Introduction
The ocelot Leopardus pardalis is the third largest of 10 felid species in the Neotropics (de Oliveira, Reference de Oliveira1994; Sunquist & Sunquist, Reference Sunquist, Sunquist, Sunquist and Sunquist2002; de Oliveira & Cassaro, Reference de Oliveira and Cassaro2005). It occurs in a variety of habitats, including tropical and subtropical forest, wetland, savannah and mangrove swamps (Kitchener, Reference Kitchener1991; Murray & Gardner, Reference Murray and Gardner1997) and historically the species was distributed from Arizona, Arkansas, Louisiana and Texas in the United States to Argentina (Tewes & Schmidly, Reference Tewes, Schmidly, Novak, Baker, Obbard and Malloch1987; Brown, Reference Brown1990). Ocelot distribution has decreased considerably during the last century (Caso et al., Reference Caso, López-González, Payán, Eizirik, de Oliveira and Leite-Pitman2008). The species is included in Appendix I of CITES but categorized as Least Concern on the IUCN Red List (Caso et al., Reference Caso, López-González, Payán, Eizirik, de Oliveira and Leite-Pitman2008). Ocelots are listed as endangered in Mexico (Norma Oficial Mexicana, 2010), where the greatest threats to their survival are habitat loss and illegal hunting (López-González et al., Reference López-González, Brown and Gallo-Reynoso2003; Aranda, Reference Aranda, Ceballos and Oliva2005).
A population of ocelots has been confirmed in the Sierra Abra-Tanchipa Biosphere Reserve (Martínez-Calderas et al., Reference Martínez-Calderas, Rosas-Rosas, Martínez-Montoya, Tarango-Arámbula, Clemente-Sánchez, Crosby-Galván and Sánchez-Hermosillo2011), the only such reserve in eastern San Luis Potosí, Mexico, which is becoming isolated from other areas of suitable habitat as a result of significant land-use changes, including slash-and-burn cropping and clearing for livestock grazing. This may pose a significant threat to this population of ocelots, the only documented population in eastern San Luis Potosí (Villordo-Galván et al., Reference Villordo-Galván, Rosas-Rosas, Clemente-Sánchez, Martínez-Montoya, Tarango-Arámbula and Mendoza-Martínez2010; Martínez-Calderas et al., Reference Martínez-Calderas, Rosas-Rosas, Martínez-Montoya, Tarango-Arámbula, Clemente-Sánchez, Crosby-Galván and Sánchez-Hermosillo2011).
Although multiple international studies of ocelot ecology, biology, behaviour and status have been undertaken, there is little information regarding ocelot population status and trends in Mexico, or valid techniques for monitoring population trends (de Oliveira et al., Reference de Oliveira, Tortato, Silveira, Kasper, Mazim, Lucherini, Macdonald and Loveridge2010). Baseline information is needed to develop management strategies for conservation of ocelots (Aranda, Reference Aranda and Anónimo1991). Hence, our objective was to estimate the abundance and density of ocelots in the Sierra Abra-Tanchipa Biosphere Reserve to provide a basis for the development of informed management strategies for the monitoring and conservation of ocelots in the Reserve and throughout Mexico.
Study area
Our study area encompassed 21,464 ha, at altitudes of 114–810 m, within and adjacent to the Sierra Abra-Tanchipa Biosphere Reserve (Fig. 1), a federal protected area located in the Huasteca region in eastern San Luis Potosí, between Ciudad Valles and Tamuin townships. The climate in the area is predominantly warm and sub-humid, with a mean annual temperature of 25.3 °C and mean annual precipitation of 1,017 mm (INEGI, 2011). The primary vegetation type in the Reserve is tropical deciduous forest, with tropical forest, oak woodlands (Quercus spp.), palms, and secondary vegetation also present (Sánchez-Ramos et al., Reference Sánchez-Ramos, Hernández, Mora, Vargas-Contreras, Lara, Zamora, Cardona, Gómez-Pompa and Dirzo1993; Chapa & Monzalvo, Reference Chapa-Vargas and Monzalvo-Santos2012). Wildlife includes puma Puma concolor, white-tailed deer Odocoileus virginianus, brocket deer Mazama temama, collared peccary Pecari tajacu, and coati Nasua narica, and several threatened species, including the jaguar Panthera onca, margay Leopardus wiedii, and military macaw Ara militaris (Villordo-Galván et al., Reference Villordo-Galván, Rosas-Rosas, Clemente-Sánchez, Martínez-Montoya, Tarango-Arámbula and Mendoza-Martínez2010). Slash-and-burn agriculture, cattle ranching, and forestry are the predominant land-uses (Sánchez-Ramos et al., Reference Sánchez-Ramos, Hernández, Mora, Vargas-Contreras, Lara, Zamora, Cardona, Gómez-Pompa and Dirzo1993). Ibarra & Galindo (Reference Ibarra and Galindo2010) reported a loss of c. 9,360 ha of original habitat types in the study area over a 32-year period (1973–2005).
