CVD and diabetes mellitus (DM) are the leading causes of mortality and morbidity in Latin American and the Caribbean (LAC), resulting in 1·05 million deaths and 18·4 millions of disability-adjusted life years lost in 2016(Reference Ordunez, Mize and Barbosa1,2) ; 41·8 % of these deaths occurred among persons younger than 70 years of age(Reference Gawryszewski and Souza Mde3). Cardiometabolic disease (CMD) burdens, including both CVD and DM, are driven by key behavioural and lifestyle risk factors that are in turn modified by socio-economic, demographic and epidemiological changes that have occurred in LAC populations over recent decades(Reference Kain, Hernández Cordero and Pineda4).
Despite increases in both total CMD mortality(Reference Rivera-Andrade and Luna5,6) and unhealthy dietary patterns in LAC,(Reference Galicia, Grajeda and de Romaña7,Reference Finck Barboza, Monteiro and Barradas8) comprehensive, detailed, quantitative estimates of the impact of intakes of key dietary factors on CMD-related mortality in LAC countries are not available, except for Brazil(Reference Otto, Afshin and Micha9). Although trends in and burdens of disease due to particular dietary factors, such as Na, sugar-sweetened beverages (SSB) and fats, have been estimated globally(Reference Mozaffarian, Fahimi and Singh10–Reference Wang, fshin and Yakoob12), and region-specific diet-related burdens have been estimated for North Africa/Middle East and South Asia(Reference Afshin, Micha and Khatibzadeh13,Reference Yakoob, Micha and Khatibzadeh14) , diet-related CMD burdens in LAC have not previously been reported by country, age, sex and over time. Comprehensive estimates of CMD-related mortality burdens attributable to suboptimal dietary intake are essential to inform national and regional health policies, priorities and interventions in LAC. To address these key gaps in knowledge, we used a comparative risk assessment analytic framework to quantify CMD mortality attributable to eleven dietary factors in thirty-two countries in LAC in 1990 and 2010.
Methods
Using a standard comparative risk assessment analytic framework, we quantified country-, age- and sex-specific CMD deaths attributable to eleven dietary factors in 1990 and 2010 across thirty-two countries in the LAC region. This analytic framework estimates the deaths that would be averted if optimal intakes of the eleven dietary factors were observed in each country.
Data sources
Dietary intake data by country, age and sex
We included eleven commonly consumed dietary factors for which there is plausible or convincing evidence of a causal relationship with CMD including CHD, stroke and type 2 DM(Reference Micha, Kalantarian and Wirojratana15,Reference Micha, Shulkin and Peñalvo16) . Protective dietary factors in this analysis included fruits, vegetables and legumes, whole grains, nuts and seeds, seafood n-3 fatty acids and polyunsaturated fatty acids (PUFA) as a replacement for saturated fatty acids (SFA), while unhealthful dietary factors included trans fats, processed meat, unprocessed red meat, SSB and Na. Table 1 characterises the eleven dietary factors, optimal exposure distributions and relevant disease outcomes included in this analysis. All dietary factors were adjusted to 8368 kJ/d(Reference Khatibzadeh, Saheb Kashaf and Micha19).
Table 1 Dietary factors, optimal intake levels, disease outcomes and aetiologic effects: inputs for comparative risk assessment model for Latin America and the Caribbean*

RR, relative risks; DM, diabetes mellitus; SBP, systolic blood pressure.
* This table has been adapted from references (Reference Wang, fshin and Yakoob12), (Reference Afshin, Micha and Khatibzadeh13), (Reference Micha, Shulkin and Peñalvo16) and (Reference Colchero, Popkin and Rivera49). The countries included in the analysis were Argentina, Antigua and Barbuda, The Bahamas, Belize, Bolivia, Brazil, Barbados, Chile, Colombia, Costa Rica, Cuba, Dominica, Dominican Republic, Ecuador, Grenada, Guatemala, Guyana, Honduras, Haiti, Jamaica, Saint Lucia, Mexico, Nicaragua, Panama, Peru, Paraguay, El Salvador, Suriname, Trinidad and Tobago, Uruguay, Saint Vincent and the Grenadines and Venezuela.
† For dietary factor optimal distributions, an sd of 10 % of the mean was utilised.
‡ Cardiometabolic diseases with convincing or probable evidence of an aetiologic association with dietary factors of interest(Reference Afshin, Micha and Khatibzadeh13).
‖ BMI was not studied as an outcome, but BMI-mediated effects were, such as on CHD, ischaemic stroke and diabetes.
§ RR were estimated from meta-analyses of randomised trials and large prospective cohort studies, including assessment of causality based on Bradford-Hill criteria, quantification of dose–response relationships, effect modification by age and assessment of external validity based on similar data from trials(Reference Micha, Shulkin and Peñalvo16).
Mean and standard deviation of dietary intakes were obtained from the Global Dietary Database (GDD) Consortium. The GDD identified national surveys or, if unavailable, subnational surveys on dietary factors from countries around the world through systematic searches of literature databases and direct contact with experts worldwide, as described elsewhere(Reference Khatibzadeh, Saheb Kashaf and Micha19,Reference Del Gobbo, Khatibzadeh and Imamura25) . Additionally, GDD retrieved, assessed and extracted annual country-level data on availability of key food items in each of the 187 countries from the United Nations FAO between 1990 and 2010(Reference Khatibzadeh, Saheb Kashaf and Micha19). To combine individual-level intake data with country-level food availability data, to harmonise different survey sampling and diet assessment methods and to capture the uncertainty in estimates of dietary intakes from measurement error, sampling uncertainty and modelling uncertainty, an age-integrating Bayesian hierarchical statistical model was employed(Reference Wang, fshin and Yakoob12,Reference Afshin, Micha and Khatibzadeh13) . Details on data collection, data representativeness and modelling methods have been described in detail elsewhere(Reference Micha, Shulkin and Peñalvo16,Reference Micha, Khatibzadeh and Shi18–Reference Afshin, Micha and Khatibzadeh20,Reference Micha, Khatibzadeh and Shi22,Reference Singh, Micha and Khatibzadeh24,Reference Del Gobbo, Khatibzadeh and Imamura25) .
