Various popular herbal teas, consumed for their pleasant taste and/or health-improving properties, contain significant amounts of polyphenols( Reference Atoui, Mansouri and Boskou 1 ). These micronutrients are involved in many biological activities that may have a positive impact on health( Reference Landete 2 , Reference Scalbert, Manach and Morand 3 ). Aloysia triphylla (L'Herit.) Britton, better known as lemon verbena, is a herbal species growing spontaneously in South America and cultivated in North Africa (Morocco) and southern Europe( Reference Carnat, Carnat and Fraisse 4 ). In these countries, it is widely used for food and medicinal purposes, its leaves reportedly possessing digestive and antispasmodic properties( Reference Pascual, Slowing and Carretero 5 ). Lemon verbena infusion is commonly consumed as a treatment for colic, diarrhoea and indigestion( Reference Pascual, Slowing and Carretero 5 ), and as a flavoured hot drink. Lemon verbena infusion contains significant amounts of polyphenols, including phenylpropanoid glycosides (mainly verbascoside) and flavone diglucuronides such as luteolin 7-diglucuronide( Reference Carnat, Carnat and Fraisse 4 , Reference Bilia, Giomi and Innocenti 6 ), and has a high antioxidant activity( Reference Valentao, Fernandes and Carvalho 7 ).
In recent years, several studies conducted in vitro or in vivo have produced evidence that polyphenols can modulate intestinal inflammation( Reference Romier, Schneider and Larondelle 8 , Reference Biasi, Astegiano and Leonarduzzi 9 ). For example, verbascoside reduced the severity of intestinal macroscopic and microscopic lesions and decreased pro-inflammatory cytokine levels in various models of murine colitis( Reference Mazzon, Esposito and Di Paola 10 , Reference Hausmann, Obermeier and Paper 11 ). The flavone luteolin attenuated dextran sodium sulphate (DSS)-induced colonic injury and inflammation in mice( Reference Nishitani, Yamamoto and Yoshida 12 ). We have also recently shown that lemon verbena infusion offered beneficial effects against DSS-induced colonic inflammation in rats( Reference Lenoir, Joubert-Zakeyh and Texier 13 , Reference Lenoir, Rossary and Joubert-Zakeyh 14 ).
The bioavailability of several subclasses of polyphenols (such as flavonols, anthocyanins and catechins) has been extensively studied( Reference D'Archivio, Filesi and Di Benedetto 15 , Reference Crozier, Del Rio and Clifford 16 ), but only limited data have been reported for some other polyphenols, such as flavones or phenylpropanoid glycosides. Absorption of polyphenols in the upper gastrointestinal tract is usually low, so a large amount of these micronutrients reach the colon and may thus be locally biologically active( Reference Dryden, Song and McClain 17 , Reference Tomas-Barberan and Andres-Lacueva 18 ). However, the impact of intestinal alterations such as colonic inflammation on the bioavailability of polyphenols has not yet been investigated. The aim of the present study was to evaluate the bioavailability and intestinal absorption of polyphenols derived from lemon verbena infusion (flavones and phenylpropanoid glycosides) in both healthy and colitic rats. Colitis was induced by daily oral administration of DSS, one of the most widely used models for inflammatory bowel disease( Reference Solomon, Mansor and Mallon 19 ).
Materials and methods
Chemicals
Verbascoside, luteolin 7-glucoside, luteolin, apigenin, chrysoeriol, diosmetin and hydroxycinnamic acid standards were obtained from Extrasynthèse. Isoverbascoside was obtained from LGC Standards. DSS (molecular weight 38 000–48 000 Da) was purchased from TdB Consultancy AB. Sodium pentobarbital was obtained from Ceva Santé Animale. β-Glucuronidase/sulphatase was supplied by Sigma-Aldrich.
Preparation of lemon verbena infusion
Crushed lemon verbena leaves (40 g; 3inature Biosphère) were infused in boiled water (1 litre) for 30 min to enhance polyphenol extraction. The infusion was then filtered, aliquoted according to the volume needed per d (50 ml/rat) and stored at − 20°C.
Animals and experimental design
Male Wistar rats (170–200 g) were purchased from Janvier. They were fed a semi-purified control diet devoid of polyphenols (630 g wheat starch/kg, 200 g casein/kg, 70 g peanut oil/kg, 50 g Alphacel/kg, 35 g AIN-93G mineral mixture/kg, 10 g AIN-93 vitamin mixture/kg, 3 g l-cystine/kg, 2·5 g choline bitartrate/kg and 0·14 g t-butyl-hydroquinone/kg; UPAE, INRA). The rats were acclimatised to the laboratory conditions for 1 week before the experiments. The present study was approved by the local Ethics Committee (registration no. CE4-08).
