and 7·43 g/kg of the diet, the mRNA abundances of Col1α1, Col1α2, TGF-β1 and Smad4 were smoothly increased and then gradually declined, respectively. With Met compositions in the diet from 2·54 to 7·43 g/kg of the diet, the Smad2 mRNA abundance increased markedly (P < 0·05), and when Met compositions were higher than 7·43 g/kg diet, that trended flat (P > 0·05). The mRNA abundance of TNF-α was highest in Met-unsupplemented group (P < 0·05); however, there was no marked difference in remaining groups (P > 0·05).

Fig. 6. Effects of dietary methionine levels on Col1α1, Col1α2, TGF-β1, Smad4, Smad2 and TNF-α gene expressions in the muscle of on-growing grass carp (Ctenopharyngodon idella). Data represent means of six fish in each group, and error bars indicate standard deviations. a,b,c Mean values with unlike letters are significantly different (P < 0·05; ANOVA and Duncan’s multiple-range tests). Dietary methionine levels (g/kg diet): ![]() , 2·54;
, 2·54; ![]() , 4·85;
, 4·85; ![]() , 7·43;
, 7·43; ![]() , 10·12;
, 10·12; ![]() , 12·40;
, 12·40; ![]() , 15·11.
, 15·11.
As shown in Fig. 7, when dietary Met supplements added to 7·43 g/kg diet, the protein level of Smad4 significantly elevated (P < 0·05), and as Met compositions in the diet were higher than 7·43 g/kg diet, that decreased gradually. With Met compositions in the diet at 10·12 and 12·40 g/kg diet, the protein abundance of t-Smad2 was higher than that in remaining four groups (P < 0·05). The p-Smad2Ser467 protein level was highest in the 10·12 g Met/kg diet group (P < 0·05) and lowest in the control group (P < 0·05), and the remaining four Met levels had no effect on that (P > 0·05). The ratio of p-Smad2:t-Smad2 was highest in the 7·43 and 10·12 g Met/kg diet groups.
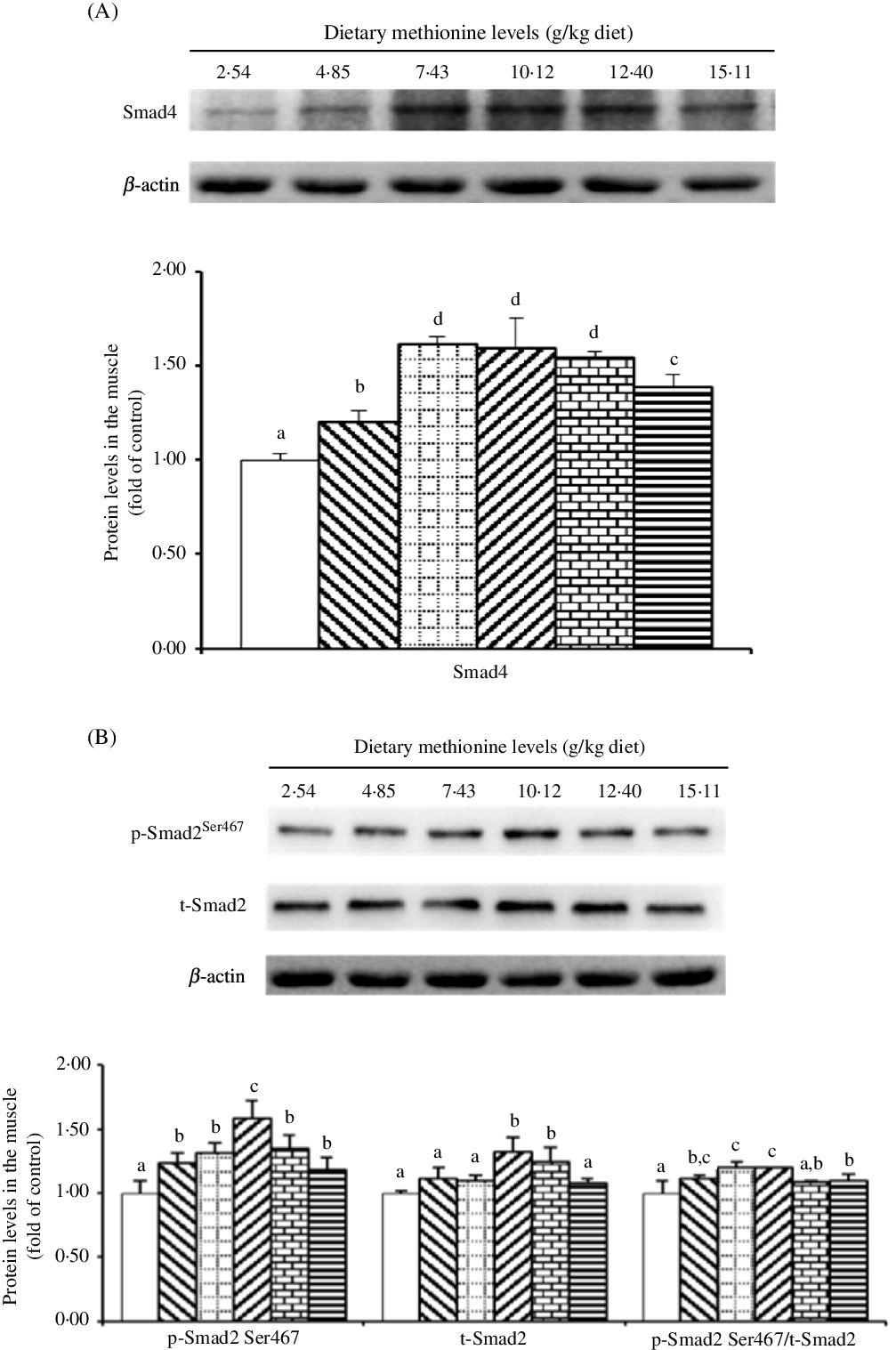
Fig. 7. Western blot analysis of protein expression of genes involved in type I collagen metabolism in the muscle of on-growing grass carp (Ctenopharyngodon idella) fed diets with graded levels of methionine (g/kg) for 60 d. Data represent means of three fish in each group, and error bars indicate standard deviations. a,b,c,d Mean values with unlike letters are significantly different (P < 0·05; ANOVA and Duncan’s multiple-range tests). Dietary methionine levels (g/kg diet): ![]() , 2·54;