Aetiologic effects of dietary exposures on cardiometabolic diseases mortality
To estimate the impact of suboptimal intake of eleven dietary factors on CMD burdens, we used published data on the dose–response aetiologic effects of dietary factors on CMD outcomes(Reference Micha, Shulkin and Peñalvo16) derived from recently published meta-analyses of randomised controlled trials or prospective cohort studies. Diet–disease pairs were assessed for probable or convincing evidence of causality based on Bradford Hill Criteria. Dose–response relationships between diet and disease were quantified, and age-specific relative risks were estimated. Validity of resulting estimates was confirmed through comparison of effect estimates with those from randomised controlled trials(Reference Micha, Shulkin and Peñalvo16,Reference Singh, Danaei and Farzadfar26) .
Optimal distribution of dietary factors
Optimal dietary intake distributions were obtained from GDD and were determined from observed dietary intake levels associated with the lowest rates of disease in published meta-analyses and found in existing global populations(Reference Micha, Kalantarian and Wirojratana15,Reference Micha, Khatibzadeh and Shi18,Reference Micha, Khatibzadeh and Shi22,Reference Singh, Micha and Khatibzadeh24) .
Cause-specific mortality by country, age and sex
Cause-specific deaths by age and sex for thirty-two countries in LAC in 1990 and 2010 were obtained from Global Burden of Diseases (GBD), Risk Factors and Injuries 2010 mortality estimates(Reference Lozano27). Outcomes included in the current analyses were IHD (ICD-10 codes I20-I25), ischaemic stroke (I63, I65–I67, I69.3), haemorrhagic stroke (I60-62, I69.0-2) and type 2 DM (E10–E14).
Statistical analysis
To estimate mortality attributable to each dietary factor, we computed the population impact fraction (PIF), which estimates the proportional reduction in mortality from each disease outcome that would occur if the usual intake distribution was reduced (for unhealthful dietary factors) or increased (for protective dietary factors) to an optimal distribution empirically known to minimise risk, also known as the theoretical minimum-risk exposure distribution(Reference Micha, Kalantarian and Wirojratana15). A standard comparative risk assessment approach was used to compute population impact fraction, using the equation below(Reference Murray, Ezzati and Lopez28):
 $$\displaylines{
{\rm{Population}}\;{\rm{impact}}\;{\rm{fraction}} = \cr
{{\int_{x = 0}^m R R\left( x \right)P\left( x \right)dx - \;\int_{x = 0}^m R R\left( x \right)P'\left( x \right)dx,} \over {\int_{x = 0}^m R R\left( x \right)P\left( x \right)dx,}} \cr} $$
$$\displaylines{
{\rm{Population}}\;{\rm{impact}}\;{\rm{fraction}} = \cr
{{\int_{x = 0}^m R R\left( x \right)P\left( x \right)dx - \;\int_{x = 0}^m R R\left( x \right)P'\left( x \right)dx,} \over {\int_{x = 0}^m R R\left( x \right)P\left( x \right)dx,}} \cr} $$
where x is the current risk factor level, P(x) is the actual distribution of risk factor in the population, P′ (x) is the optimal level of risk factor distribution in the population, RR(x) is the relative risk of cause-specific mortality at risk factor level x and m is the maximum risk factor level. We calculated the number of disease-specific deaths attributable to each dietary factor by multiplying the estimated population impact fraction with total disease-specific mortality (CHD, stroke and DM). For Na and SSB, we included effects mediated through systolic blood pressure and BMI, respectively. To compute total attributable mortality due to an individual dietary factor, we summed the deaths attributable to that dietary factor across different disease outcomes. We further estimated mortality burdens related to overall suboptimal diet by computing the multiplicative interaction among population attributable fractions for all dietary risk factors included in the analysis, assuming independence of each risk factor(Reference Otto, Afshin and Micha9,Reference Yakoob, Micha and Khatibzadeh14) :
where r is the individual dietary risk factor and R is the number of risk factors.
All analyses were conducted across twelve age- and sex-specific strata (female and male ages 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79 and ≥80 years) within each country in LAC for 1990 and 2010. Due to joint distributions and shared pathways through interaction or mediation, attributable deaths cannot be summed across dietary factors(Reference Otto, Afshin and Micha9).
Estimation of uncertainty and sensitivity analyses
We used Monte Carlo simulation to propagate uncertainty from each data input into the final burden estimates. We drew 1000 observations from the gamma distribution of each dietary exposure, the log-normal distribution of disease-specific relative risks, the distribution of optimal intake levels and the normal distribution of cause-specific mortality and input them in the comparative risk assessment framework to generate 1000 mortality estimates for each country–age–sex group and dietary factor, from which we report the mean and 95 % uncertainty interval (UI) based on the 2·5th and 97·5th percentiles of the resulting distribution of attributable deaths(Reference Otto, Afshin and Micha9). In addition, we performed a sensitivity analysis using the optimal level of 1·0 g/d for dietary Na to assess impact on resulting disease burdens. All analyses were conducted using R version 3.3.2.
