Pathogens can persist for several hours on hands Reference Aiello, Cimiotti, Della-Latta and Larson1,Reference Kampf and Kramer2 and up to several months on surfaces, Reference Bhalla, Pultz and Gries3 and they can be acquired at a high rate through contact with environmental surfaces. Reference Patel, Mantey and Mody4 Residual hand moisture is associated with increased microorganism transfer from hands to surfaces. Reference Patrick, Findon and Miller5 Thus, the process of hand drying is essential in minimizing the risk of pathogen spread. Reference Boyce and Pittet6,Reference Best, Parnell and Wilcox7
Previously, we examined the risk of environmental bacterial contamination in hospital toilets associated with different hand-drying methods. Reference Best, Parnell and Couturier8 We observed less droplet and/or microbe dispersion, and consequently lower level of toilet surfaces contamination, following hand drying with paper towels compared to a jet air dryer. These observations showed the impact of the hand-drying method on the risk of contamination of the washroom and toilet environment. However, whether these differences could also affect the spread of pathogens beyond the toilets remains unknown, especially as in hospitals, these are used by staff, visitors, and patients. Because hand washing is not always performed according to guidelines, Reference Boyce and Pittet6,9 we aimed to determine whether pathogens remaining on hands following inadequate hand washing can transfer across the hospital.
We utilized a bacteriophage as an indicator of microbial contamination in a pilot study to investigate whether microorganisms that remain present on poorly washed hands and/or contaminate the user during hand drying in the toilet, can be transferred beyond the washroom environment to hospital and surfaces near patients.
Methods
Study power calculation
The study was powered to detect a difference in the transmission load to the first surface touched after hand drying, using 2 methods. Assuming that data were not normally distributed and analysis would be performed using a Mann-Whitney test, we determined that, to find a difference of 1 log10 copies/µL (or 10 fold) with 90% power and alpha error rate of 0.05%, with a population standard deviation of 0.4 between the 2 arms, a sample size of 4 per arm would be required (https://www.benchmarksixsigma.com/calculators/sample-size-calculator-for-mann-whitney-test/).
Hand contamination and drying
Bacteriophage PR772 (BAA-769-B1) and host-strain Escherichia coli K12 (BAA-769) were obtained from the American Type Culture Collection (ATCC) and prepared following ATCC recommendations. In total, 4 healthy adult volunteers took part in the study. Each volunteer performed the assay twice, once drying the hands with paper towels and once with a jet air dryer. Hand drying was performed in a washroom/toilet of Leeds General Infirmary (UK) used by hospital staff, visitors, and patients. Volunteers had the option to wear nitrile gloves (StarLab, Blakelands, Milton Keynes, UK) or to use their bare hands. Gloves were inspected for holes before use. All volunteers sanitized their hands or gloved hands with 70% alcohol hand gel disinfectant (Sterillium, Heidenheim, Germany) before immersion in ˜200 mL of 107 pfu/mL PR772 filtrate. Hands were shaken thrice to remove excess liquid and dried using either paper towels (Hand Towels H3, Tork, York, UK) or a jet air dryer (Airblade, Dyson, Malmesbury, UK) (Fig. S1). Volunteers wore a plastic apron to enable the measurement of body or clothing contamination during hand drying. Each volunteer’s nondominant hand (palm and finger tips) was sampled immediately after drying to measure (baseline levels of) hand contamination before environmental sampling.
Surface sampling
The volunteers then walked from the toilet on a preset route that included public and clinical areas, and samples were collected from environmental surfaces following contact with either their dominant (still contaminated) hand or apron. To investigate microbial transfer from clothing, the volunteers placed a stethoscope around their neck, leaving chest piece and earpiece in contact with the apron for ˜7 minutes. Volunteers also crossed their arms across their chest for 2 minutes, followed by resting them on the arms of a chair for 3 minutes. Each surface was swabbed with a 3M sponge-stick moistened with neutralizing buffer (Scientific Laboratory Supplies, Nottingham, UK). Surfaces were disinfected with chlorine wipes before and after sampling, followed by routine cleaning.
