Food insecurity is lack of access at all times to enough food for an active, healthy life( Reference Coleman-Jensen, Rabbitt and Gregory 1 ). Between 10 and 15 % of the US population experiences food insecurity each year( Reference Coleman-Jensen, Rabbitt and Gregory 1 ). Many food-insecure persons are at or below the poverty line, measured as poverty-income ratio by the US Bureau of the Census. Certain characteristics of food-insecure persons reflect the symptoms of Cu toxicity: depression, anaemia, mental agitation, restlessness, anxiety, insomnia, irritability and CVD( Reference Wilson 2 – Reference Potera 4 ).
Due to coping strategies that result from food insecurity, food habits may be altered for food sources that could be detrimental to health( Reference Fram, Ritchie and Rosen 5 ). Food-insecure persons most frequently consume processed foods such as canned fruits and vegetables, sauce, fruit juice, canned meats and processed cereals( 6 ). They may also consume heavy metal-rich water sources( Reference Sharma, Simran and Nagpal 7 ). Even though specific sources of Cu exposure in food-insecure persons are hard to ascertain, people become exposed to Cu through corroded hot-water Cu pipes, Cu cookware, canned foods, Cu-based fungicides, e-cigarettes, Cu intra-uterine devices, oral contraceptive use, Cu jewellery, dental prosthesis, brewed beverages and drinking-water( Reference Ashish, Neeti and Himanshu 3 , Reference Potera 4 , 8 – Reference Berg, Kohlmeier and Brenner 10 ). Cu competes for absorption and interferes with the metabolism of other microminerals such as Cr, Co, Fe, Mg, Mn, Se and Zn( Reference Wilson 2 , Reference Ashish, Neeti and Himanshu 3 ). Low intake of these microminerals thus results in increased Cu absorption due to lack of competitive inhibition( Reference Wilson 2 , Reference Ashish, Neeti and Himanshu 3 ).
The effects of Cu overload tend to show in the brain, liver, nervous system, adrenal glands, reproductive system and erythrocytes( Reference Wilson 2 – Reference Potera 4 ). Other effects of Cu excess include overproduction of adrenaline, noradrenaline, dopamine and oestrogen, and a decrease in histamine production. The consequences of the high levels are mood swings, depression, mental agitation, overstimulation, restlessness, anxiety, insomnia, irritability, racing mind, growth and developmental delays, attention deficit disorder, autism and related brain disorders( Reference Wilson 2 – Reference Potera 4 ). Preference for sweet foods and salty foods are reported characteristics of food-insecure persons, also seen in people with Cu overload( Reference Wilson 2 , Reference Hampton 11 , Reference Drewnowski and Specter 12 ).
Cu overload in women has been associated with depression and ovarian syndromes including endometrial cysts, carcinoma, low sex drive and premenstrual syndrome( Reference Crayton and Walsh 13 , Reference Marinov, Tsachev and Doganov 14 ). One effect of high blood Cu level in women is an enormous rise in oestrogen level, a potent carcinogen( Reference Wilson 2 , Reference Ashish, Neeti and Himanshu 3 ). In men, among the symptoms of high blood Cu level are prostate infection and enlargement, prostate cancer, erectile dysfunction, depression, testicular pain, testicular cancer, and in some cases violent behaviour( Reference Wilson 2 , Reference Ashish, Neeti and Himanshu 3 ). From the above, it seems clamant to examine Cu levels in population sectors that could experience greater Cu exposure. The current study examined serum Cu to ascertain any associations with adult household food security status.
Methods
Source of data and study sample
Data from the phlebotomy sample of the 2011–2014 waves of the US National Health and Nutrition Examination Survey (NHANES) were analysed for the present study. In the NHANES, participants are selected through a complex, multistage, probability cluster sampling to obtain a representative sample of the non-institutionalized US civilian population( 15 ). In the present study, participants’ serum Cu data were merged with their household food security and demographic data to obtain a single composite data set( 16 ). The final sample comprised 2780 participants: 1360 men and 1420 women. Participants were included after satisfying the following inclusion criteria: had data on household food security status, serum Cu, gender and age. Those aged 18–65 years were included in the study to preclude any possible influence of ageing on serum mineral concentration.