, 2·54; ![]() , 4·85;
, 4·85; ![]() , 7·43;
, 7·43; ![]() , 10·12;
, 10·12; ![]() , 12·40;
, 12·40; ![]() , 15·11.
, 15·11.
Discussion
Methionine improved fish growth performance
In this study, we discovered that the optimal level of Met enhanced the feed intake, feed efficiency, PWG and the activities of glutamate-oxaloacetate transaminase and glutamate-pyruvate transaminase in the hepatopancreas and muscle as well as decreased the plasma ammonia content of on-growing grass carp, indicating that Met improved amino acid utilisation and growth performance of fish. Meanwhile, the optimal dietary Met requirement of on-growing grass carp (178–626 g) was 9·56 g/kg of the diet (corresponding to 33·26 g/kg protein of diet, Fig. 1) for maximum PWG. This value was slightly different with our previous research for on-growing grass carp, which was 10·33 g/kg of the diet (34·43 g/kg protein of diet, calculated PWG by their results)(Reference Tang, Feng and Liu20). That slight difference in Met requirements between our two studies might be partially relevant to diverse types and amounts of protein sources (fishmeal: 6·80 %, gelatin: 4·00 %, soyabean protein concentrate: 11·00 % in current experiment v. fishmeal: 7·80 %, gelatin: 3·99 %, casein: 3·00 % in our previous experiment) and the variation in concentrations of crystal amino acid mix (14·28 % in current experiment v. 20·96 % in our previous experiment) in these two experiments. Additionally, fish growth is mainly due to muscle growth, which is closely relevant to the deposition of nutrients and the growth of muscle fibre and surrounding intramuscular connective tissue in the muscle(Reference Jiang, Wu and Tang4,Reference Johnston, Bower and Macqueen9) . Therefore, we next investigated the effects of dietary Met on these processes in the muscle of on-growing grass carp.