Each volunteer performed the assay twice, once after drying their hands with paper towels and once after using the jet air dryer. Two volunteers did their first assay with paper towels and the other 2 volunteers started with the jet air dryer. Sampling was spaced over a 5-week period.
Sample processing
Sponges were processed on the same day of sampling. DNA extraction was performed in duplicate from 1 mL fluid using the QIAamp UltraSens Virus kit (Qiagen, Manchester, UK) as recommended by the manufacturer. Samples were quantified and normalized to 5 ng/µL.
The genes P3 and P12 of bacteriophage PR772 were amplified using primer pairs previously validated for real-time quantitative PCR (qPCR) (Supplementary Table S1 online). The qPCR conditions are detailed in the Supplementary Material (online).
Data analysis
The changes in bacteriophage levels were calculated based on logarithms of gene copy numbers to achieve normal distribution. Statistical significance was assessed in SPSS version 23 software (IBM, Armonk, NY) using a 2-sided Wilcoxon signed rank to compare samples within the same method or assay and using a 2-sided Mann-Whitney U test to compare samples between the jet air dryer and paper towel methods, or samples between different assays. Both tests were performed using a 95% confidence interval. P ≤ .05 was considered statistically significant.
Ethical approval
The recruitment of volunteers following informed consent was approved by the School of Medicine Research Ethics Committee, University of Leeds (reference MREC 18-094). Authorization to perform the study in the hospital was approved by the Leeds Teaching Hospitals NHS Trust Research and Innovation Department.
Results
Hand and body contamination following hand drying
In total, 1 woman and 3 men participated in the study voluntarily. Paper towel drying was performed for an average of 12 seconds, using 3–5 towels, and jet air drying lasted 10 seconds on average. Each sample collection period lasted 73 minutes on average. In addition, 3 volunteers chose to wear gloves and 1 volunteer preferred to immerse their hands directly into the phage solution. There was no significant difference in bacteriophage recovery between assays performed with and without gloves (Supplementary Fig. S2 online). The results for the P3 gene are discussed here, and results for the P12 gene are provided in Supplementary Figs. S3–S5 (online).
Both the jet air dryer and the paper towel methods significantly (P < .05) reduced bacteriophage contamination of the hands by 2 log10 copies/µL and 3 log10 copies/µL, respectively (Fig. 1). Apron (simulated trunk or clothing) contamination by bacteriophage during hand drying was significantly higher (P < 0.05) after jet air dryer use. The bacteriophage levels detected on the volunteers’ hands at the end of the experiments suggested gross persistence of bacteriophage contamination throughout the sampling period (Fig. 1).

Fig. 1. Real-time quantitative PCR (qPCR) results for detection of the gene P3 of bacteriophage PR772 from surfaces exposed to bacteriophage during hand drying. *P < .05 on the Wilcoxon signed rank; # P < .05, Mann-Whitney U test.
Surface contamination following hand contact
All surfaces (n = 8) investigated following jet air dryer use had bacteriophage contamination above the limit of detection, whereas this occurred for only 5 surfaces after paper towel use (Fig. 2). For all samples, there was a significantly (P < .05) higher level of surface contamination following hand drying with the jet air dryer than with paper towels. Samples obtained from smaller surface areas, namely elevator and ward access buttons, showed lower bacteriophage contamination. Interestingly, simulated use of a hospital phone for 10 seconds resulted in detectable contamination only following jet air dryer use. The average surface contamination following hand contact was >10-fold higher after jet air dryer use than after paper towel use: 4.1 log10 copies/µL versus 2.9 log10 copies/µL, respectively.