Results
In 2010, a total of 953 377 CMD deaths occurred among adults in countries in LAC, including 465 406 (48·8 %) from CHD, 154 622 (16·2 %) from haemorrhagic stroke, 166 411 (17·4 %) from ischaemic stroke and 166 938 (17·5 %) from DM. Men in LAC had more CMD-related deaths (total n 481 094; 52·2 % CHD, 16 % haemorrhagic stroke, 16·3 % ischaemic stroke and 15·5 % DM) than women (total n 472 283; 45·4 % CHD, 16·4 % haemorrhagic stroke, 18·6 % ischaemic stroke and 19·6 % DM). Approximately 48 % of total CMD (453 000 deaths) occurred prematurely, below age 70 years (Table 2).
Table 2 Cardiometabolic deaths attributable to dietary factors in countries in Latin America and the Caribbean (2010)*

CMD, cardiometabolic disease; UI, uncertainty interval.
* The Latin America and the Caribbean region includes thirty-two countries: Argentina, Antigua and Barbuda, The Bahamas, Belize, Bolivia, Brazil, Barbados, Chile, Colombia, Costa Rica, Cuba, Dominica, Dominican Republic, Ecuador, Grenada, Guatemala, Guyana, Honduras, Haiti, Jamaica, Saint Lucia, Mexico, Nicaragua, Panama, Peru, Paraguay, El Salvador, Suriname, Trinidad and Tobago, Uruguay, Saint Vincent and the Grenadines and Venezuela.
† Cardiometabolic deaths include the following outcomes: CHD (ICD-10 codes I20-I25), ischaemic stroke (I63, I65-I67, I69.3), haemorrhagic/other non-ischaemic stroke (I60–62, I69.0–2) and diabetes mellitus (E10–E14).
‡ To calculate the UI for percentage CMD mortality, we generated 1000 mortality estimates for each age–sex group and dietary factor, divided by total mortality for each outcome, and report the 95 % UI based on the 2·5th and 97·5th percentiles of the resulting distribution of percentages.
§ Following guidelines of the US Institute of Medicine and to be consistent with prior studies evaluating regional CMD burdens, we used the level of 2000 mg/d.
‖ Mediated effect through blood pressure.
¶ PUFA as a replacement of SFA.
** Mediated effect through BMI.
†† Calculated based on computing the combined population impact fraction of individual dietary factors assuming that the contribution of each component is multiplicative and independent.
Consumption distributions of eleven dietary factors in Latin America and the Caribbean
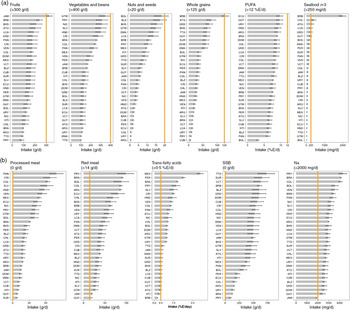
Between 1990 and 2010, intakes of protective dietary factors increased across the five sub-regions of LAC (see online supplementary material, Supplemental Figs. S1–S5). However, in 2010, almost all countries in LAC still had suboptimal intakes of these factors, except for fruits, whole grains and n-3, for which Jamaica, Barbados and Chile had intakes at optimal levels (Fig. 1(a)). The lowest intakes of protective dietary factors were in Paraguay (fruits 83·8 ± 104·6 g/d), Trinidad and Tobago (vegetables 100·4 ± 101·7 g/d), Argentina and Uruguay (nuts 0·6 g/d), Cuba (whole grains 6·3 ± 44 g/d) and Bolivia (PUFA 3·1 ± 1·5 %E/d and seafood n-3 fatty acids 15·6 ± 99·2 mg/d). Women in LAC had higher intakes than men of fruit, vegetables and nuts with an ascending trend by age. Consumption of other protective dietary factors was similar among men and women across age groups (see online supplementary material, Supplemental Figs. S6–S11).

Fig. 1 National distribution of dietary factors in thirty-two countries in Latin America and the Caribbean in 2010. Optimal levels of intake are indicated by numbers in parentheses and the solid orange line; (a) distribution of protective dietary factors and (b) distribution of unhealthful dietary factors. ARG, Argentina; ATG, Antigua and Barbuda; BHS, The Bahamas; BLZ, Belize; BOL, Bolivia; BRA, Brazil; BRB, Barbados; CHL, Chile; COL, Colombia; CRI, Costa Rica; CUB, Cuba; DMA, Dominica; DOM, Dominican Republic; ECU, Ecuador; GRD, Grenada; GTM, Guatemala; GUY, Guyana; HND, Honduras; HTI, Haiti; JAM, Jamaica; LCA, Saint Lucia; MEX, Mexico; NIC, Nicaragua; PAN, Panama; PER, Peru; PRY, Paraguay; SLV, El Salvador; SUR, Suriname; TTO, Trinidad and Tobago; URY, Uruguay; VCT, Saint Vincent and the Grenadines and VEN, Venezuela. SSB, sugar-sweetened beverages
Among unhealthful dietary factors, changes in intake between 1990 and 2010 were less consistent. For example, in Southern Latin America, the intake of red meat decreased by 18 %, while the intake of SSB increased by 7·5 % over two decades (see online supplementary material, Supplemental Figs. S1–S5). Furthermore, men had a consistently higher consumption of unhealthful dietary factors than women, with the exception of SSB (see online supplementary material, Supplemental Figs. S12–S16). Intakes of processed meat and SSB were inversely related to age among both men and women. In 2010, all countries in the region had suboptimal intakes of unhealthful dietary factors (Fig. 1(b)). The highest intakes were observed in Panama (processed meat 66·1 ± 62·4 g/d), Paraguay (red meat 98·4 ± 18·8 g/d and Na 4·3 ± 1·6 g/d), Mexico (trans fat 3·6 ± 1·6 %E/d) and Trinidad and Tobago (SSB 727 ± 583 g/d). Overall, no single country showed consistently optimal levels of consumption across all dietary factors included in the current analysis.