In vivo study
A total of twelve male Wistar rats were individually housed in metabolism cages fitted with urine/faeces separators in a temperature-controlled room (22°C), with a controlled dark period from 07.00 to 19.00 hours. They were given free access to the semi-purified control diet. The rats were randomly divided into two groups (n 6 per group): a control group and a verbena infusion group. For 14 d, the control rats received tap water, while the rats in the verbena infusion group received lemon verbena infusion in order to mimic the regular intake of infusion. The rats consumed the drink ad libitum, and the drink not consumed was discarded every day. From day 15 to day 22, the rats received 4 % (w/v) DSS in their drink. Animal weight, diet and beverage consumption were measured daily. On days 14 and 21, urine and faecal samples were collected over 24 h. Thereafter, 24 h urinary excretion of polyphenols was determined, and urine samples were rapidly acidified with 10 mm-acetic acid and frozen at − 80°C until analysis. Faecal samples were weighed and rapidly frozen at − 80°C until analysis. At day 22, the rats were anaesthetised by intraperitoneal administration of sodium pentobarbital (40 mg/kg body weight) and killed by decapitation. Blood was collected in heparinised tubes, and urine present in the bladder was collected.
In situ intestinal perfusion
A total of eighteen male Wistar rats were housed two per cage in a temperature-controlled room (22°C), with a controlled dark period from 20.00 to 08.00 hours and access to food from 16.00 to 08.00 hours. They were fed the semi-purified control diet for 2 weeks. The rats were randomly divided into three groups (n 6 per group): verbena group (V); verbena-plus-DSS group (VD); inflamed-plus-verbena group (IV). The V and IV groups received verbena infusion in their perfused solution, and the intestine of the VD group was perfused with a solution containing both verbena infusion and DSS as described below. During the last 7 d of the experiment, the IV group received 4 % (w/v) DSS in their drinking-water to induce colonic inflammation. The rats fasted for 24 h were anaesthetised with sodium pentobarbital and kept alive under anaesthesia throughout the experiments. A perfusion was made into the jejuno-ileal segment of the intestine (from 5 cm distal from the duodeno-jejunal flexure up to the ileo-caecal valve) after installing a cannula at each extremity of the bile duct. This segment was continuously perfused in situ at 37°C for 45 min at a flow rate of 0·75 ml/min with a buffer containing 5 mm-KH2PO4, 2·5 mm-K2HPO4, 5 mm-NaHCO3, 50 mm-NaCl, 40 mm-KCl, 2 mm-CaCl2, 1 mm-MgSO4, 10 mm-K3C6H5O7, 12 mm-glucose, 2 mm-glutamine and 1 mm-taurocholic acid (pH 6·6), as described previously( Reference Talavéra, Felgines and Texier 20 ). For the V and IV groups, verbena infusion was extemporaneously diluted 50-fold in the perfusion buffer. For the VD group, 4 % DSS was dissolved in the verbena infusion, and the solution obtained was extemporaneously diluted 50-fold in the perfusion buffer. The contents of the intestine were washed during the first 25 min of perfusion. Effluents were directly collected at the exit of the ileum during the last 5 min of perfusion. Effluent volume was estimated by weighing. Bile was collected throughout the perfusion period (45 min). At the end of the experiment, urine present in the bladder was collected. The perfused solution, effluents and urine samples were acidified with 10 mm-acetic acid and immediately stored at − 80°C until analysis.
To determine the stability of polyphenols derived from lemon verbena infusion throughout the in situ perfusion experiment (at 37°C, pH 6·6), an aliquot of the perfused solution maintained at 37°C was collected at the beginning (t= 0), at t= 25 min and at the end of the perfusion period (t= 45 min), and lemon verbena polyphenols were analysed by HPLC as described below. The overall percentage of the degradation of various lemon verbena polyphenols, calculated by the decrease in their concentrations between 0 and 45 min, was less than 2·5 %. Thus, the amount of each polyphenol perfused was determined from the mean of its concentration in the perfused solution at t= 0 and 45 min.