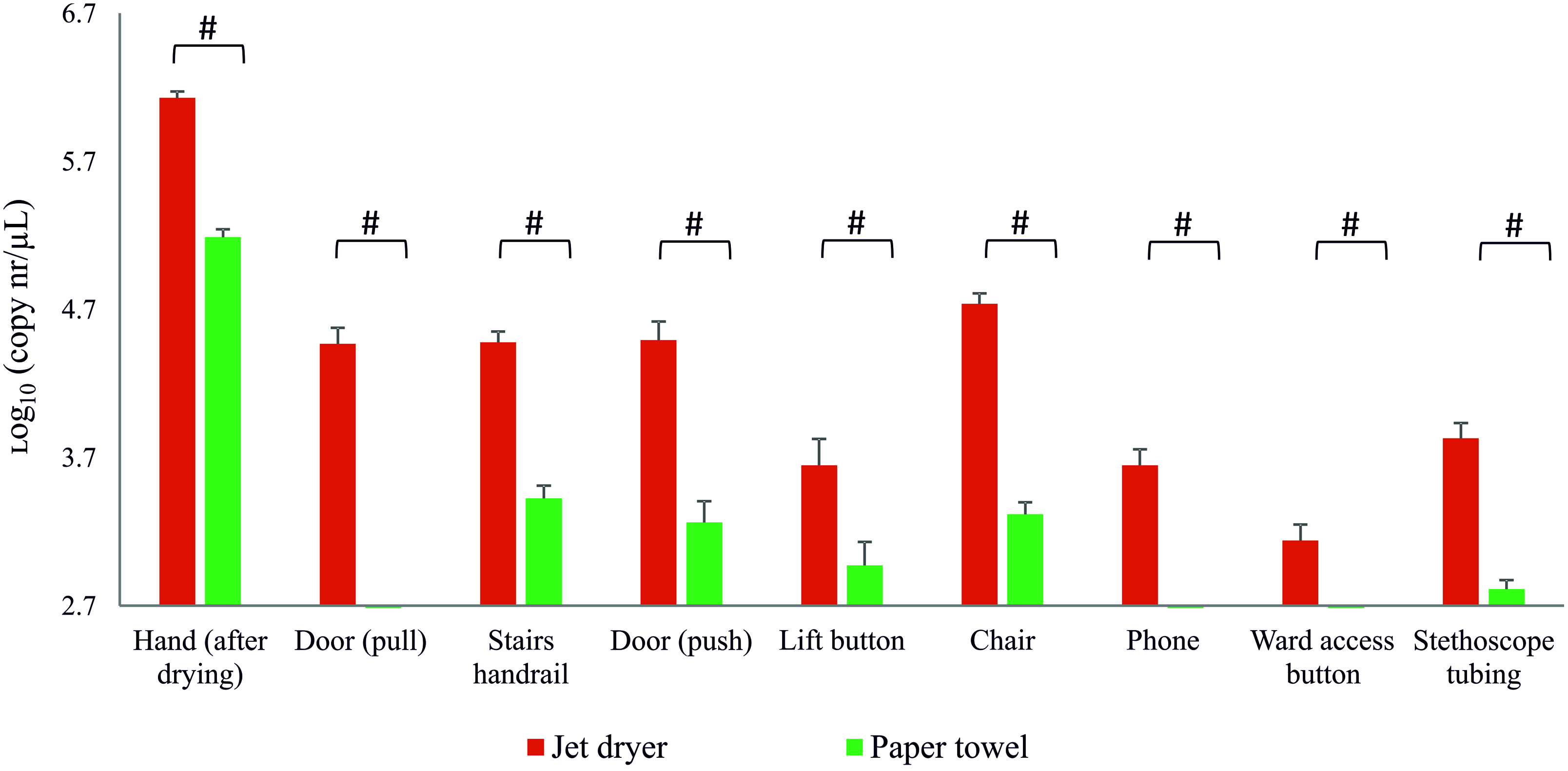
Fig. 2. Real-time quantitative PCR (qPCR) results for detection of gene P3 of the bacteriophage PR772 from environmental samples following contact with contaminated hand. # P < .05, Mann-Whitney U test.
Bacteriophage transfer from apron/clothing
Phage dispersal to volunteers’ aprons was observed after the use of a jet air dryer and paper towels; however, bacteriophage transfer from aprons was detected only on surfaces sampled following jet air dryer use (Fig. 3). Chair arm samples were collected following indirect contact with the apron, that is, after the volunteers’ crossed arms contacted the apron and then touched the chair arms. The increased level of contamination observed in this surface following jet air dryer versus paper towel use was nonsignificant for the P3 gene (P < .076) but was significant for the P12 gene (Fig. S5).