Age- and sex-specific cardiometabolic disease mortality attributable to diet in Latin America and the Caribbean
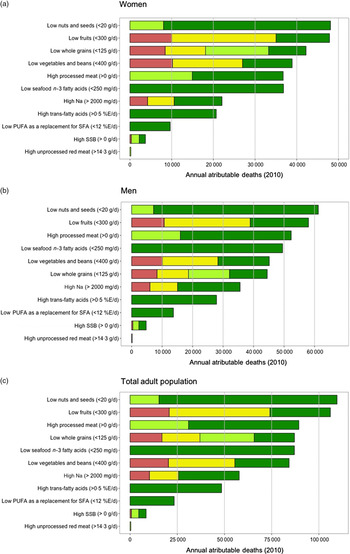
Among women, overall suboptimal diet was responsible for 233 048 (95 % UI 187 459–254 395; 49·3 %) of CMD-related mortality in the region (Table 2). The individual dietary factors among women that resulted in greatest CMD mortality burdens were low intake of nuts and seeds (48 389 (95 % UI 30 572–54 353); 10·2 % deaths), low intake of fruits (47 925 (95 % UI 41 951–52 659); 10·1 % deaths) and low intake of whole grains (42 604 (95 % UI 32 660–46 114); 9 % deaths) (Fig. 2(a) and Table 2). In men, overall suboptimal diet was responsible for 278 143 (95 % UI 226 918–298 631) CMD-related mortality or 57·8 % of all cardiometabolic deaths. The top three dietary factors associated with greatest CMD mortality burdens in men were low intake of nuts and seeds (61 427 (95 % UI 38 690–68 139); 12·8 % deaths), low intake of fruits (58 255 (95 % UI 50 107–62 807); 12·1 % deaths) and high intake of processed meat (52 441 (95 % UI 47 662–57 589); 10·9 % deaths) (Fig. 2(b) and Table 2). Proportional mortality attributable to CMD deaths was higher among men than among women for most dietary factors, ranging from 5·6 to 61·5 % higher for whole grains and Na intake, respectively. Across all dietary factors, adults aged 45–70 years in LAC had higher total CMD mortality attributable to dietary factors compared with the youngest (25–44 years) and oldest (>70 years) age groups; however, proportional mortality for CMD deaths attributable to diet was higher among younger adults (25–44 years) for all dietary factors, with the exception of Na (Table 2). Overall in LAC, deaths attributable to excess Na intake included the highest proportion of premature CMD deaths (89 % of all Na attributable deaths), and deaths attributable to suboptimal vegetable intake included the lowest proportion of premature CMD deaths (48 %) (see online supplementary material, Supplemental Fig. S17).
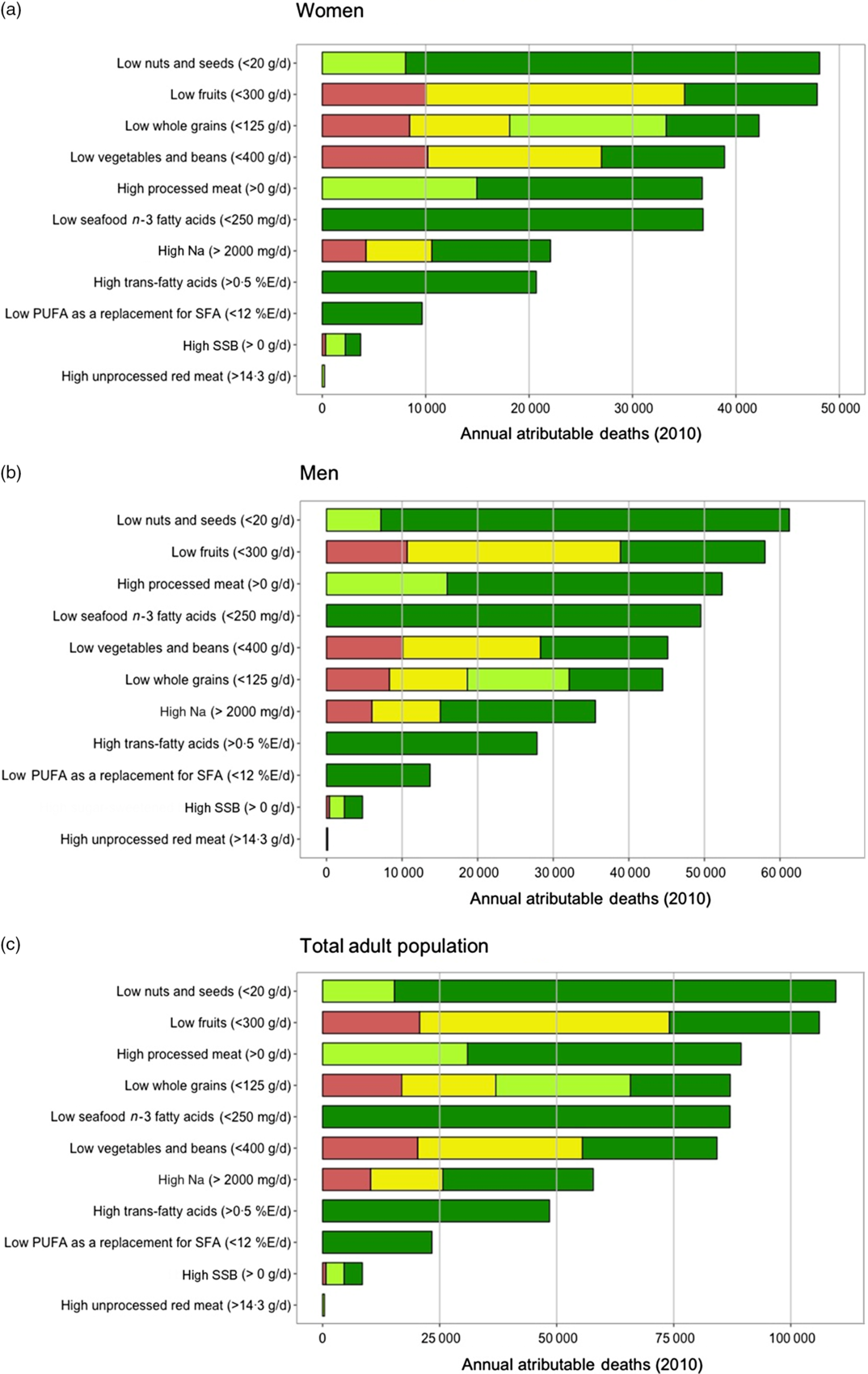
Fig. 2 Cardiometabolic deaths attributable to dietary intakes in 2010, by outcome and sex. Data are from thirty-two countries in LAC and the age of the participants raged from 25 to 80+ years; (a) women, (b) men and (c) total adult population. (a–c) ![]() , CHD;
, CHD; ![]() , diabetes;
, diabetes; ![]() , Haemorrhagic stroke;
