Diabetes mellitus disproportionately affects low-income Americans, who experience higher disease prevalence and complication rates( Reference Gaskin, Thorpe and McGinty 1 – Reference Agardh, Allebeck and Hallqvist 3 ). Food insecurity is one mechanism by which low income may increase risk of poor diabetes outcomes( Reference Seligman, Bindman and Vittinghoff 4 ). Food insecurity refers to limited or uncertain access to adequate food at the level of the household( 5 ). It is an independent risk factor for poor intermediate health outcomes, including glycaemic control, in adults with diabetes( Reference Seligman, Jacobs and Lopez 6 – Reference Wang, McGinnis and Fiellin 8 ). Food insecurity may impact diabetes self-management through varied mechanisms: reliance on inexpensive, shelf-stable foods which are generally poor for glycaemic control; binge eating when food becomes available; competing demands between food and health-care expenditures; and reduced capacity to manage the complexity of diabetes self-care when confronted with the immediacy of inadequate food( Reference Seligman, Bindman and Vittinghoff 4 , Reference Silverman, Krieger and Kiefer 9 ).
Food insecurity is categorized by degree of severity: low food security or very low food security( 5 ). Low-food-secure households generally experience diets that are reduced in quality or variety, while very-low-food-secure households also experience reduced food intake( Reference Coleman-Jensen, Nord and Andrews 10 ). In 2012, 14·5 % of US households (33 million adults) were food insecure, of which 8·8 % met criteria for low food security and an additional 5·7 % for very low food security( Reference Coleman-Jensen, Nord and Andrews 10 ). Low and very low food security may differ in ways that are important for diabetes self-management( Reference Anater, McWilliams and Watkin 11 – Reference Drewnowski and Darmon 13 ). For example, adults from very-low-food-secure households are more likely than those from low-food-secure households to have disrupted food supplies, including going hungry, reducing food intake and losing weight( Reference Coleman-Jensen, Nord and Andrews 10 ).
Although previous studies have examined food security and diabetes self-management, these studies have generally lacked adequate numbers of very-low-food-secure participants to permit stratified analysis by severity of food insecurity( Reference Berkowitz, Baggett and Wexler 14 , Reference Seligman, Davis and Schillinger 15 ). As part of a pilot study of food pantry-based interventions for diabetes support( Reference Seligman, Lyles and Marshall 16 ), we were able to reach a large number of very-low-food-secure individuals who are historically under-represented in clinic-based samples. The high prevalence of very low food security allowed us to examine the differential impact of low and very low food security on diabetes self-management.
Methods
Study design
We conducted a cross-sectional survey of adults with diabetes at food pantries in Sonoma County, California, Columbus, Ohio and Corpus Christi, Texas, USA. Baseline surveys for a diabetes self-management intervention located at the food pantries were administered between March 2012 and March 2014. Inclusion criteria included age ≥18 years; English or Spanish language fluency; and point-of-care glycated Hb (HbA1c) percentage greater than or equal to 6·5 %, or self-reported diagnosis of diabetes with prescription bottles of oral hypoglycaemic medications or insulin on-hand. Point-of-care HbA1c testing was performed with Bayer A1CNow® testing kits. Exclusion criteria included pregnancy, hearing impairment and cognitive impairment. Trained bilingual staff conducted the survey in person (58 %) or over the telephone (42 %) in the participants’ preferred language.
Measures
Food security status was determined using the six-item short form of the US Department of Agriculture’s Household Food Security Survey Module( Reference Coleman-Jensen, Nord and Andrews 10 ). By convention, we categorized participants as food secure if they affirmed zero or one item, low food secure if affirming two to four items, and very low food secure if five or six items were affirmed.
We examined eight indicators of diabetes self-management: (i) HbA1c; (ii) diabetes self-efficacy; (iii) diabetes distress; (iv) medication non-adherence; (v) severe hypoglycaemia; (vi) depressive symptoms; (vii) medication affordability; and (viii) food–medicine purchasing trade-offs.
Self-efficacy describes an individual’s cognitive perception of his/her ability to actively manage his/her chronic disease. Previous studies have linked self-efficacy to diabetes self-care behaviours and glycaemic control( Reference Johnston-Brooks, Lewis and Garg 17 – Reference Talbot, Nouwen and Gingras 20 ). We measured self-efficacy using an eight-item instrument( Reference Kavanagh, Gooley and Wilson 21 ) and calculated mean scores from Likert response options (range of 1–10). A higher score indicates greater self-efficacy, which is generally correlated with lower HbA1c values( Reference Kavanagh, Gooley and Wilson 21 ).