Sample preparation
Lemon verbena infusion
Phenylpropanoid glycosides (verbascoside and isoverbascoside) were quantified directly on infusions diluted 10-fold in water using their respective commercial standards. Since flavone diglucuronides found in the lemon verbena infusion were not commercially available, they were quantified as aglycones after enzymatic hydrolysis. We have previously shown that flavone diglucuronides were completely hydrolysed under the described conditions. Infusion samples were diluted 50-fold in water, acidified with 10 μl of 0·6 m-acetic acid/100 μl sample to obtain pH 5, incubated with 6 × 106U β-glucuronidase/l plus 5 × 104U sulphatase/l (from Helix pomatia) for 30 min at 37°C, and then treated with 2·8 volumes of methanol containing 100 mm-HCl. The resulting mixture was centrifuged at 4500 g for 5 min at room temperature. The supernatant was diluted with 0·5 volumes of water and analysed by HPLC as described below.
Urine
Urine samples were centrifuged at 12 000 g for 5 min. A fraction of the supernatant was diluted 6-fold in water–methanol (50:50, v/v) plus 200 mm-HCl and analysed by HPLC, as described below, to detect native polyphenols. Another fraction was treated with β-glucuronidase/sulphatase, as described above, and then analysed by HPLC for the detection of flavone aglycones and hydroxycinnamic acids.
Faeces
Whole faecal samples were thawed, crushed with a grinder, dehydrated by the addition of absolute ethanol (15 ml/g) in an ultrasound bath for 2 min, and then dried by evaporation under reduced pressure. Faecal samples were then treated twice with hexane (20 ml/g) for 10 min at 50°C, and then again for 10 min in an ultrasound bath to remove lipids and non-polar substances. After filtration, the residue was dried for polyphenol extraction. Flavone glycosides were extracted twice from the residue with absolute ethanol (20 ml/g) for 10 min at 50°C, and then again for 10 min in an ultrasound bath. After filtration, the filtrates were pooled, evaporated to dryness and weighed. To extract more hydrophilic compounds such as phenolic acids, the residue was then extracted twice with 50 % ethanol as described previously. The filtrates were then pooled, dried and weighed. Phenolic compounds in the dried extracts were then dissolved in 50 % ethanol before HPLC analysis. Faecal excretion of phenolic compounds is expressed as nmol/24 h.
Intestinal effluents
After centrifuging at 12 000 g for 8 min, the supernatants of intestinal effluents were diluted twice in distilled water or 50 % ethanol for analysis of verbascoside and isoverbascoside or flavones, respectively, and analysed by HPLC as described below. Another fraction of the supernatants was treated with β-glucuronidase/sulphatase as described above before HPLC analysis. All the concentrations measured in the effluent samples were corrected by taking into account the intestinal absorption of water. Water absorption was estimated by calculating the difference between effluent flow (estimated by effluent weight) and perfusion flow (0·75 ml/min)( Reference Talavéra, Felgines and Texier 20 ). Absorption through the intestinal barrier was estimated by calculating the difference between the amount of various polyphenols perfused through the intestinal segment and the amount recovered at the end of the ileal segment. These amounts were determined during the last 5 min of perfusion.
HPLC analysis
Flavones and phenolic acids were analysed by HPLC using a DAD 200 photodiode array detector (Perkin-Elmer) and a 785A UV/Vis detector (Perkin-Elmer) at 350 and 320 nm, respectively, except for 3-coumaric acid, which was detected at 280 nm. Samples were injected onto an Uptisphere C18 5 μm column (150 × 4·6 mm) protected by a guard column (Uptisphere C18 5 μm, 10 × 4 mm) (Interchim). Elution was performed using water–H3PO4 (99:1) as solvent A and acetonitrile as solvent B at a flow rate of 1·0 ml/min. Analysis was carried out with linear gradient conditions from 95 % A to 75 % A for 40 min for flavone diglucuronides and phenolic acids and from 90 % A to 70 % A for 40 min for flavone aglycones. Flavones were quantified as luteolin, apigenin and diosmetin equivalents, unless otherwise stated. Lemon verbena phenylpropanoid glycosides and urinary hydroxycinnamic acids were quantified as their respective equivalents.
Statistical analysis
Results are expressed as means with their standard errors. Data analysis was performed using the Statistica software program (version 5.0; Statistica). Statistical analysis was performed using a paired t test to compare data before and after administration of DSS. Results from in situ intestinal perfusion were subjected to one-way ANOVA followed by Fisher's least significant difference test. A value of P< 0·05 was considered to be statistically significant.