Participants in the NHANES 2011–2014 were grouped based on their food security status (food secure, marginal food security, low food security, very low food security). To improve sample size, those whose food security status was low and very low were combined into one category: food insecure. Participants were categorized as food secure if they had no reported indications of food-access problems or limitations, whereas those categorized as having marginal food security had one or two reported indications, typically of anxiety over food sufficiency or anxiety over food shortage in the household, but had little or no indication of adverse changes in diets or food intake. Those who were categorized as low food security reported reduced quality, variety or desirability of diet, although most often had little or no indication of reduced food intake. If a participant was in the very low food security category, then there were reports of multiple indications of disrupted eating patterns and decreased food intake( Reference Coleman-Jensen, Rabbitt and Gregory 1 ). Therefore, those categorized as food insecure had reduced food quality, variety, desirability, intake, and disrupted eating pattern. Most of the food insecure in the present study received governmental food assistance in the form of food stamps.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Review Board of the National Center for Health Statistics, Centers for Disease Control and Prevention of the US Department of Health and Human Services( 15 ). Written informed consent was obtained from all subjects. Like all NHANES data released for public use, all the data for the present study had been de-identified.
Serum copper level and copper status
During the NHANES, serum total Cu concentration (serum Cu) was determined using inductively coupled plasma–dynamic reaction cell–mass spectroscopy (ICP-DRC-MS). The capabilities of ICP-DRC-MS include a multilevel analytical capacity for trace-level elemental analysis( 17 ). The laboratory method used for the serum Cu analysis is publicly available( 18 ). Participants’ serum Cu data were obtained from the NHANES 2011–2014 MEC (mobile examination centre) laboratory data files( 16 ). The normal serum Cu range is reported as 63·70–140·12 µg/dl( Reference Sloan, Feyssa and Staros 19 , Reference Murray, Jacob, Varghese, Bender and Botham 20 ). Participants’ serum Cu status and risk of toxicity were defined as serum Cu greater than 140·12 µg/dl, the upper value of this normal range( Reference Sloan, Feyssa and Staros 19 , Reference Murray, Jacob, Varghese, Bender and Botham 20 ). Other biomarkers such as measurement of serum ceruloplasmin protein and activity, free serum Cu, serum total Cu, liver Cu, hair Cu, plasma malondialdehyde, benzylamine oxidase activity, erythrocyte superoxide dismutase activity and 24 h urinary Cu excretion have been utilized by other researchers for Cu status assessment( Reference Turnlund, Jacob and Keen 9 , Reference Sloan, Feyssa and Staros 19 , Reference Murray, Jacob, Varghese, Bender and Botham 20 ). In the current study, serum total Cu was used due to easy assessment, better relationship to recent dietary intake and high enough levels for a robust comparison between groups.
Data analysis
To account for NHANES’ MEC complex probability sampling design and to apply the MEC sampling weights, the statistical software package Stata version 14.0 was used to estimate all descriptive and inferential statistics( 21 ). In all analyses, because NHANES 2011–2014 encompasses two surveys, 2011–2012 and 2013–2014, the MEC sample weights were halved and applied( 21 ). The serum Cu data were adequately normally distributed to enable the calculation of mean values for each food security category and to include Cu as a continuous variable in the multivariable regression analyses.
To substantiate any associations between serum Cu and food security status we applied a two-pronged approach: (i) using a clinical cut-off for normal serum Cu; and (ii) using percentile ranking. In the first approach, we used a clinical cut-off for normal serum Cu to determine risk of Cu toxicity. Gender-stratified logistic regression models were used to estimate the likelihood (adjusted OR) to have serum Cu greater than the upper value (140·12 µg/dl) of the normal range and thus risk of Cu toxicity in the marginal food security and food insecure categories, using the food secure category as the referent( Reference Sloan, Feyssa and Staros 19 , Reference Murray, Jacob, Varghese, Bender and Botham 20 ). In the second approach, percentile ranking was used to group participants based on their serum Cu, notably ≤25th, 50th and ≥75th centiles stratified by gender. Proportions of participants in the marginal food security and food insecure categories who had serum Cu in the fourth quartile (≥75th centile or greater), and thus had high serum Cu, were compared with their counterparts in the food secure category by applying logistic regression models. These two approaches were used to present robust evidence for any association between food security status and risk of Cu toxicity for the following reasons: (i) because of the higher serum Cu levels in women, most of whom had serum Cu in the upper range of the clinical cut-off; and (ii) due to scarcity of a well-defined serum Cu clinical cut-off and the wide range of the existing serum Cu clinical cut-off.