, Haemorrhagic stroke; ![]() , ischaemic stroke. SSB, sugar-sweetened beverages
, ischaemic stroke. SSB, sugar-sweetened beverages
Across LAC, evaluation of the joint effects of the eleven dietary factors included in the current analysis on CMD mortality indicates that 513 371 (95 % UI 423 286–547 841) cardiometabolic deaths, or 53·8 % of all CMD-related mortality in LAC is attributable to suboptimal diet (Table 2). Among individual dietary factors, greatest CMD mortality burdens were attributable to low intake of nuts and seeds (109 831 deaths (95 % UI 71 920–121 079)), low intake of fruits (106 285 deaths (95 % UI 94 904–112 320)) and high intake of processed meat (89 381 deaths (95 % UI 82 984–97 196)), accounting for 11·5, 11·1 and 9·4 % of total CMD deaths in LAC, respectively (Fig. 2(c) and Table 2).
Country-specific cardiometabolic disease mortality attributable to diet
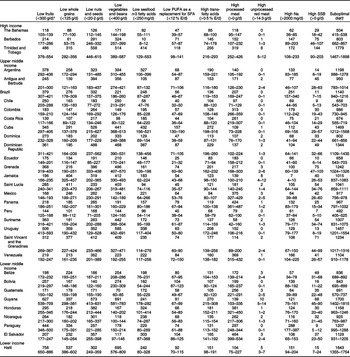
Among LAC countries, the highest absolute CMD burdens in 2010 (deaths/year per million adults) attributable to suboptimal diet were in Trinidad and Tobago (1779 deaths/year per million adults (95 % UI 1467–1898)) and Guyana (1700 deaths/year per million adults (95 % UI 1402–1815)) and the lowest were in Peru (492 deaths/year per million adults (95 % UI 405–525)) and The Bahamas (504 deaths/year per million adults (95 % UI 415–538)) (Table 3). Among protective dietary factors, greatest diet-related CMD death rate attributable to low intake of fruits (758 deaths/year per million adults (95 % UI 650–886)), to whole grains (537 deaths/year per million adults (95 % UI 386–602)) and to vegetables (695 deaths/year per million adults (95 % UI 576–809)) was in Haiti; greatest CMD death rate attributable to low intake of nuts (573 deaths/year per million adults (95 % UI 413–631)) was observed in Guyana; greatest CMD death rates due to low intake of seafood n-3 fatty acids (432 deaths/year per million adults (95 % UI 156–521)) and to low intake of PUFA (157 deaths/year per million adults (95 % UI 130–190)) were observed in Cuba.
Table 3 Cardiometabolic deaths (per year per million adults) attributable to dietary risk factors in thirty-two countries in Latin American and Caribbean (2010)

* Values provided in range indicate optimal levels of intake.
† Based on the estimation of joint population impact fraction of individual dietary factors.