Results
Lemon verbena infusion polyphenols
As shown in Fig. 1(a), phenolic acids were identified as verbascoside (peak 4) and an isomer of verbascoside was identified as isoverbascoside (peak 5) in the lemon verbena infusion. Furthermore, flavone derivatives were identified as diglucuronides of the aglycones luteolin (peak 6), apigenin (peak 7) and diosmetin (peak 8) (Fig. 1(b)). Luteolin 7-diglucuronide (peak 1) and apigenin 7-diglucuronide (peak 2) have already been identified in lemon verbena infusion by NMR( Reference Carnat, Carnat and Chavignon 21 ) and HPLC–MS( Reference Bilia, Giomi and Innocenti 6 ). Diosmetin diglucuronide (peak 3) was identified as the aglycone after enzymatic hydrolysis and by LC–MS/MS analysis (positive-ion mode, respective m/z values of its parent and product ion: 653/301; C Felgines, D Fraisse, B Lyan and O Texier, unpublished results), and is reported for the first time in lemon verbena.

Fig. 1 (a) Representative HPLC chromatogram of lemon verbena infusion at 320 nm. The analysis was carried out with linear gradient conditions from 5 to 25 % acetonitrile in 40 min. (b) Representative HPLC chromatogram of lemon verbena infusion hydrolysed with β-glucuronidase/sulphatase at 350 nm. The analysis was carried out with linear gradient conditions from 10 to 30 % acetonitrile in 40 min. The peaks obtained are as follows: 1, luteolin 7-diglucuronide; 2, apigenin 7-diglucuronide; 3, diosmetin diglucuronide; 4, verbascoside; 5, isoverbascoside; 6, luteolin; 7, apigenin; 8, diosmetin. (a, b) Retention times of the peaks differ due to differing chromatographic conditions.
In vivo study
Body weight was not significantly affected by the consumption of the lemon verbena infusion. However, body weight gain decreased after the administration of DSS in both the control and verbena groups (data not shown). On day 14, food consumption was 27·2 (sem 2·1) and 25·7 (sem 0·9) g in the control and verbena groups, respectively. After 7 d of DSS administration (day 21), it was significantly decreased in the control and verbena groups (19·1 (sem 2·0) and 14·4 (sem 2·0) g, respectively; P< 0·05).
On the last day of the experiment, both control and verbena groups exhibited bloody stools and diarrhoea as a result of DSS administration. As bioavailability of lemon verbena polyphenols was evaluated in the same rats before and after colitis induction, colonic markers of inflammation could not be analysed.
Quantification of lemon verbena infusion polyphenols
Lemon verbena infusion consumed by rats contained 1268 μm-verbascoside and 270 μm-isoverbascoside. Flavone concentrations were 217 μm-luteolin, 88 μm-apigenin and 674 μm-diosmetin.
Daily consumption of polyphenols
The 24 h consumption of lemon verbena infusion on day 14 (without DSS) and day 21 (with DSS) was 22·5 (sem 1·2) and 29·5 (sem 1·7) ml, respectively (P< 0·05). Thus, on days 14 and 21, rats ingested 34·6 and 45·4 μmol/d of phenolic acids and 21·9 and 28·9 μmol/d of flavones, respectively (P< 0·05). Therefore, total polyphenol consumption was 56·5 and 74·3 μmol/d, respectively (P< 0·05).
Urinary excretion of polyphenols
The control diet did not contain any flavonoids or phenolic acids. Hence, as expected, polyphenols were not present in the urine of the control group; however, polyphenols were present in the urine of rats that received the lemon verbena infusion. Before enzymatic hydrolysis, 24 h urine samples contained small amounts of verbascoside and isoverbascoside, but did not contain native lemon verbena flavone diglucuronides or luteolin or apigenin. A small amount of free diosmetin (aglycone) was also detected. However, it was not found in the urine collected directly from the bladder, thus indicating that this small fraction of diosmetin resulted from the hydrolysis of diosmetin conjugates during the collection of urine in metabolism cages. Urinary excretion profiles also contained non-identified peaks that could correspond to various glucurono- and/or sulpho-conjugated metabolites. After enzymatic hydrolysis, hydroxycinnamic acids (caffeic, ferulic, isoferulic, 3-coumaric and 4-coumaric acids) and flavone aglycones (luteolin, apigenin and diosmetin) were identified (Fig. 2(a) and (b)). Traces of chrysoeriol, an isomer of diosmetin, were detected in some samples, but its amount was too low to allow quantification. Similar HPLC profiles were observed before and after DSS administration. Peaks that were not numbered and with retention times longer than 6 min (Fig. 2(a) and (b)) were not present in the urine of the control group, and corresponded to unidentified compounds.