Diabetes distress, a measure of the emotional burden an individual associates with managing her/his disease, is independently associated with glycaemic control( Reference Fisher, Glasgow and Strycker 22 , Reference Fisher, Mullan and Arean 23 ). We assessed diabetes distress using a two-item screening tool( Reference Fisher, Glasgow and Mullan 24 ). We averaged scores of the two six-point Likert items to generate a summary score between 1 and 6, with a higher score indicating greater distress.
We assessed medication non-adherence using the four-item Medication Adherence Questionnaire, with Likert response options from 0 to 4( Reference Krapek, King and Warren 25 , Reference Morisky, Green and Levine 26 ). A higher score indicates lower adherence.
Hypoglycaemia is associated with food insecurity and is often indicative of poor diabetes self-management( Reference Seligman, Davis and Schillinger 15 , Reference Seligman, Jacobs and Lopez 27 ). We dichotomized (‘0 times’ or ‘≥1 time’) responses to the following item: ‘In the past 4 weeks, how many times have you had a severe low blood sugar reaction, such as passing out or needing help to treat the reaction?’
Several studies have linked depression to poor glycaemic control and diabetes self-care( Reference Gross, Olfson and Gameroff 28 – Reference Lustman, Anderson and Freedland 30 ). We used the Patient Health Questionnaire-2 (PHQ-2) to assess depressive symptoms. Participants who answered affirmatively to one or both items were considered to have depressive symptoms.
We assessed participants’ ability to afford medications with the following item: ‘In the last 12 months, how often did you take less medicine than you were supposed to because you could not afford to buy more?’
We considered participants to have made trade-offs between food and medications or diabetes supplies if they answered any of the following four items affirmatively (‘often’ or ‘sometimes’ on a four-point Likert scale of ‘often’, ‘sometimes’, ‘rarely’ or ‘never’), queried over the last 12 months: ‘How often have you …’ (i) ‘…put off buying food so that you would have money to buy medicines?’, (ii) ‘… put off buying medicines so that you would have money to buy food?’, (iii) ‘… put off buying diabetes supplies, like test strips or lancets, so that you would have money to buy food?’ and (iv) ‘… put off buying food so that you would have money to buy diabetes supplies, like test strips or lancets?’
We measured five covariates, including age, gender, race/ethnicity, education and study site. We assessed race/ethnicity in order to capture the diversity of our sample and to adjust for known racial/ethnic differences in item response distribution for some of our variables( Reference Gaskin, Thorpe and McGinty 1 , Reference Sarkar, Fisher and Schillinger 19 ). Race/ethnicity was determined by participant selection of ‘Latino or Hispanic’, ‘White’, ‘Black or African American’, ‘Native American’, ‘Asian/Pacific Islander’ or ‘Other’. Due to very small sample sizes for Native American (n 46) and Asian/Pacific Islander (n 9) participants, we collapsed the variable into ‘Latino or Hispanic’, ‘White’, ‘Black or African American’ and ‘Native American, Pacific Islander or other.’
Statistical analysis
We compared baseline demographic characteristics using χ 2 or t tests for categorical and continuous variables, respectively. We analysed unadjusted associations between food security status and diabetes variables using χ 2 or one-way ANOVA tests. Participants who did not provide responses to question items pertaining to an individual measure were excluded from analysis of that measure, but included in other analyses. We used logistic or linear regression models depending on whether the self-management outcome was categorical or continuous and included age, gender, race/ethnicity, education and study site as covariates. In a sensitivity analysis, we additionally adjusted for depressive symptoms in all regression models. Statistical analyses were performed using the statistical software package Stata version 12.0 with a significance level of P=0·05.
Results
Study population
Of 1495 eligible food pantry clients, 1237 provided informed consent and participated in the survey (83 % response). More than 98 % of the sample responded to all six of the food insecurity items and all respondents answered at least four items. Most of the sample was food insecure, with 42 % reporting low food security and 42 % very low food security. Almost all (98 %) participants identified as having diabetes with point-of-care HbA1c testing were previously aware of a diabetes diagnosis (i.e. few new cases of diabetes were identified). There were statistically significant differences in age, gender, education, race/ethnicity, BMI and tobacco use by level of food insecurity (Table 1). Survey non-response to each of the diabetes self-management measures ranged from 1 % (depressive symptoms) to 7 % (medication non-adherence).
Table 1 Sociodemographic characteristics, clinical characteristics and study site distribution of participants: a convenience sample of adults with diabetes receiving food assistance at community-based food pantries in California, Ohio and Texas, USA, March 2012–March 2014

GED, General Education Diploma.
Clinical characteristics
There was no statistically significant difference in mean HbA1c (Table 1) or percentage of participants with HbA1c level above 8·5 % (data not shown) by level of food security. Mean BMI was greatest among the very-low-food-secure group and differed significantly from that of the food-secure group (35·0 v. 32·7 kg/m2, P=0·009). Tobacco use was more than twice as frequent in the very low-food-secure compared with the food-secure group (31 % v. 12 %, P<0·001).