Among unhealthful dietary factors, the greatest CMD death rate due to high intake of trans-fatty acids (272 deaths/year per million adults (95 % UI 198–316)) was observed in Cuba; the greatest CMD death rate due to high intake of processed meat (464 deaths/year per million adults (95 % UI 399–534)) was observed in El Salvador; greatest CMD death rate due to high intake of unprocessed red meat (10 deaths/year per million adults (95 % UI 5–14)) was observed in Guyana; the greatest CMD death rate due to high intake of SSB (144 deaths/year per million adults (95 % UI 90–203)) was observed in Trinidad and Tobago and the greatest CMD death rate attributable to high intake of Na (288 deaths/year per million adults (95 % UI 177–387)) was observed in Paraguay. Guyana and Trinidad and Tobago presented highest rates of overall CMD mortality (see online supplementary material, Supplemental Fig. S18). The lowest diet-related attributable CMD mortality was observed in Peru, which had the lowest CMD death rate attributable to both trans fat and SSB (Table 3 and online supplementary material, Supplemental Fig. S18).
Time trends: total cardiometabolic disease deaths and diet-attributable cardiometabolic disease burdens
The LAC adult population increased by 90 % between 1990 and 2010, from 192 838 511 to 367 264 161; and the total number of CMD deaths concomitantly increased by 53 %, from 624 911 to 953 377. During this period, the CMD death rate per million adults decreased by approximately 20 %. From 1990 to 2010, greatest decline in diet-attributable CMD mortality was observed for fruit (35 %), polyunsaturated fats (30·2 %) and vegetables (29·5 %); smallest declines were observed for unprocessed red meat (8·6 %), while CMD mortality attributable to SSB consumption increased over this time period by 7·2 % (see online supplementary material, Supplemental Figs S19–S20 and Supplemental Tables S1–S3). Further, across the region, diet-related burdens increased between 1990 and 2010 for particular dietary factors in certain countries. CMD deaths attributable to low intake of fruits increased by 83·5 % in Honduras. In Nicaragua, CMD deaths attributable to low intake of nuts, low seafood n-3 consumption and low vegetable consumption increased by 123, 149 and 119 %, respectively. In Paraguay, deaths attributable to PUFA and whole grains increased by 119 and 207 %, respectively. Among unhealthful dietary factors, largest increases in CMD burdens between 1990 and 2010 were observed in Guatemala where deaths attributable to excess consumption of red meat, processed meat and SSB increased by 233, 205 and 604 %, respectively. Largest increases in CMD burdens attributable to trans fat were apparent in the Dominican Republic (118 % increase); and largest increase in CMD burdens attributable to Na was apparent in Jamaica (148 % increase) (see online supplementary material, Supplemental Tables S4–S15).
Sensitivity analysis
Overall, regional CMD mortality attributable to Na in LAC increased by 54 % (62·1 % in women and 48·4 % in men) after lowering the optimal level for Na intake from 2000 to 1000 mg/d. In addition, using the reference level of 1000 mg/d, we found that 89 326 (95 % UI 55 778–118 683) total deaths would be attributable to Na compared with 58 121 (95 % UI 35 806–78 257) attributable deaths at the reference level of 2000 mg/d (see online supplementary material, Supplemental Table S16 and Supplemental Fig. S21).
Discussion
These results highlight the impact of eleven commonly consumed dietary factors on CHD, stroke and DM mortality in thirty-two countries in LAC. Overall, suboptimal diet was related to 53·8 % of all cardiometabolic deaths (n 513 371) in this region. Among the individual dietary risk factors examined, suboptimal intakes of nuts and seeds, fruits and high intake of processed meat resulted in the greatest CMD mortality burdens in LAC in 2010. Men had larger absolute diet-related CMD burdens than women, and younger populations had greater diet-related proportional mortality than older populations. Our investigation provides insights into the heterogeneities in diet-related CMD burdens across countries within the LAC region and emphasises nation-specific dietary priorities for CMD prevention.
The current study advances current understanding of the distribution and impact of dietary factors on CMD-related mortality among LAC countries in several ways. First, we present country-, age- and sex-specific intake distributions and trends among eleven commonly consumed dietary factors in comparison with optimal intake levels. Previous literature on dietary consumption patterns in the region provided limited information on consumption based on percentage contribution of cereals, red meat, trans-fatty acids, fruits and vegetables to dietary energy intake(Reference Bermudez and Tucker29,Reference Barria and Amigo30) . An analysis using country-level intake data from eight Latin American countries reported an overall mean energy intake of 8196·456 kJ/d with more than 25 % of this energy intake from food sources rich in sugar and fat(Reference Kovalskys, Fisberg and Gómez31). In contrast, our current analyses provide detailed data on actual dietary intakes in thirty-two countries by age and sex. Second, our study estimates attributable and proportional mortality for each country–age–sex group and dietary factor for 1990 and 2010, including uncertainty, while prior Global Burden of Disease studies solely allocated ranks to dietary factor impacts on disease burdens and did not provide detailed numeric estimates of such burdens(Reference Lim, Vos and Flaxman32–Reference Stanaway, Afshin and Gakidou35). In spite of some methodological differences with prior Global Burden of Disease studies, including distributional assumptions, mediating effects and theoretical minimum-risk exposure distribution ranges(Reference Lim, Vos and Flaxman32–Reference Stanaway, Afshin and Gakidou35), our findings are largely consistent with results from these prior studies reporting that among protective dietary factors, low intake of fruits, nuts and seeds and whole grains was the leading dietary risk factors for CMD mortality in LAC. Similarly, among unhealthful dietary factors, high consumption of Na was the leading dietary risk factor for CMD mortality. Our work extends and expands upon these prior studies by using the best available evidence for distributions of dietary factors, most recent evidence on aetiologic effects of diet on disease and comparative risk assessment methods tailored to nutritional exposures(Reference Micha, Penalvo and Cudhea36).