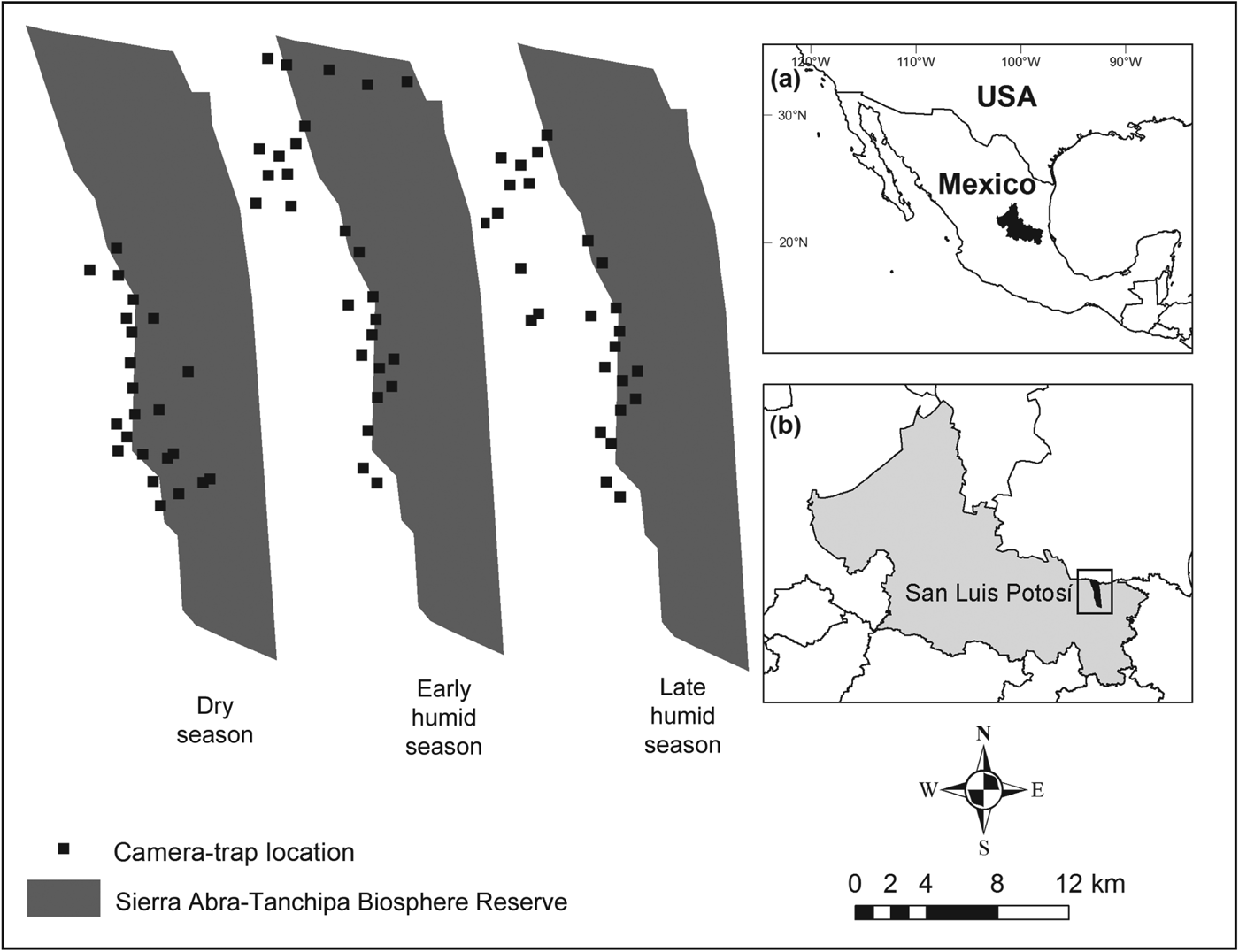
Fig. 1 Location of camera-trapping sites for the three surveys carried out in the greater Sierra Abra-Tanchipa Biosphere Reserve region, San Luis Potosí, Mexico. The shaded area in (a) indicates the location of San Luis Potosí state, and the rectangle in (b) indicates the location of the main map.