Fig. 2 Representative HPLC chromatograms of 24 h urine samples hydrolysed with β-glucuronidase/sulphatase and faecal samples from rats receiving lemon verbena infusion. (a) For hydroxycinnamic acids, the analysis of urine was carried out with linear gradient conditions from 5 to 25 % acetonitrile in 40 min and detection was carried out at 320 nm. For flavone aglycones, the analysis of (b) urine and (c) faeces was carried out with linear gradient conditions from 10 to 30 % acetonitrile in 40 min and detection was carried out at 350 nm. The peaks obtained are as follows: 1, caffeic acid; 2, 4-coumaric acid; 3, ferulic acid; 4, isoferulic acid; 5, luteolin; 6, apigenin; 7, chrysoeriol; 8, diosmetin.
Urinary excretion of verbascoside and isoverbascoside was too low to be accurately quantified. The mean urinary excretion rates of lemon verbena polyphenol derivatives over a 24 h period are presented in Tables 1 and 2. Urinary excretion of luteolin and apigenin was based on their respective ingestion. Urinary excretion of diosmetin was based on luteolin-plus-diosmetin ingestion, since diosmetin could result not only from verbena diosmetin diglucuronide, but also from the methylation of luteolin( Reference Chen, Chen and Pan 22 ). Urinary excretion of flavones was not significantly affected by DSS administration. Urinary excretion of hydroxycinnamic acids was related to verbascoside-plus-isoverbascoside ingestion. Urinary excretion of caffeic acid and total hydroxycinnamic acids was significantly higher in the DSS-treated rats, whereas that of 3-coumaric acid was significantly decreased (P< 0·05). Total polyphenols excreted in the urine accounted for about 7·2 (sem 1·0) and 12·1 (sem 1·6) % of ingested lemon verbena polyphenols before and after DSS administration, respectively (P< 0·05).
Table 1 24 h urinary excretion of flavones derived from lemon verbena infusion in rats* (Mean values with their standard errors; n 6)
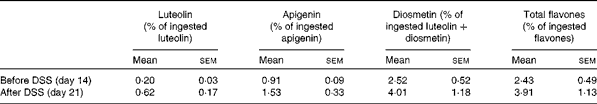
DSS, dextran sodium sulphate.
* Comparison was made between day 14 and day 21 by paired t test. There were no significant differences.
Table 2 24 h urinary excretion of hydroxycinnamic acids derived from lemon verbena infusion in rats (% of the ingested amount of verbascoside+isoverbascoside) (Mean values with their standard errors; n 6)

DSS, dextran sodium sulphate.
* Mean value was significantly different from that of before DSS treatment (P< 0·05).
Faecal excretion of polyphenols
Since the DSS-treated rats exhibited bloody stools and diarrhoea, accurate collection of faeces was not possible on day 21. Accordingly, faecal excretion of polyphenols was evaluated only for faeces collected before DSS administration (on day 14). Both extracts of faeces (with absolute ethanol or 50 % ethanol) qualitatively presented the same HPLC profile. Neither native diglucuronides of flavones nor phenylpropanoid glycosides were detected in the faeces. Luteolin and diosmetin aglycones were detected and quantified (Fig. 2(c)). However, neither apigenin nor hydroxycinnamic acids (caffeic or ferulic acid) were identified in the faecal extracts. On day 14, 279 (sem 114) nmol/24 h of luteolin and 444 (sem 181) nmol/24 h of diosmetin were excreted in the faeces. Excretion of luteolin and diosmetin corresponded to 5·4 (sem 2·1) % of the ingested luteolin derivative and 2·8 (sem 0·9) % of the ingested diosmetin derivative, respectively. Total flavones excreted in the faeces accounted for 3·2 (sem 1·0) % of ingested lemon verbena flavones.
In situ intestinal perfusion
Perfused lemon verbena polyphenols
The perfused lemon verbena solution contained 21·7 μm-verbascoside, 6·1 μm-luteolin 7-diglucuronide, 0·84 μm-apigenin 7-diglucuronide and 11·4 μm-diosmetin diglucuronide. Isoverbascoside concentration in the perfused solution was too low to allow accurate evaluation of its intestinal absorption.