These results highlight heterogeneities in dietary priorities between world regions. In comparison with diet-related burdens in South Asia and North Africa/Middle East(Reference Afshin, Micha and Khatibzadeh13,Reference Yakoob, Micha and Khatibzadeh14) where low consumption of fruit and whole grains was the leading causes of CMD mortality; in the LAC region, largest diet-related burdens were from low intake of nuts/seeds and fruits and high intake of processed meat. The burden of cardiometabolic disease attributable to low intakes of protective dietary factors in LAC was similar to countries such as Australia and Ethiopia, where diets low in fruits, vegetables, nuts and seeds and whole grains contributed the most to non-communicable chronic diseases mortality(Reference Melaku, Renzaho and Gill37,Reference Melaku, Wassie and Gill38) . Such between-region heterogeneities in dietary-related CMD burdens could be related to differences in cultural preferences, affordability and accessibility of foods, among other factors.
Low consumption of nuts and seeds in the LAC could be driven by cultural preferences in dietary habits and lower availability(Reference Micha, Khatibzadeh and Shi22,39) . In 2016, LAC produced only 5 % (approximately 200 000 metric tonnes) of the worldwide net total of nuts and consumed only 2 % of available nuts compared with other regions(39). A study conducted in six Latin American countries found that the low intake of fruits could be driven by four factors: a preference for fast food (sweet and salty fatty snacks), the perception that fruits do not satisfy hunger, the lack of preference for fruit in daily dietary customs and cost(Reference Olavarría and Zacarías40).
High consumption of processed meat in some LAC countries, such as Panama, Costa Rica and Colombia, could be related to economic development in the region over the past years, which increased population purchasing power and accessibility of foods of animal origin(Reference de Carvalho, Cesar and Fisberg41); the optimisation of local food-production capacities that has increased the availability of low-priced processed meats; and marketing/advertising strategies used by the food industry to motivate the consumption of processed meat(Reference Uauy and Monteiro42).
Low intake of seafood n-3 fatty acids across the region could be related to industrialisation of commercial fishing for export instead of local consumption. Further, traditional dietary habits related to seafood have diminished in part due to the ready availability of cheap beef products and the expense of fish products, especially in lower income areas of the region(43). In addition, rural inland areas face the challenge of inadequate preservation methods and under-developed fish markets. These and other factors have influenced consumption patterns among the population towards readily available products, for instance, preserved sardines and tuna fish, especially among the poorer segments of the population in countries throughout LAC(43,44) .
Currently, countries in LAC are experiencing key challenges associated with the nutrition transition, in particular, the double burden of disease(Reference Rivera, Barquera and González-Cossío45,Reference Popkin46) . The spectrum of diet-related ill-health in LAC varies from populations challenged primarily with stunting, underweight and micronutrient deficiencies, to those experiencing the double burden of undernutrition and nutrition-related noncommunicable diseases, to those mainly faced with chronic disease(Reference Rivera, Barquera and González-Cossío45,Reference Tirado, Galicia and Husby47) . Presently, many LAC countries have ongoing programmes and public policies to promote healthy diet among their populations(Reference Tirado, Galicia and Husby47). A comprehensive study identified 204 interventions across the region, 53 % in South America, 35 % in Central America and 12 % in the Caribbean. Commonly implemented interventions involved media and public health campaigns to promote use of nutritious foods, incentivisation of family farming to reduce food insecurity and food labelling. Such strategies have been promoted and implemented through community education, local and national media and conditional cash transfer programmes. Less-commonly implemented interventions focused on valorisation of traditional culinary culture in the Caribbean countries(48). Examples of key national policies in specific countries include taxation of SSB (approximately 10 % cost increase) and nonessential highly energy-dense food (approximately 8 % cost increase) in Mexico(Reference Colchero, Popkin and Rivera49), front-of-package labelling warning foods high in sugar, salt, energy content and fat (Ecuador, Mexico and Chile)(Reference Tirado, Galicia and Husby47,Reference Kanter, Vanderlee and Vandevijvere50) , and bans on advertising unhealthy foods and beverages to children and adolescents (Peru, Uruguay, Bolivia, Brazil, Chile, Colombia, Ecuador and Mexico)(Reference Tirado, Galicia and Husby47,Reference Colon-Ramos, Monge-Rojas and Campos51) . Furthermore, Bolivia, Chile, Colombia, Costa Rica, Mexico, Brazil, Ecuador and Argentina have national plans to reduce the amount of salt/Na and trans-fatty acids contained in their foods(Reference Tirado, Galicia and Husby47,Reference Colon-Ramos, Monge-Rojas and Campos51,Reference Monge-Rojas, Colon-Ramos and Jacoby52) .
Thus far, successful examples of diet-related public policy in LAC come from Mexico, Argentina and Chile(Reference Monge-Rojas, Colon-Ramos and Jacoby52–Reference Boza54). After the implementation of a tax on SSB purchases of 1 peso/l in 2014, Mexico achieved a 6·3 % reduction in purchases of SSB and 16·2 % increase in water purchases. The magnitude of these changes was greater in lower income and urban households(Reference Colchero, Popkin and Rivera49). In 2006, Argentina implemented mandatory labelling of artificial trans fatty acids (TFA) in food with the aim that industrially produced TFA in food should not exceed 2 % of total fats in vegetable oils and margarines by the end of 2014(Reference Rubinstein, Elorriaga and Garay55). A recent study found that foods in Buenos Aires showed a significant decrease in the content of TFA from 12·6 to 34·8 % range in 2011–2012 to nearly 0 % in 2015–2016(Reference Monge-Rojas, Colon-Ramos and Jacoby52). In Chile, a 2016 policy included mandatory warning labels on unhealthy foods (high in salt, sugar, fat and energy) with restrictions on unhealthy food marketing (prohibited from using any licenced or brand character, toy or child-targeted imagery)(56). Preliminary results showed that out of a total 8000 products in the country, 1550 have been reformulated to reduce at least one critical nutrient, indicating that the local food industry has modified one of every five products to make it healthier(Reference Boza54).