Methods
We used photographic recaptures from camera-trapping grids to develop mark–recapture models to estimate ocelot abundance (Karanth, Reference Karanth1995; Karanth & Nichols, Reference Karanth and Nichols1998). We established camera-trapping grids in three seasons during April 2011–March 2012, with one survey during the dry season, a second at the beginning of the following humid season and a third at the end of the humid season, deploying 22, 27 and 26 camera stations, respectively (Table 1). Cameras used included 31 WildView Xtreme (Stealth Cam LLC, Grand Praire, USA), 16 digital Stealth Cam (Stealth Cam LLC, Grand Prairie, USA), 11 DeerCam 200 (DeerCam, Park Falls, USA), and four Bushnell Trophy Cam (Bushnell, Overland Park, USA). Within the grid, stations were placed systematically on trails, streams and other routes where there was evidence of ocelot sign (scat, tracks), to maximize the capture probability of individuals. Most stations were located outside the protected area because a previous study within the protected area yielded a low number of photographic captures of ocelot (Martinez-Calderas et al., Reference Martínez-Calderas, Rosas-Rosas, Martínez-Montoya, Tarango-Arámbula, Clemente-Sánchez, Crosby-Galván and Sánchez-Hermosillo2011; Fig. 1). In most cases (60, 67 and 58% for the dry, early and late humid seasons, respectively), we used two cameras at each station to increase the likelihood of obtaining photographs of both sides of each animal. Each camera was placed 0.45 m above ground level and stations were spaced at a mean spacing of 1.5 km, based on the area of activity of ocelots in Mexico (Caso, Reference Caso1994; Martínez-Meyer, Reference Martínez-Meyer1997). Cameras were programmed to be active for 24 hours and record the time and date of each capture. Our camera grids covered > 45 km2, following the recommendations of Dillon & Kelly (Reference Dillon and Kelly2007) and Maffei & Noss (Reference Maffei and Noss2008). We limited seasonal surveys to < 90 days to meet the assumption of a closed population, following Karanth & Nichols (Reference Karanth and Nichols1998). We checked cameras every 15 days to change batteries, and film or memory cards.
Table 1 Details of camera-trap surveys for the ocelot Leopardus pardalis in the Sierra Abra-Tanchipa Biosphere Reserve, San Luis Potosí, Mexico (Fig. 1), during the dry and early and late humid seasons, with survey duration and numbers of cameras, camera-trap stations and trap-days.
We identified individual ocelots by their morphological characteristics, as described by Trolle & Kéry (Reference Trolle and Kéry2003), and identified gender by the presence or absence of a testicular sack. We excluded distorted pictures or those that lacked clarity for individual identification.
We used CAPTURE (Otis et al., Reference Otis, Burnham, White and Anderson1978; White et al., Reference White, Anderson, Burnham and Otis1982) to estimate abundance, under the assumption of a closed population (Otis et al., Reference Otis, Burnham, White and Anderson1978; White et al., Reference White, Anderson, Burnham and Otis1982; Rexstad & Burnham, Reference Rexstad and Burnham1991). We used 5-day intervals to develop capture histories for each ocelot photographed during sampling, which resulted in 17, 16 and 6 potential capture events for the dry, early and late humid seasons, respectively. We selected the most appropriate model for each survey season, using the discriminant model selection function of CAPTURE (Otis et al., Reference Otis, Burnham, White and Anderson1978; Rexstad & Burnham, Reference Rexstad and Burnham1991).
We determined population density, using full maximum likelihood spatially-explicit capture–recapture (Borchers & Efford, Reference Borchers and Efford2008) in DENSITY 5.0 (University of Otago, New Zealand). We assumed a Poisson distribution of home range centres and used a buffer distance of 7,500 m, corresponding with home range estimates in Mexico (Caso, Reference Caso1994). We contrasted half normal, hazard, and negative exponential detection functions and three alternative capture probabilities (g 0): g 0(.)σ(.), where all individuals have the same capture probability; g 0(b)σ(.), where individuals show a behavioural response to first capture; and g 0(h2)σ(.), where individuals exhibit heterogeneity in capture probability. We did not consider more complex models because our data were sparse. We compared models, using AICc (Burnham & Anderson, Reference Burnham and Anderson1998), and considered models with AICc < 5 competing models. Lastly, we compared resultant density estimates, using parametric bootstrapping (Efron & Tibshirani, Reference Efron and Tibshirani1993).