Effluent analysis
The HPLC profile of the effluents was qualitatively similar to that of the perfused solution (data not shown). Intestinal absorption of verbascoside was similar, irrespective of the conditions tested (Table 3). The effluents contained native diglucuronides of flavones, but could also contain some other glucurono- and/or sulpho-conjugated derivatives of flavones among the small non-identified peaks observed on the HPLC profile. However, enzymatic hydrolysis released flavone aglycones from both native lemon verbena diglucuronides and conjugated metabolites. Hence, the concentration of total aglycones (A t) was the sum of the concentrations of aglycones from lemon verbena diglucuronides (A g) and of aglycones from conjugated metabolites (A m): A t= A g+A m. To determine A g in the effluents, we quantified the diglucuronides of flavones in the perfused conjugate-free solution as aglycones after enzymatic hydrolysis (C H) and directly as diglucuronides (C D). These two values were not exactly identical since diglucuronides were expressed as a closely related commercially available compound (luteolin 7-glucoside). We then calculated the ratio R= C H/C D for each flavone. Next, we quantified flavone diglucuronides in the effluents as luteolin 7-glucoside equivalents and applied the coefficient R to this amount to obtain A g and to calculate the amount of metabolites: A m= A t− A g. Only a very small amounts of conjugated metabolites were thus determined (luteolin derivatives: 0·3–1·5 μmol/l, diosmetin derivatives: 0–1·7 μmol/l, apigenin derivatives: not detected). Intestinal absorption of lemon verbena flavones was wide-ranging among rats and did not significantly differ between the experimental conditions (Table 3).
Table 3 Polyphenol absorption after perfusion of lemon verbena infusion through the intestinal lumen of rats* (Mean values with their standard errors; n 6)
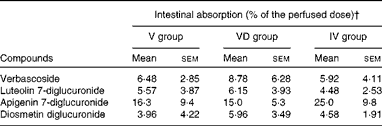
V group, rats perfused with lemon verbena solution; VD group, rats perfused with lemon verbena solution-plus-DSS; IV group, rats perfused with lemon verbena solution after treatment with 4 % DSS for 7 d.
* Comparison was made between the groups using one-way ANOVA. There were no significant differences.
† These amounts were determined during the last 5 min of the perfusion.
Discussion
Until now, the bioavailability and metabolism of lemon verbena polyphenols have only been scantly evaluated( Reference Funes, Fernández-Arroyo and Laporta 23 ). The present study aimed to evaluate the urinary and faecal excretion and metabolism of lemon verbena polyphenols and to investigate their intestinal absorption using an in situ intestinal model in rats. The present study also evaluated, for the first time, the impact of colonic inflammation on the fate of ingested polyphenols and was thus carried out in both healthy and colitic rats.
Neither lemon verbena flavone diglucuronides nor free flavone aglycones were present in the urine after consumption of lemon verbena infusion. Flavones were detected in the urine only after the hydrolysis with β-glucuronidase/sulphatase, thus indicating that they were excreted in the urine as glucurono- and/or sulpho-conjugated derivatives. The following four flavones were identified in the urine: luteolin; apigenin; diosmetin; chrysoeriol. Luteolin and apigenin resulted from luteolin 7-diglucuronide and apigenin 7-diglucuronide present in the infusion, respectively. As previously reported( Reference Chen, Li and Lu 24 ), urinary excretion of luteolin was lower than that of apigenin. Also, urinary excretion of flavones was lower than that reported after the administration of flavones as 7-glucosides( Reference Chen, Li and Lu 24 ). On the one hand, as has been already reported for some other polyphenols( Reference Crozier, Del Rio and Clifford 16 , Reference Galvano, La Fauci and Vitaglione 25 ), the nature of glycosylation may thus influence the absorption and excretion of these compounds. On the other hand, it has been shown that phase II catechol-O-methyl transferase enzyme catalyses the methylation of luteolin on both 3′- and 4′-hydroxyl groups, yielding chrysoeriol and diosmetin, respectively( Reference Chen, Chen and Pan 22 , Reference Chen, Kong and Song 26 ). In the present study, only trace amounts of chrysoeriol were detected in the urine after consumption of lemon verbena infusion. Although methylation of flavonoids usually occurs preferentially on the 3′-hydroxyl group( Reference de Boer, Dihal and van der Woude 27 , Reference Felgines, Texier and Besson 28 ), it has been shown that luteolin is mainly 4′-O-methylated in vitro ( Reference Chen, Chen and Pan 22 ). In the present study, the major flavone present in the urine was diosmetin. This flavone could thus result not only from lemon verbena diosmetin diglucuronide, but also from the methylation of luteolin (Fig. 3(a)). It has also been reported that flavones such as luteolin and apigenin can be transformed to their corresponding flavanones (eriodictyol and naringenin) by the gut microbiota( Reference Hanske, Loh and Sczesny 29 – Reference Braune, Gutschow and Engst 31 ). However, we did not detect any such metabolites in the urine. As has been hypothesised previously( Reference Falé, Madeira and Florêncio 32 ), flavones excreted in the urine could be produced by deglucuronidation of lemon verbena flavone diglucuronides, followed by glucuronidation at a different position and/or sulphatation. The present study and the study by Falé et al. ( Reference Falé, Madeira and Florêncio 32 ) differ from most reports on the in vivo administration of flavonoids in that flavones were administered as glucuronide derivatives, as commonly found in some herbal teas( Reference Fecka and Turek 33 ), instead of their aglycones or glucosides.