Methionine promoted the deposition of nutrients in the muscle of fish
To our knowledge, in fish, muscle is the main site of nutritive deposition(Reference Mommsen48), while muscle protein and lipid are the main DM(Reference Fauconneau, Alami-Durante and Laroche49). First, our current result in on-growing grass carp displayed that compared with Met insufficiency, optimal level of Met increased muscle protein content, as the same results for juvenile grouper(Reference Luo, Liu and Mai7) and Pseudobagrus ussuriensis (Reference Wang, Che and Tang50). The increased protein content in the muscle was potentially in part contacted to the TORC1 signalling pathway. As the core component of mTORC1, phosphorylation of mTOR at Ser2448 is stimulated by amino acids, which is an reasonable indicator for the activation status of mTOR(Reference Chiang and Abraham51). In addition, ribosomal S6 kinase (S6K1) is a key signalling molecule that regulates protein synthesis, which can activate 40S ribosomal protein S6 through phosphorylation to improve the translation efficiency of some mRNA(Reference Jefferies, Fumagalli and Dennis52,Reference Aoki, Blazek and Vogt53) , while eukaryotic translation initiation factor 4E binding protein 1(4E-BP1), as a negative regulator of translation, can inhibit the initiation of translation by combining with the eukaryotic translation initiation factor 4E’s mRNA cap binding subunit(Reference Gingras, Raught and Sonenberg54). It has been reported that mTORC1 can modify S6K1 and 4E-BP1 by direct phosphorylation to promote protein translation process(Reference Laplante and Sabatini55). Our current observations indicated that optimal Met level elevated muscle TOR mRNA abundance, the total and phosphorylation of TOR protein levels of on-growing grass carp. These results were consistent with reports in cobia Rachycentron canadum (Reference He, Chi and Tan56), songpu mirror carp(Reference Cheng, Wang and Xu57) and broiler(Reference Wen, Wu and Chen58) demonstrating that Met could increase TOR (mTOR) mRNA abundance in the muscle, as well as in grass carp(Reference Su, Wu and Feng59), showing that Met could advance the protein levels of total and phosphorylated TOR in the intestine. Also, we found that optimal Met level reduced the ratio of p-TORSer2448/t-TOR in the current experiment, which was inconsistent with that in cobia(Reference He, Chi and Tan56) and turbot Scophthalmus maximus L.(Reference Jiang, Bian and Zhou60). That result might be due to the fact that the effect of Met on translation was greater than activation to TOR in our result, and similar result could be found in the study of bovine mammary epithelial cells(Reference Hu, Chen and Cortes61). In addition, optimal Met level enhanced the protein level of p-S6K1Ser389 and the ratio of p-S6K1Ser389: t-S6K1, while we failed to test any obvious change in the level of p-4E-BP1Thr37/46 and the ratio of p-4E-BP1Thr37/46: t-4E-BP1, as the similar results in cobia(Reference He, Chi and Tan56). Such a discrepancy between the phosphorylation status of these two proteins (S6 and 4E-BP1) has already been discovered in the muscle of rainbow trout(Reference Belghit, Skiba-Cassy and Geurden17) and in the liver of rats(Reference Sikalidis, Mazor and Kang62) in response to dietary Met intake and highlights the complexity of the signalling network associated with the regulation of the activation of these translation initiation factors. By correlation analysis, we found positive correlations between the content of muscle protein and the protein level of p-TORSer2448 and p-S6K1Ser389 (online Supplementary Table S2). These data indicated that optimal Met level promoting muscle protein content might be in part associated to elevate muscle protein synthesis via the TORC1/S6K1 (not 4E-BP1) signalling pathway in fish. We envisaged that the regulating effect of Met on TORC1 signalling might be partially relevant to incremental muscle free amino acid contents, particularly Met, arginine, threonine and leucine. Therefore, we next investigated the effects of dietary Met on free amino acid content in the muscle of on-growing grass carp.
In the muscle, previous studies reported that Met in rainbow trout(Reference Belghit, Skiba-Cassy and Geurden17), arginine in Jian carp Cyprinus carpio var. Jian(Reference Chen, Feng and Kuang31) and threonine and leucine in hybrid catfish Pelteobagrus vachelli × Leiocassis longirostris (Reference Zhao, Jiang and Zhou63,Reference Zhao, Li and Jiang64) could stimulate protein synthesis via the TORC1 signalling pathway. In the present study, we found that compared with the control group, optimal Met level increased muscle-free Met, arginine, threonine and leucine contents of on-growing grass carp, supporting our hypothesis. Meanwhile, in the present study, we also found that optimal level of Met enhanced muscle-free total amino acid and total essential amino acid contents of on-growing grass carp, which might be to some extent associated with increased amino acids absorption. In fish, most AA absorption required an extracellular Na ion concentration gradient(Reference Nitzan, Rozenberg and Cnaani65). It has been reported that Na+, K+-ATPase was located on the basolateral membrane and could carry out the uptake of Na+ in most higher eukaryotes(Reference Kaplan66). Our previous study in on-growing grass carp reported that optimal Met level increased the activity of Na+, K+-ATPase in the intestine (proximal intestine and mid intestine)(Reference Tang, Feng and Liu20), supporting our assumption.