Results
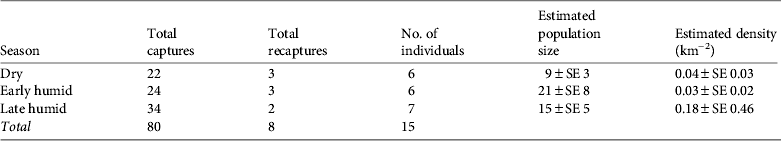
We obtained 80 photographic captures of ocelots during 7,786 camera-days. Of these, 22 were recorded in the dry season, 24 in the early humid season and 34 in the late humid season. Most captures were nocturnal, with most activity during 18.00–06.00 (Fig. 2), which was consistent among surveys (χ2 = 0.56, P < 0.05, df = 2). Peak activity was during 20.00–02.00 (Fig. 2).
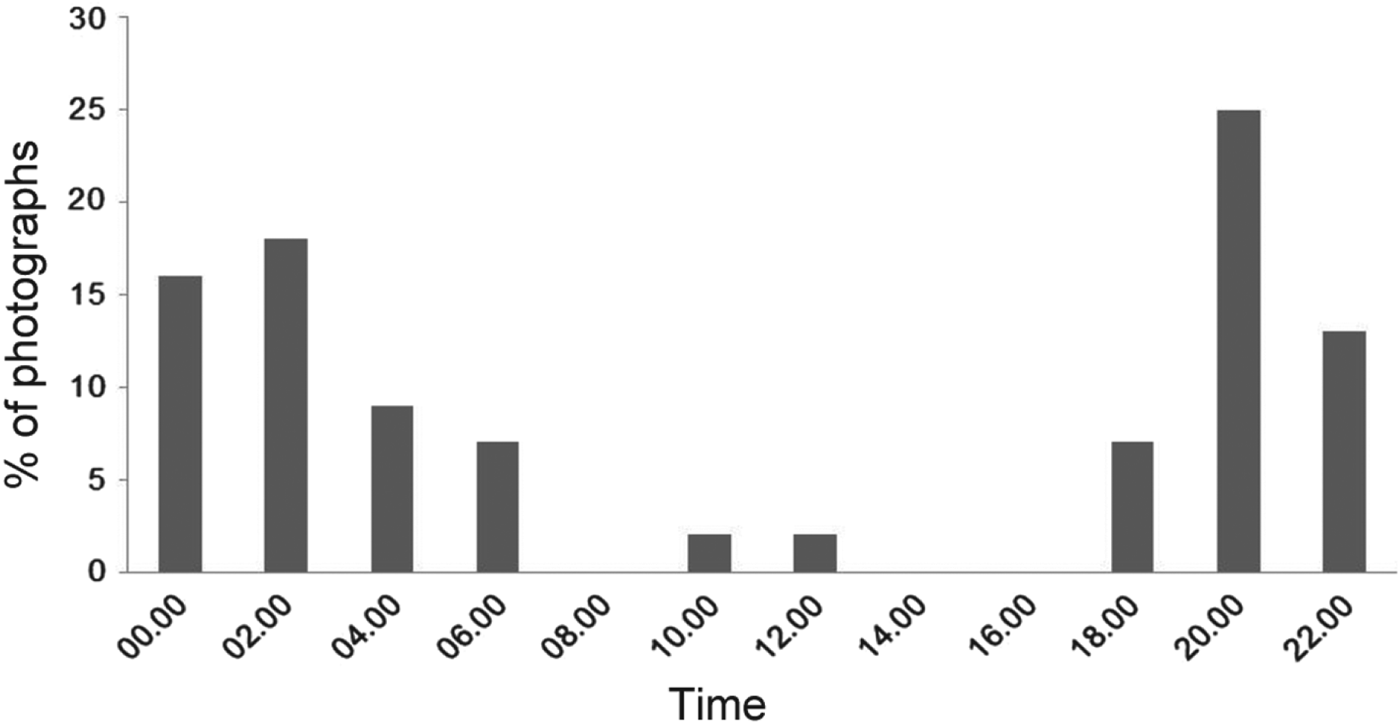
Fig. 2 Activity patterns of all ocelots Leopardus pardalis (n = 55) documented in the greater Sierra Abra-Tanchipa Biosphere Reserve region, San Luis Potosí, Mexico.