Fig. 3 (a) Structure of lemon verbena flavones 7-diglucuronides and flavone aglycones identified after hydrolysis with β-glucuronidase/sulphatase in the urine of rats receiving lemon verbena infusion. The structure of diosmetin diglucuronide identified in lemon verbena infusion is not presented here since the position of the diglucuronyl residue on the aglycone could not be determined by the LC–MS/MS analysis. Lut, luteolin; digluc, diglucuronide; Api, apigenin; Dios, diosmetin; Chrys, chrysoeriol. (b) Structure of verbascoside and hydroxycinnamic acids that resulted from its metabolism.
It has been suggested that apigenin may be absorbed more efficiently at the intestinal level than luteolin( Reference Chen, Li and Lu 24 , Reference Chen, Tu and Sun 34 ). After intestinal perfusion of lemon verbena infusion, we obtained a similar result for luteolin and apigenin diglucuronides, which could explain the lower urinary excretion of luteolin compared with that of apigenin. Using the rat everted small intestine, Shimoi et al. ( Reference Shimoi, Okada and Furugori 35 ) showed that luteolin 7-glucoside was absorbed after hydrolysis to luteolin and that luteolin was converted to glucuronides during its transit through the intestinal mucosa. In the present study, no free flavone aglycones were detected in the effluents, but small amounts of glucurono- and/or sulpho-conjugated derivatives were re-excreted into the effluents, thus indicating hydrolysis of flavone diglucuronides and then conjugation of aglycones at the intestinal level. Using a similar model of in situ intestinal perfusion, Crespy et al. ( Reference Crespy, Morand and Besson 36 ) showed that 41 % of luteolin was absorbed at the intestinal level. The absorption of lemon verbena luteolin was much lower (5·6 %). However, we noted that the experiment of Crespy et al. ( Reference Crespy, Morand and Besson 36 ) was carried out with aglycones. This again underlines the fact that glycosylation may affect the absorption and excretion of flavones.
Verbascoside is a phenylpropanoid glycoside characterised by caffeic acid and hydroxytyrosol bound to a glucose moiety through ester and glycosidic bonds, respectively, with a rhamnose unit linked to the glucose molecule. Lemon verbena phenolic acids such as verbascoside and isoverbascoside have been detected in the urine only in low and unquantifiable amounts. However, their presence in the urine indicated that these compounds could be, at least in a minor proportion, absorbed intact. Verbascoside has been reported to be rapidly absorbed from the gastrointestinal tract and then detected in rat plasma, but its oral bioavailability was quite low( Reference Funes, Fernández-Arroyo and Laporta 23 , Reference Wu, Lin and Sung 37 ). Similarly, urinary excretion of complex phenolic acids such as rosmarinic or chlorogenic acids in their intact forms is low( Reference Gonthier, Verny and Besson 38 , Reference Baba, Osakabe and Natsume 39 ). Intestinal absorption of verbascoside (V group 6·48 %) was close to that reported previously for chlorogenic acid( Reference Lafay, Morand and Manach 40 ). After ingestion of lemon verbena infusion, caffeic acid was detected in the urine accompanied by methylated derivatives such as ferulic and isoferulic acids, and dehydroxylated compounds such as 3-coumaric and 4-coumaric acids (Fig. 3(b)). Formation of these metabolites from verbascoside and isoverbascoside requires the ester bond to be cleaved. These hydroxycinnamic acids were not detected before the hydrolysis with β-glucuronidase/sulphatase, indicating that they were excreted in the urine as conjugated derivatives. After entering the systemic circulation, caffeic acid is most probably methylated during the first liver passage( Reference Wittemer, Ploch and Windeck 41 ). In the present study, ferulic acid, the 3-O-methylated derivative of caffeic acid, was the main phenolic acid excreted in the urine, indicating that a high proportion of caffeic acid was methylated preferentially at the 3-hydroxyl group as described previously( Reference Gonthier, Verny and Besson 38 ). 3-Coumaric acid detected in the urine may have resulted from the dehydroxylation of caffeic acid due to its metabolism by the gut microflora, as reported previously( Reference Gonthier, Verny and Besson 38 ). To our knowledge, production of hydroxycinnamic acids such as caffeic acid from flavones has not yet been demonstrated. Urinary excretion of hydroxycinnamic acids is therefore related to the ingestion of phenylpropanoid glycosides.