On the other hand, from current study, we found that in comparison with Met deficiency, optimal Met level also increased on-growing grass carp muscle lipid content, which was similar to the report by Luo et al.(Reference Luo, Liu and Mai7). Meanwhile, it has been reported that the nutritive value of lipid mainly depended on the contents and species of its fatty acid, and decreased SFA and increased unsaturated fatty acids in the muscle could improve the nutritive value of on-growing grass carp(Reference Jiang, Wu and Tang4). In the present research, we for the first time found that optimal Met level decreased the SFA (C14 : 0, C16 : 0, C18 : 0, C20 : 0, C23 : 0) concentrations and increased the unsaturated fatty acids (MUFA, such as C16 : 1, C17 : 1 and C18 : 1n-9c and PUFA, such as C20 : 5 n-3, C18 : 3 n-6 and C20 : 3 n-6) concentrations in on-growing grass carp muscle. We speculated that these results might be partially related to FAS. Ganguly et al. demonstrated that FAS played a crucial role in catalysing the synthesis of PUFA in the muscle of Indian shad hilsa Tenualosa ilisha (Reference Ganguly, Mahanty and Mitra67). Our present data observed that optimal Met level elevated the activity of FAS in the muscle of on-growing grass carp, supporting our assumption.
In addition, muscle growth was also relevant to the growth of muscle fibre and the formation of intramuscular connective tissue(Reference Nishimura8), which were primarily involved with the hyperplastic and hypertrophic growth of muscle fibre and the synthesis of type I collagen, respectively. Therefore, we next examined the effects of Met on these processes as well as related molecular mechanisms (TGF-β1/Smads and TORC1 signalling) in the muscle of on-growing grass carp.
Methionine promoted muscle fibre hypertrophy and type I collagen synthesis in fish
Generally, it was well known that muscle morphology was an important auxiliary mean in fish nutrition research(Reference Michelato, Vidal and Xavier11). Previous study reported that the processes of hyperplasia and hypertrophy would maintain active for a long time to muscle growth in large-sized fish with rapid growth(Reference Buzollo, Sandre and Neira68). It has been reported that a new fibre arised by hyperplasia was relatively small(Reference Zimmerman and Lowery69), of which the diameter of fibres <20 µm always represented the fibres recruited by hyperplasia, and those exceeding this diameter represented fibres that have been subsequently grown by hypertrophy(Reference Rowlerson, Veggetti and Johnston70). Our current observations in on-growing grass carp displayed that fibres <20 µm in diameter occurred in all treatments, and compared with Met unsufficient, optimal level of Met significantly decreased the frequency of fibres <20 µm in diameter and increased the frequency distribution of muscle fibres with >50 µm of diameter. These data indicated that first, hyperplasia occurred in all treatment groups, and this process was also affected by the dietary Met concentrations and second, optimal level of Met promoted muscle fibre growth mostly by hypertrophy in fish.