We identified 15 individuals: 10 males, one female and four of indeterminate sex. Models M o and M h were usually the best-supported models (Table 2). We report abundance using estimates from M h because of the robustness of the jackknife estimator to violations of model assumptions, following Karanth & Nichols (Reference Karanth and Nichols1998), in combination with goodness-of-fit tests that did not reject M h as a candidate model (Table 2). The low capture rate of females suggests heterogeneity in capture probabilities among ocelots, leading us to favour M h. We estimated there were 9 ± SE 3, 21 ± SE 8 and 15 ± SE 5 ocelots during the dry, early and late humid season surveys, respectively. Our data fit the assumption of closure (z < |0.28|, P > 0.610; Otis et al., Reference Otis, Burnham, White and Anderson1978; White et al., Reference White, Anderson, Burnham and Otis1982).
Table 2 Model selection criteria scores and goodness-of-fit tests of hypothesis of M h vs not-M h for ocelot mark–recapture models for three camera-trap surveys in the greater Sierra Abra-Tanchipa Biosphere Reserve region (Fig. 1).
Model g 0(.)σ(.) using either half normal or negative exponential detection functions was clearly superior to other models of ocelot density (for all others ΔAICc ≥ 14) and both detection functions were approximately equally supported (Table 4). Model-averaged estimates of ocelot density were 0.04 ± SE 0.03 (95% CI 0.01–0.15), 0.03 ± SE 0.02 (95% CI 0.01–0.11) and 0.18 ± SE 0.46 (95% CI 0.01–2.78) ocelots per km2 for the dry, early and late humid surveys, respectively (Table 3). Density of ocelots was similar among surveys (P > 0.612) because of large variance in the late humid season estimate. Capture probabilities at the home range centre were 0.05 ± SE 0.06, 0.01 ± SE 0.01 and 0.03 ± SE 0.05 for the dry, early and late humid season surveys, respectively.
Table 3 Camera-trapping and model data for three surveys of ocelots in the Sierra Abra-Tanchipa Biosphere Reserve (Fig. 1), with total numbers of captures and recaptures, number of individuals captured, estimated population size from closed mark–recapture, and estimated density from model-averaged full maximum likelihood spatially-explicit capture–recapture.
Discussion
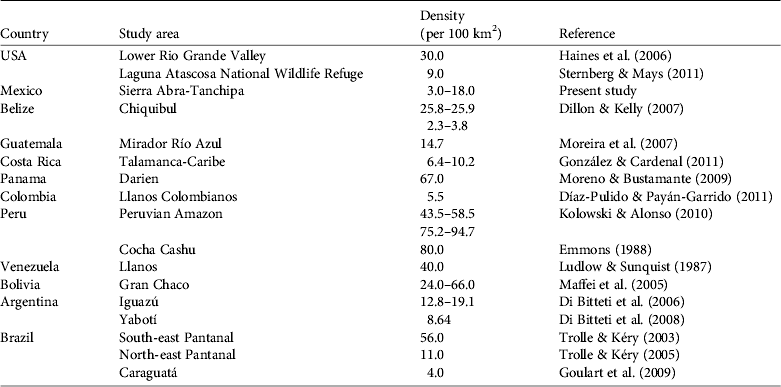
Density of ocelots reported throughout the Americas is variable (Table 4), with differences associated with habitat type, number of camera-trap stations, and the distance between them, the calculation of the effective area of sampling, sampling effort, and the method of estimation (e.g. camera-trapping vs radio-telemetry). Although comparison between studies is difficult for these reasons our density estimates are towards the lower range of published estimates (Table 5). This supports the hypothesis that ocelot density decreases with decreasing precipitation and increasing distance from the equator (Di Bitetti et al., Reference Di Bitetti, Paviolo, De Angelo and Di Blanco2008). This relationship is probably attributable to decreased primary productivity and, consequently, prey base. The habitat quality in our study area was probably reduced by fragmentation as a result of increased slash-and-burn agriculture and conversion of grassland for cattle ranching (Villordo-Galván et al., Reference Villordo-Galván, Rosas-Rosas, Clemente-Sánchez, Martínez-Montoya, Tarango-Arámbula and Mendoza-Martínez2010). Furthermore, the Sierra Abra-Tanchipa Biosphere Reserve is becoming increasingly isolated from the mountain massif of the Sierra Madre Oriental, where ocelots are common (Ávila-Nájera et al., Reference Ávila-Nájera, Rosas-Rosas, Tarango-Arámbula, Martínez-Montoya and Santoyo2011). Isolation and fragmentation of habitat together limit dispersal movements of ocelots (and prey) as well as decreasing the availability and quality of suitable habitat (Di Bitetti et al., Reference Di Bitetti, Paviolo, De Angelo and Di Blanco2008).