Only flavone aglycones (luteolin and diosmetin) were detected in the faeces, and their faecal excretion was low (3·2 % of total ingested flavones). Similarly, Chen et al. ( Reference Chen, Li and Lu 24 ) found aglycones only in the faeces after ingestion of luteolin and apigenin glucosides, but they reported a higher faecal excretion (about 30 % each). Also, rat faecal excretion was higher after gastric administration of apigenin (15·2 %) than after the administration of apigenin 7-apiosylglucoside (4 %)( Reference Griffiths and Smith 42 ). These results suggest that glycosylation and the nature of the glycosidic moiety may also affect the faecal excretion of flavones. Herein we observed a high inter-individual variability for faecal excretion of flavones (0·7–13 %). This excretion also varied across days. Collection of faeces over several days could help estimate faecal excretion more accurately. Neither verbascoside nor isoverbascoside were detected in the faeces. These compounds are not stable, and could have been degraded into substances such as caffeic acid. Under the analytical conditions of the present study, no such compound was detected in the faeces. Gut microflora could have continued to cause the degradation of these phenolic acids into smaller compounds such as hydroxybenzoic acids( Reference Gonthier, Verny and Besson 38 ), which we did not look for.
Several studies have focused on the beneficial role of polyphenols on the development of intestinal inflammation in various in vitro or in vivo models( Reference Romier, Schneider and Larondelle 8 , Reference Biasi, Astegiano and Leonarduzzi 9 ), but to our knowledge, bioavailability of polyphenols in a colitic situation has not yet been evaluated. HPLC profiles of urinary excretion were similar before and after DSS ingestion. Urinary excretion of flavones was low and not significantly affected by DSS consumption. Their intestinal absorption was also not affected by colonic inflammation. This could be related to the site of DSS action. DSS mainly affects the colon, particularly its distal segment, and the small intestine is not altered by DSS( Reference Solomon, Mansor and Mallon 19 ). Hence, in cases of colonic inflammation, absorption and excretion of lemon verbena flavones are not affected. Also, co-perfusion of lemon verbena polyphenols and DSS enabled us to demonstrate that polyphenol absorption was not affected by DSS itself. Intestinal absorption of verbascoside was not affected by colonic inflammation. Urinary excretion of caffeic acid (as well as total phenolic acids) was increased after DSS ingestion. Thus, this could have resulted from increased formation from verbascoside and isoverbascoside (by hydrolysis of the ester bond) and/or increased absorption at the colonic level. The colon is an important site for absorption of phenolic acids. Increased mucosal permeability at the colonic level has been observed in DSS-treated rats by evaluating passive permeation of mannitol using distal colon sheets mounted in Ussing chambers( Reference Venkatraman, Ramakrishna and Pulimood 43 ). Thus, alteration of colonic structure in DSS-treated rats may increase colonic permeability and thus facilitate the absorption of small molecules such as caffeic acid. DSS may also affect colonic microflora, in particular increase the numbers of Enterobacteriaceae, Bacteroidaceae and Clostridium spp. and decrease the levels of Bifidobacterium and Lactobacillus spp.( Reference Perse and Cerar 44 – Reference Lara-Villoslada, Debras and Nieto 46 ), which could later alter the metabolism and absorption of polyphenols. The decrease in the urinary excretion of 3-coumaric acid could therefore result from decreased formation of this phenolic acid at the colonic level under inflammatory conditions.
In conclusion, the present study shows that urinary and faecal excretion of lemon verbena polyphenols is low. Also, colonic inflammation did not affect intestinal absorption and urinary excretion of lemon verbena polyphenols and flavones, respectively, but increased urinary excretion of phenylpropanoid glycoside metabolites. The various microbial metabolites derived from lemon verbena polyphenols still need to be identified, and the impact of colonic inflammation on their metabolism warrants further investigation.
Acknowledgements
The present study received no specific grant from any funding agency, commercial or not-for-profit sectors.
The authors' contributions are as follows: C. F. and O. T. designed the research, analysed the data and wrote the paper; C. F., O. T., D. F. and C. B. conducted the research; M.-P. V. managed the ECREIN research team.
The authors declare that there are no conflicts of interest.