Fig. 3. Real-time quantitative PCR (qPCR) results for detection of gene P3 of the bacteriophage PR772 from environmental samples obtained after contact with contaminated apron. # P < .05, Mann-Whitney U test.
Discussion
Using a bacteriophage as surrogate for bacterial pathogens, we investigated whether residual microbial contamination of hands and body following hand drying in toilets facilitates microbe dispersal beyond the washroom into hospital public and clinical areas. Bacteriophage dispersal across hospital surfaces was more frequently detected after hands were dried using a jet air dryer than using paper towels. On average, the levels of contamination were 10-fold higher following jet air dryer use than after paper towel use. This finding suggests a higher potential for microbial spread through the hospital following jet air dryer use, which is concerning because objects and surfaces can serve as reservoirs for microorganisms that can be acquired via hand contact. Reference Kampf and Kramer2–Reference Patel, Mantey and Mody4 The significantly greater contamination of items that are in close contact with healthcare professionals and patients, such as phones or stethoscopes, following jet air dryer use is particularly concerning. As previously reported, Reference Best, Parnell and Wilcox7,Reference Best and Redway10 we also observed significantly greater microbial contamination of the user body or trunk following jet air dryer use. Importantly, such contamination from the study participant’s apron or trunk or arms to environmental surfaces was directly and indirectly transferred onto surfaces following jet air dryer use only. These observations likely reflect the increased risk of participant contamination during hand drying by a jet air dryer due to splattering. Reference Best and Redway10
Transmission of (multidrug-resistant) pathogens and virus in healthcare settings can occur via contaminated hands of patients or hospital workers. Reference Aiello, Cimiotti, Della-Latta and Larson1–Reference Patel, Mantey and Mody4 We found that drying of hands that were still contaminated (as often occurs after poor washing) reduced the microbial burden, with a significantly greater effect seen following paper towel use compared with jet air dryer use. Importantly, recommended handwashing practices for healthcare workers are often not followed, with an average adherence of 40%, as previously reported. Reference Boyce and Pittet6,9 Thus, it is important to understand how the choice of an appropriate hand drying method can complement good hand hygiene and help reduce the contamination remaining on the hands following inadequate hand washing. Our results support the recommendation of paper towel use in healthcare settings. Reference Boyce and Pittet6,9
Our study has several limitations. This was a preliminary study, and the testing numbers were modest, but they were supported by a power calculation that yielded reproducible, significant differences in levels of surface contamination according to hand-drying method. Blinding of volunteers to the method of hand drying was not possible, but we altered the order in which volunteers used each method to minimize ‘learning’ during the study. Also, we purposely enumerated the levels of surface contamination only after completing the volunteers’ journeys and sampling. In this study, we focused on hand drying of inadequate washed hands, and a handwashing step was not part of the study design. Future studies should investigate how the method and length of handwashing may affect the degree of bacteriophage transfer and surface contamination. Although 3 volunteers used gloves, 1 elected to use bare hands. Thus, we demonstrated that there was no significant effect of gloving on the results obtained. Hence, the excess risk of surface contamination following hand (and body) contact, associated with choice of hand drying method, can reasonably be extrapolated from results (primarily) using gloved hands to results for real-life nongloved hands.
Microbial contamination of hands or trunk remaining or occurring during hand drying in the washroom or toilet, can result in microbe dissemination to multiple surfaces in the hospital environment via hand and clothing or skin contact. This phenomenon is significantly more likely to occur after hand drying with a jet air dryer as opposed to a paper towel. A fundamental principle of infection prevention practice is to minimize the potential for microbe dispersal. Thus, our findings question the use of hand drying with jet air dryers in a hospital setting.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2021.43
Acknowledgments
The authors thank all volunteers who took part in the study, as well as the LTHT facilities and ward staff, for their contribution.
Financial support
The study was supported by the European Tissue Symposium (ETS). The funder had no involvement in project design, data collection, analysis or interpretation, or manuscript preparation.
Conflicts of interest
M.H.W. has received honoraria from the ETS for microbiological advice and lectures and travel expenses to attend meetings. All other authors report no conflicts of interest relevant to this article.