Table 4 Candidate models for full maximum likelihood spatially explicit capture–recapture models of ocelot density in three seasons in the Abra-Tanchipa Biosphere Reserve (Fig. 1), with model selection criteria and number of parameters.
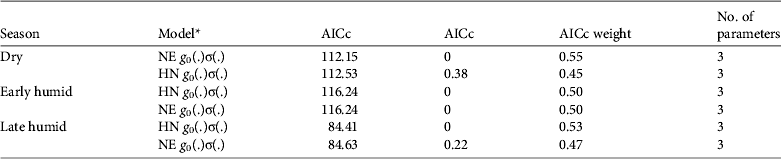
* NE, negative exponential detection function; HN, half normal detection function
Table 5 Density of ocelots recorded in studies in North, Central and South America.
Similar to other camera-trapping studies of carnivores (Silver et al., Reference Silver, Ostro, March, Maffei, Noss and Kelly2004; Gray & Prum, Reference Gray and Prum2012), we had fewer detections of female ocelots than males. Our traps were spaced at c. 1.5 km intervals, and only three ocelots were photographed at more than one station, all of which were males. Thus, the distance between traps may have been too great, particularly for females. However, trap spacing was set in accordance with the movement parameters of our density models (σ = 1.1–5.0 km, seasonally) and ocelot home range sizes recorded in Mexico (8–10 km2; Caso, Reference Caso1994), indicating that the lower capture rate for females is probably not attributable to the trap spacing (Efford et al., Reference Efford, Warburton, Coleman and Barker2005; Gray & Prum, Reference Gray and Prum2012). Thus, differences in the number of individuals of each sex that were photographed may be related to differing movement patterns of males and females and the elusive behaviour of female ocelots (Silver et al., Reference Silver, Ostro, March, Maffei, Noss and Kelly2004; Gray & Prum, Reference Gray and Prum2012). Males have a higher probability of being captured because they have larger territories, they tend to use higher-use roads and trails, where camera traps are located, and they are more active than females (Martínez-Meyer, Reference Martínez-Meyer1997). These behaviours limit captures of females in studies of felids. Consequently, we recommend that sampling be designed and conducted in a way that will maximize the potential for capturing females in the study area, such as including all possible unpaved roads and trails (Sterenberg & Mays, Reference Sternberg and Mays2011; Foster & Harmsen, Reference Foster and Harmsen2012). Additionally, spacing of camera grids should be conservative in cases where home ranges are unknown.
We also observed differences in the probability of detection of ocelots between the dry and humid seasons. Based on mean population estimates, the probability of photographing an ocelot in the dry season was 0.67 (i.e. six individuals of an estimated nine were photographed) but in the late humid season was < 0.50 (i.e. six of an estimated 21 and seven of an estimated 15 were photographed in the early and late humid season, respectively). These differences may be related to decreased movement during the dry season. In similar habitats in Jalisco, Mexico, Martínez-Meyer (Reference Martínez-Meyer1997) found that males and females had smaller home ranges during the dry season, with overlap in activity patterns. In areas where the dry season is severe, such as the Sierra Abra-Tanchipa Biosphere Reserve, predators such as ocelots tend to concentrate in areas close to permanent water, where vegetation density remains relatively high and prey are likely to be more abundant and vulnerable (Martínez-Meyer, Reference Martínez-Meyer1997). This behaviour may explain the higher detection rate during the dry season.
Differences in detection between the dry and wet seasons indicate that long-term monitoring must be seasonally consistent over time. Otherwise, differences in ocelot numbers between years may be confounded by seasonal differences. Because of the higher probability of detection and the more conservative density estimate we observed, we recommend that long-term monitoring be conducted using camera-trapping during the dry season in Sierra Abra-Tanchipa and similar areas.