Statistically significant differences in serum Cu between the food secure and the other food security categories were tested using the native t test component of the multiple regression models. The test for statistically significant differences in the proportions of participants in each food security category was done using Pearson’s χ 2 test of independence. During the multiple regression analyses, we controlled for age, BMI, annual household income, level of education, marital status and race/ethnicity because these variables seemed to predict serum Cu level. In all analyses, food-secure men and women were the referent for their counterparts, and statistical significance was tested at P < 0·05.
Results
Table 1 shows the background information on the participants. In summary, the average age of the participants was about 41 years. The median serum Cu for men, 103·5 (95 % CI 100·8, 106·2) μg/dl, was lower than that for women, 132·8 (95 % CI 129·9, 135·6) μg/dl (Table 2). In women, the median serum Cu was 23·8 (95 % CI 21·6, 26·0) μg/dl greater than in men (P < 0·001). This translates to about 23·0 % higher serum Cu in women than in men. About 20·5 % of the participants had serum Cu that was above the upper value of the clinical cut-off for normal serum concentration.
Table 1 Demographic characteristics by food security status: nationally representative sample of US adults aged 18–65 years (n 2780; 1360 men and 1420 women), National Health and Nutrition Examination Survey (NHANES) 2011–2014

† The food insecure category comprised participants in the low and very low food security categories.
‡ Other includes those divorced, widowed or never married.
§ PIR, poverty-income ratio; expressed as a ratio of the federal poverty threshold provided by the US Bureau of the Census.
Table 2 Serum total copper concentrations, proportions above normal range and likelihood of having serum copper above the normal range, by food security status and gender, in a nationally representative sample of US adults aged 18–65 years (n 2780; 1360 men and 1420 women), National Health and Nutrition Examination Survey (NHANES) 2011–2014

* Significantly higher than the food secure category, P < 0·05.
† OR have been adjusted for age, BMI, marital status, race/ethnicity, income and educational level. NHANES sampling weights were applied in all analysis.
‡ The food secure category was the referent.
Genders combined, marginal food security, 4·0 (95 % CI 1·29, 6·86) μg/dl (P = 0·004), and food insecurity, 6·4 (95 % CI 3·8, 9·1) μg/dl (P < 0·001), were associated with greater serum Cu compared with their food-secure counterparts. In both men and women, serum Cu appeared to increase as food insecurity worsened (Fig. 1). In the gender-stratified regression analysis, only food-insecure men, 4·7 (95 % CI 2·1, 7·3) μg/dl (P < 0·001), and marginally food-secure women, 4·3 (95 % CI 1·4, 8·5) μg/dl (P = 0·03), associated with significantly greater serum Cu, compared with food-secure counterparts.

Fig. 1 Box-and-whisker plot categorizing serum total copper concentration by food security status (1, food secure; 2, marginal food security; 3, food insecure) and gender in a nationally representative sample of US adults aged 18–65 years (n 2780; 1360 men and 1420 women), National Health and Nutrition Examination Survey (NHANES) 2011–2014. The bottom and top edge of the box represent the first and third quartiles (interquartile range); the line within the box represents the median; and the ends of the bottom and top whiskers represent the minimum and maximum values
In both men and women, significantly greater proportions of food-insecure persons had serum Cu that was greater than the upper value of the normal range compared with their food-secure counterparts (Table 2). Generally, the likelihood of having serum Cu that was above the upper value of the normal range was significantly greater for those in the marginal food security (OR = 1·6, P = 0·028) and food insecurity (OR = 1·55, P = 0·038) categories. Food-insecure men (OR = 1·79, P < 0·001) and marginally food-secure women (OR = 1·82, P = 0·016) had significantly greater likelihood to have serum Cu that was above the upper value of the normal range (Table 2).