Meanwhile, except for muscle fibre, the collagen was an important protein that constituted muscle connective tissue and had the characteristics of maintaining structural stability and integrity of fibre in previous study of sea bass Dicentrarchus labrax L.(Reference Periago, Ayala and López-Albors33). Its content increase in fish muscle could result in higher mechanical strength(Reference Gordon and Hahn71). Johnston et al.(Reference Johnston, Li and Vieira72) reported that measuring the content of Hyp could relatively quantify the collagen amount in fish. Our present research in on-growing grass carp displayed that compared with Met deficiency leading to decreased muscle collagen content, optimal Met level significantly enhanced the content of collagen in the muscle. We supposed that this result might be partially linked to de novo synthesis of collagen. In grass carp, the main collagen in the muscle intramuscular connective tissue was type I collagen (included Col1α1 and Col1α2 peptide chains)(Reference Yu, Liu and Wang14). As demonstrated in this study, optimal Met level advanced the mRNA abundances of Col1α1 and Col1α2 in the muscle of on-growing grass carp. The elevated mRNA abundance of Col1α2 was in line with the report for rainbow trout(Reference Alami-Durante, Bazin and Cluzeaud3). In addition, we also found significant positive correlations via correlation analysis on the basic of muscle collagen content and Col1α1, Col1α2 mRNA abundances (online Supplementary Table S2). These data suggested that optimal Met level increased muscle collagen content, which was partially due to up-regulated type I collagen de novo synthesis in the muscle of fish. On the basic of muscle collagen content, the Met requirement was recommended to be 9·28 g/kg diet (32·29 g/kg protein of diet, Fig. 2), which was close to or slightly lower than the optimal Met requirement for growth (9·54 g/kg diet). Similar observation could be found in the study of tryptophan for on-growing grass carp(Reference Jiang, Wen and Liu26). Furthermore, recent research has displayed that type I collagen expression in the muscle could be modulated by the TGF-β1/Smads signalling pathway in grass carp(Reference Yu, Ma and Ji15). Therefore, we next explored the impact of Met on TGF-β1/Smads in the muscle of on-growing grass carp.
As the TGF-β1/Smads signalling pathway, it should be noted that TGF-β1 bound to the TGF-β type II and type I receptor in turn, and the activated TGF-β type I receptor interacted with Smad2 to phosphorylate it, and then, phosphorylated Smad2 bound to Smad4 forming the Smad2/4 complex, and finally, the complex was transferred to the nuclear to work(Reference Mincione, Di Marcantonio and Tarantelli73). In addition, previous studies showed that Smad2 and Smad4 played important regulatory role in type I collagen expression in mammals and grass carp(Reference Yu, Ma and Ji15,Reference Loeffler, Liebisch and Allert74–Reference Liu, Wang and Xie76) . For the first time, our present results for on-growing grass carp displayed that in comparison with control group, optimal level of Met significantly elevated the mRNA abundances of TGF-β1, Smad4, Smad2 and the protein levels of Smad4, t-Smad2 and p-Smad2Ser467 as well as the ratio of p-Smad2Ser467: t-Smad2 in the muscle. We also found positive correlations between Col1α1 mRNA abundance and TGF-β1 mRNA abundance, t-Smad4, p-Smad2Ser467 protein levels via correlation analysis and Col1α2 possessed the similar trend (as shown in online Supplementary Table S2). The results studied above manifested that optimal Met level increased the gene expression of type I collagen via regulating the TGF-β1/(Smad4 and Smad2) signalling pathway. We speculated that there were two pieces of evidence for the Met-regulated TGF-β1/(Smad2 and Smad4) signalling pathway. First, Met acting as a functional signalling molecule directly up-regulated the expression of TGF-β1, and similar result could be found in the study by Pan et al.(Reference Pan, Feng and Jiang77), further activated the downstream signalling pathway involved with Smads. However, this speculation needs deeper exploration. Second, that potentially related to TNF-α. Previous scholars have demonstrated that TNF-α could depress the protein expression of TGF-β type II receptor in human dermal fibroblasts(Reference Yamane, Ihn and Asano78) and prevent the phosphorylation and nuclear translocation of Smads in mice fibroblast cell lines(Reference Bitzer, von Gersdorff and Liang79). Our present research with on-growing grass carp revealed that optimal level of Met increased muscle TNF-α mRNA abundance, which supports our assumption.
Furthermore, type I collagen synthesis was also regulated by mTORC1 signalling in human dermal fibroblasts(Reference Shegogue and Trojanowska18). As described in the current study, optimal level of Met increased the mRNA level, phosphorylation and total protein levels of TOR in the muscle of on-growing grass carp. Also, we discovered that there were positive correlations between Col1α1, Col1α2 mRNA abundances and p-TORSer2448 protein level (as shown in online Supplementary Table S2), indicating that optimal Met level promoted type I collagen synthesis might in part by regulating the TORC1 signalling pathway. The regulation of TORC1 by Met might be partially in connection with protein kinase casein kinase 2 (CK2). CK2 is a constitutively active and highly conserved serine/threonine protein kinase, which includes two catalytic (α and/or α‘) and two regulatory (β) subunits(Reference Litchfield80). CK2 is commonly expressed in subcellular compartments of all eukaryotes and phosphorylates over 300 substrates and regulates several different metabolic events as a result(Reference Meggio and Pinna81). Study reported that CK2 inhibition down-regulated the expression of type I collagen in NIH-3T3 fibroblasts(Reference Bagchi, Wang and Jahan82). Also, in human glioblastoma cells, CK2 depletion led to the reduction of phosphorylation level of mTOR(Reference Olsen, Svenstrup and Guerra83). Our data found that compared with Met deficiency, optimal level of Met elevated muscle CK2α and CK2β mRNA abundances of on-growing grass carp. We also discovered that there were positive correlations between p-TORSer2448 protein level and CK2α, CK2β mRNA abundances (as shown in online Supplementary Table S2), supporting our assumption.