Ocelots in the Reserve were mainly nocturnal, as reported in other studies (Ludlow & Sunquist, Reference Ludlow and Sunquist1987; Emmons, Reference Emmons1988; Maffei et al., Reference Maffei, Noss, Cuéllar and Rumiz2005; Di Bitetti et al., Reference Di Bitetti, Paviolo and De Angelo2006; Dillon & Kelly, Reference Dillon and Kelly2007; Goulart et al., Reference Goulart, Graipel, Tortato, Ghizoni, Oliveira-Santos and Cáceres2009). This corresponds to a strategy of avoiding high temperatures during the day (Stoner & Timm, Reference Stoner, Timm, Dirzo, Young, Mooney and Ceballos2010). Nocturnal activity coincides with the period of activity of much of the ocelot's prey (Ludlow & Sunquist, Reference Ludlow and Sunquist1987; Emmons, Reference Emmons1988).
Ocelot abundance is influenced by prey density, habitat quality, presence of competitors, predators, and environmental variables, especially those related to primary production and prey availability (Di Bitetti et al., Reference Di Bitetti, Paviolo, De Angelo and Di Blanco2008; de Oliveira et al., Reference de Oliveira, Tortato, Silveira, Kasper, Mazim, Lucherini, Macdonald and Loveridge2010). Land conversion for agriculture and cattle ranching in the Reserve is resulting in increased soil erosion, changes in vegetation cover type, and the loss or fragmentation of natural habitats. Similar to other species, the ocelot is susceptible to this kind of disturbance. Given their need for dense vegetation cover (Harveson et al., Reference Harveson, Tewes, Anderson and Laack2004), the persistence of the ocelot could be threatened by the opening of habitats for agricultural development (Martínez-Calderas et al., Reference Martínez-Calderas, Rosas-Rosas, Martínez-Montoya, Tarango-Arámbula, Clemente-Sánchez, Crosby-Galván and Sánchez-Hermosillo2011). These challenges may be especially critical in our study area, given the small, relatively low-density ocelot population, because Sierra Abra-Tanchipa is the only reserve in eastern San Luis Potosí and our small study population is the only known population. Increasing fragmentation could further isolate this population and decrease connectivity with other viable habitats, both of which would increase the likelihood of extirpation (Martínez-Calderas et al., Reference Martínez-Calderas, Rosas-Rosas, Martínez-Montoya, Tarango-Arámbula, Clemente-Sánchez, Crosby-Galván and Sánchez-Hermosillo2011). We recommend continued dry-season monitoring of the status of this population, surveys of adjacent areas to determine presence or absence of ocelots, as well as studies of movement and habitat associations of ocelots in this and adjacent populations (if present). This information is needed to determine the viability of this population, identify adjacent populations and identify areas necessary to maintain connectivity between populations and habitats to conserve the ocelot in eastern Mexico.
Since 2012 ocelots have been monitored, by camera-trapping, in and south of the Sierra Abra-Tanchipa Biosphere Reserve to estimate abundance and identify suitable habitat. There appears to be a lack of habitat connectivity in states such as Hidalgo and Puebla, where ocelots are less common. Long-term monitoring, including genetic studies in southern regions, is necessary to investigate connectivity for ocelots in north-east Mexico.
Acknowledgements
We thank the Consejo Nacional de Ciencia y Tecnología, Mexico, for granting a scholarship for graduate studies to Abraham Martínez-Hernández, and Colegio de Postgraduados, Campus San Luis Potosí, the personnel of the Sierra Abra-Tanchipa Biosphere Reserve, Idea Wild, and the Department of Extension Animal Sciences and Natural Resources, New Mexico State University, for funding and other support. We acknowledge all project personnel, volunteers and ranchers for their help with field research activities.
Biographical sketches
Abraham Martínez-Hernández is interested in carnivore ecology, population dynamics and wildlife management. He has worked on felids and other mammal species of Mexico. Octavio César Rosas-Rosas is interested in predator–prey relationships, community conservation, and big game management. Fernando Clemente-Sánchez, Luis Antonio Tarango-Arámbula, Jorge Palacio-Núñez and José Guadalupe Herrera-Haro are interested in game and non-game ecology, biodiversity, habitat management and experimental statistics. Louis C. Bender's main interests are applied population and habitat management.