Our investigation has several strengths. To our knowledge, this is the first study to report detailed country-, age- and sex-specific changes in diet over time and to asses diet-attributable CMD-related mortality in countries in LAC. We used comprehensive and consistent data on risk factor distributions, aetiologic effects and cause-specific deaths, by age, sex and time such that results are comparable across countries and world regions. The current work utilises the best available effect sizes of diet–disease relationships, models dietary data using gamma distributions to take into consideration skewness(Reference Micha, Penalvo and Cudhea36), incorporates and quantifies uncertainty using Monte Carlo simulation and includes sensitivity analyses to test alternative optimal intake levels of Na.
Limitations of the current work must also be considered. Uncertainty exists in each of the data inputs into the comparative risk assessment framework. To account for this uncertainty in our final estimates, we utilised a simulation approach to propagate uncertainty from each data input through the comparative risk assessment framework and into the final burden estimates; however, some sources of uncertainty in source data may remain unaccounted for. Despite the use of plausible and convincing aetiologic effects adjusted for major confounders, the possibility of residual confounding (typically leading to overestimation of effects) and measurement error (typically leading to underestimation of effects) cannot be excluded. In addition, attributable mortality reported here may be underestimated due to errors or underreporting in national cause-of-death data sets(Reference Micha, Penalvo and Cudhea36). Our estimates do not include children, and hence underestimates the true overall population-level effects of suboptimal diet; current efforts include collection of data on children, which will facilitate future assessment of dietary impacts in child and adolescent populations. Estimates of changes in diet-related burdens over time could partially be due to changes in the demographic structure of LAC from 1990 to 2010, and future work should update these findings with more recent data. Our findings estimate the impact of individual dietary factors and do not account for synergistic effects dietary components. Methods used to estimate overall CMD mortality burdens due to multiple dietary factors rely on the assumption of independence between risk factors and may therefore overestimate the overall disease burden. Novel methods are needed in the future to model joint distributions of multiple correlated risk factors(Reference Yakoob, Micha and Khatibzadeh14,Reference Micha, Penalvo and Cudhea36) .
Public health implications
Our study has important implications for key components of the 25 × 25 targets of the WHO for the region, including reducing CVD, diabetes and obesity burdens, reducing population Na intake and reducing population blood pressure, each of which is heavily impacted by diet(57). In light of our results, at the regional and national level, policies and interventions need to be developed or strengthened to improve the availability, affordability and acceptability of healthier foods including nuts and seeds, seafood n-3 fatty acid, fruits and vegetables to reduce population consumption of processed meat, Na, trans-fatty acids and sugars in LAC. In addition, many LAC countries could benefit from strengthening policies to reduce the availability or encourage reformulation of ultra-processed food products, as these are major sources of Na, added sugars and refined carbohydrates(Reference Tirado, Galicia and Husby47,58) .
Further work is necessary to update these burden estimates and evaluate recent time trends in consumption of the eleven dietary factors investigated here; the GDD Consortium is currently collecting and updating new data for this region. Currently, policy evaluations, longitudinal food retail studies, impacts of food price on diet and effects of digital marketing on diet/health are largely absent across the region(Reference Pérez-Ferrer, Auchincloss and Carvalho de Menezes59). Thus, further analyses and efforts are necessary to fill these gaps in knowledge in order to reduce CMD burdens in LAC(Reference Pérez-Ferrer, Auchincloss and Carvalho de Menezes59,Reference Llanos, Oyarzun and Bonvecchio60) .
In conclusion, we estimated CMD mortality attributable to eleven important and commonly consumed dietary factors in thirty-two LAC countries. These results highlight, at the national and regional levels, patterns and heterogeneities in consumption of and disease burdens from key dietary factors by age, sex and time, underscoring relevant dietary priorities for sustainable, scalable and cost-effective population-level policies and interventions to effectively reduce CMD disease burdens in LAC.
Acknowledgements
Acknowledgements: The authors would like to thank to Rajaram Lakshminarayan and Robert Goldberg for their comments and suggestions, which led to an improvement in the manuscript. Financial support: I.S. was supported by the Higher Education, Science, Technology and Innovation of the government of Ecuador and Universidad San Francisco de Quito. G.M.S. was supported by a grant from the National Heart Lung and Blood Institute (R00HL124321). R.B. was supported by the National Center for Complementary and Integrative Health (K23AT009374) of the National Institutes of Health. R.R. was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award No. UL1TR001064. The contents of the current manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institues of Health (NIH). Conflict of interest: There are no conflicts of interest. Authorship: I.S. conducted, analysed and wrote the manuscript. E.A.-G., R.M.F., M.D.J., G.L.M., R.S. and R.R.B. assisted in the manuscript writing. F.G. and R.R. assisted in the analysis and writing of the manuscript. D.M. and G.M.S. formulated the research question, designed the study and assisted in the manuscript writing. Ethics of human subject participation: No research involving human participants.
Supplementary material
For supplementary material accompanying this, please visit https://doi.org/10.1017/S1368980020000646