Diabetes self-management
We observed poorer diabetes self-management in the food-insecure groups compared with the food-secure group. All unadjusted associations examined were statistically significant with P values <0·001 (Table 2). After adjusting for age, gender, race/ethnicity, education and study site, we found statistically significant associations between food insecurity and seven of the diabetes self-management measures in the very-low-food-secure group and four measures in the low-food-secure group (Table 3; reference group is the food-secure group). Diabetes self-efficacy scores were on average 0·51 units lower (95 % CI −0·85, −0·17) among very-low-food-secure participants compared with food-secure participants and the mean diabetes distress score was 0·79 points higher (95 % CI 0·54, 1·04) in the very-low-food-secure compared with the food-secure group. Compared with food-secure participants, those identified as having very low food security had average medication non-adherence scores 0·31 units higher (95 % CI 0·12, 0·50). The adjusted odds of reporting an episode of severe hypoglycaemia among very-low-food-secure participants was 2·6 times greater than among participants who were food secure (OR=2·63; 95 % CI 1·42, 4·85). Both the low- and very-low-food-secure groups had significantly higher odds of having depressive symptoms, experiencing challenges around affordability of medications and diabetes supplies, and making trade-offs between food and medications and medical supplies, compared with their food-secure counterparts. In a sensitivity analysis, significant associations in Table 3 remained statistically significant after additionally controlling for depressive symptoms, with the exception of diabetes distress in the low-food-secure group and medication non-adherence in the very-low-food-secure group (data not shown).
Table 2 Unadjusted associations between food insecurity status and diabetes self-management among a convenience sample of adults with diabetes (n 1237) receiving food assistance at community-based food pantries in California, Ohio and Texas, USA, March 2012–March 2014
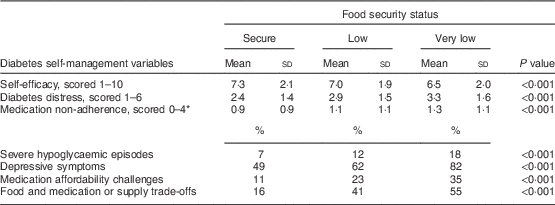
* Higher scores indicate poorer medication adherence.
Table 3 AdjustedFootnote associations between food insecurity status and diabetes self-management among a convenience sample of adults with diabetes (n 1237) receiving food assistance at community-based food pantries in California, Ohio and Texas, USA, March 2012–March 2014

Adjusted for age, gender, race/ethnicity, education and study site.
* Denotes the 95 % CI does not include the null value.
Discussion
In this community sample of adults with diabetes seeking assistance at food pantries, we identified many adults living in very-low-food-secure households. Our data suggest a dose–response relationship between severity of food insecurity and barriers to diabetes self-management.
Recruitment from food pantries allowed us to reach participants from very-low-food-secure households who we have been unable to reach easily in clinical settings, including clinical settings traditionally serving vulnerable and marginalized populations( Reference Seligman, Jacobs and Lopez 6 , Reference Seligman, Jacobs and Lopez 27 ). The discrepancy between food insecurity reports in clinical settings and community-based settings suggests that members of food-insecure households may not access clinical care as regularly as less-food-insecure groups. Interventions to engage marginalized groups in clinical care should focus on this underserved population and food pantries may be an ideal venue.
There is a growing capacity for food banks (which provide food, and often infrastructure, to food pantries which distribute food directly to clients) to conduct this work( 31 ) and our research supports this trend. A number of food banks now employ dietitians and are beginning to deliver health-care support services( 31 ). Our ongoing intervention to provide diabetes self-management support through food pantry networks similarly seeks to address this apparent gap in care experienced by food-insecure groups( Reference Seligman, Lyles and Marshall 16 ). Prescription food programmes, currently being used in some health-care systems, may also be effective interventions in this group( Reference Baronberg, Dunn and Nonas 32 – Reference Herman, Harrison and Afifi 34 ) to assist in providing healthy, diabetes-appropriate foods to low- and very-low-food-secure patients who are engaged in clinical care. Our findings support the notion that food pantries are well positioned to deliver this type of lifestyle content, rather than relying solely on clinic-based approaches.
Participants from very-low-food-secure households did not have significantly higher HbA1c values (8·2 %) than those from low-food-secure (8·0 %) or food-secure (8·0 %) households. The overall mean HbA1c across the entire study population was 8·1 %, almost an entire percentage point greater than the national average HbA1c among people with diabetes (7·2 %) reported in the National Health and Nutrition Examination Survey( 35 ). This may suggest that current clinical interventions to improve glycaemic control in low-income populations, widespread over the last decade, are not effectively reaching many high-risk adults. Furthermore, we do not yet know whether food-secure, low-food-secure and very-low-food-secure populations will respond to self-management support similarly. Prior studies in clinical settings suggest that food-insecure adults may respond differentially to self-management support( Reference Lyles, Wolf and Schillinger 18 ). Understanding the distinct self-management challenges of food-insecure populations will inform the development and implementation of self-management strategies across the spectrum of food security status.