Methionine excess decreased fish growth
Met was a sulphur-containing amino acid, the excessive intake of which could result in poor growth performance and feed utilisation for juvenile Chinese sucker Myxocyprinus asiaticus (Reference Chu, Gong and Lin84). Our present data displayed that compared with Met-insufficiency (2·54 g/kg diet), although excess Met level (15·11 g/kg diet) led to better growth performance, feed utilisation and muscle growth, while in comparison with optimal Met level (7·43 g/kg diet), these indicators significantly decreased. That consequence could be explained by the following possibility that excessive Met intake might (1) reduce the palatability of feed(Reference Griffin, White and Brown85); (2) reduce the ability of digestion and absorption in fish(Reference Wu, Tang and Jiang2,Reference Tang, Wang and Jiang27) ; (3) occupy a large number of amino acid transporters, affecting the transportation and absorption of other amino acids(Reference Espe, Hevrøy and Liaset86,Reference Bogé, Roche and Balocco87) and (4) increase the accumulation of oxidised Met, leading to oxidative stress and reducing the antioxidant capacity(Reference Feng, Yang and Zhu88), which ultimately reduced the growth performance of fish. However, this speculation needs further research.
Conclusion
In short, similar to our previous study, our current research showed that optimal Met level also improved the growth performance and feed ulitisation of on-growing grass carp. Meanwhile, we for the first time systematically revealed that optimal Met level promoted the growth of muscle in fish by regulating the deposition of nutrients, muscle fibre growth and type I collagen synthesis as well as related signalling molecules, as displayed in the following aspects: (1) optimal Met level promoted the deposition of muscle nutrients such as protein, lipid, total free amino acid and unsaturated fatty acid contents, and elevated muscle protein content partly linked to the TORC1/S6K1 signalling pathway; (2) optimal Met level accelerated muscle growth by muscle fibre hypertrophy; (3) optimal Met level enhanced muscle collagen content by increasing type I collagen synthesis in part relevant to (TGF-β1/(Smad4 and Smad2)) and (CK2α, β/TORC1) signalling pathways. Additionally, on the basic of growth indicator (PWG) and muscle collagen content, the Met requirements for on-growing grass carp (178–626 g) were estimated to be 9·56 g/kg of the diet (33·26 g/kg protein of diet) and 9·28 g/kg of the diet (32·29 g/kg protein of diet), respectively.
Acknowledgements
The authors would like to express their sincere thanks to the personnel of these teams for their kind assistance.
This research was financially supported by National Key R&D Program of China (2018YFD0900400, 2019YFD0900200), National Natural Science Foundation of China for Outstanding Youth Science Foundation (31922086), the Young Top-Notch Talent Support Program of National Ten-Thousand Talents Program, the Earmarked Fund for China Agriculture Research System (CARS-45), Outstanding Talents and Innovative Team of Agricultural Scientific Research (Ministry of Agriculture), Foundation of Sichuan Youth Science and Technology Innovation Research Team (2017TD0002), Key Research and Development Plan in Sichuan Province (2018NZ0007) and Supported by Sichuan Science and Technology Program (2019YFN0036).
X.-Q. Z. and L. F. designed the study; C.-C. F., W.-D. J. and L. F. conducted the study and analysed the data; Y. L., P. W., S.-Y. K., L. T. and X.-A. L. participated in the interpretation of the results; C.-C. F. and W.-D. J. wrote the manuscript; X.-Q. Z. had primary responsibility for the final content of the manuscript. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520002998
