The high BMI among this population may also be reflective of the unique setting in which the study was conducted: food pantries serving a highly vulnerable population. This finding further highlights the importance of studying community-based populations in order to improve our understanding of very low food security and its tight link to high BMI, depressive symptoms, and other barriers to good health in general and diabetes self-management in particular.
We also found high rates of diabetes distress in our sample, which has been linked to diabetes self-care behaviours and glycaemic control( Reference Fisher, Glasgow and Strycker 22 , Reference Fisher, Mullan and Arean 23 ). The very-low-food-secure group had a mean distress score >3, which has been interpreted as indicative of need for clinical intervention( Reference Polonsky, Fisher and Earles 36 ). Our findings of the highest distress scores in the very-low-food-secure groups may further signal the low penetration of diabetes support into these high-risk groups.
Similarly, we observed a high frequency of severe hypoglycaemic episodes among the very-low-food-secure group. We suspect this high frequency reflects missed meals which accompany exhaustion of food budgets in the very-low-food-secure household and inadequate training in how to manage diabetes medications in the setting of reduced dietary intake( Reference Seligman, Davis and Schillinger 15 , Reference Seligman, Bolger and Guzman 37 ).
Previous studies of depression and food insecurity have predominantly focused on women’s and maternal health( Reference Corcoran, Heflin and Siefert 38 – Reference Whitaker, Phillips and Orzol 42 ). One study of depression and anxiety in mothers stratified by food security status observed proportions of depressive symptoms of 17, 21 and 30 % in the food-secure, low-food-secure and very-low-food-secure groups( Reference Whitaker, Phillips and Orzol 42 ) – substantially lower proportions than we observed in our sample (49, 62 and 82 %, respectively). Diabetes and food insecurity are separately associated with depression( Reference Hanson and Olson 29 , Reference Heflin, Siefert and Williams 39 , Reference Whitaker, Phillips and Orzol 42 , Reference Anderson, Freedland and Clouse 43 ), so the high prevalence we observed is unsurprising and emphasizes the ongoing need for mental health services in this population. The interrelationships among depression, diabetes and food insecurity are complex and interventions directed towards food-insecure populations to address depression and diabetes simultaneously are likely to offer advantages over interventions addressing each in isolation( Reference Bogner, Morales and de Vries 44 ).
There are several limitations to the present study. It was a cross-sectional analysis and we therefore cannot infer causality. We were not able to control for all potential confounders, including medical co-morbidities, health literacy, substance use and access to care. Food insecurity is a household-level measure, not an individual measure; however, most adults residing in food-insecure households individually experience the effects of food insecurity. All diabetes self-management measures were self-reported, and some were reported via face-to-face interviews (58 %) and others via telephone (42 %), constituting potential sources of bias in our data( Reference Glasgow, Ory and Klesges 45 ). Finally, the interplay among each of the investigated diabetes self-management measures is presumably more complex than our models can depict.
The present study is important in its ability to characterize the most vulnerable subgroup of food-insecure adults with diabetes. We identified independent associations between food insecurity and barriers to diabetes self-management, with greater food insecurity amplifying these challenges. Diabetes self-management support programmes for this population must address not only diabetes self-care and food affordability, but also low self-efficacy, emotional distress and mental health, and barriers to medication adherence. Non-clinical settings may effectively reach the most food-insecure adults with diabetes.
Acknowledgements
Financial support: This work was supported by NIH/NCRR/OD UCSF-CTSI Grant Number KL2 RR024130, AHRQ Career Development Award R00HS022408, and The Bristol-Myers Squibb Foundation. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number T32GM066691. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funders had no role in the design, analysis or writing of this article. Conflict of interest: H.K.S. serves on the board of directors of the S.F.-Marin Food Bank but receives no financial support from them. All other authors report no conflict of interest. Authorship: M.M.I. drafted the manuscript and analysed and interpreted data; C.R.L. analysed and interpreted data, provided statistical expertise and critically revised the manuscript; K.P. contributed to project conception and design, acquisition of funding, collection of data and critical revision of the manuscript; E.W. and M.B.M. contributed to project conception and design, acquisition of funding, collection and interpretation of data and critical revision of the manuscript; H.K.S. provided project supervision and contributed to project conception and design, acquisition, analysis and interpretation of data, and manuscript drafting and revision. All authors had complete access to the data. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Copernicus Group Institutional Review Board. Written informed consent was obtained from all subjects.