Significantly greater proportions of those in the food insecure and marginal food security categories had serum Cu that was above the upper quartile (Fig. 2). Genders combined, food insecurity significantly associated with greater likelihood (OR = 1·65, P = 0·020) to have serum Cu that was in the upper quartile (≥ 75th centile) compared with their food-secure counterparts. Gender-stratified analysis showed food-insecure men (OR = 3·3, P < 0·001) and marginally food-secure women (OR = 1·70, P = 0·028) associated with greater likelihood to have serum Cu that was in the upper quartile compared with their food-secure counterparts.
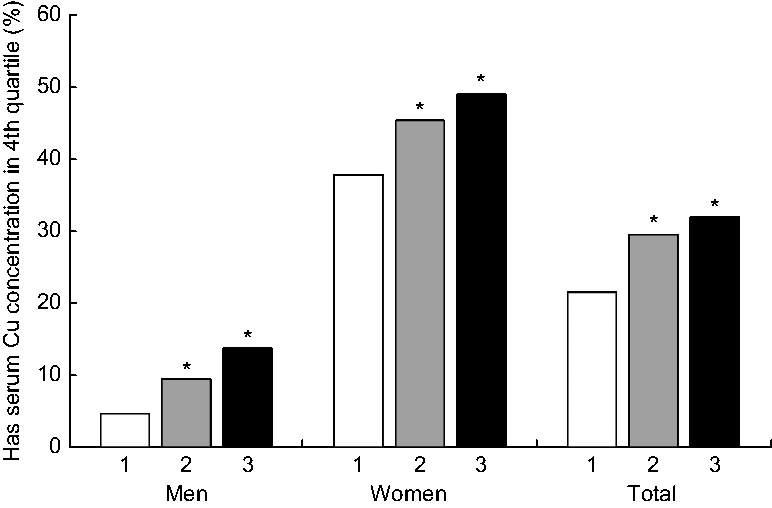
Fig. 2 Proportions of participants having serum total copper concentration in the fourth quartile (≥ 75th centile), by food security status (1, food secure; 2, marginal food security; 3, food insecure) and gender, in a nationally representative sample of US adults aged 18–65 years (n 2780; 1360 men and 1420 women), National Health and Nutrition Examination Survey (NHANES) 2011–2014. NHANES sampling weights were applied. *Significantly higher than the food secure category, P < 0.05
Discussion
The current study presents evidence for significant association between food insecurity and high serum Cu. Irrespective of whether clinical cut-offs or percentile ranking was applied, food-insecure men associated with higher-than-normal serum Cu. Similarly, marginally food-secure men and women associated with higher-than-normal serum Cu. Individuals in this category have less intense issues about food access, mainly anxiety over food sufficiency or anxiety over food shortage in the household, and yet they associated with higher-than-normal serum Cu level( Reference Coleman-Jensen, Rabbitt and Gregory 1 ). Adults who worry about food shortage may adopt coping strategies that could influence serum Cu level either via the intake route or via metabolic changes( Reference Wilson 2 – Reference Potera 4 , Reference Walls, Walls and Benke 22 ). It can thus be inferred that food-insecure persons are at risk of Cu toxicity or of the harmful effects of Cu excess. Even though food insecurity associated with higher-than-normal serum Cu in women, their high serum Cu could have dulled our ability to detect differences because the women referent group also had high serum Cu. However, higher proportions of food-insecure women associated with serum Cu that was higher than the upper quartile of this study population.
Several reasons can be forwarded in our attempt to explain why food-insecure persons may associate with higher than the upper value of the normal serum Cu range. Cu-rich food sources include plant-based ones such as nuts, beans, other seeds and grains( Reference Wilson 2 , Reference Ashish, Neeti and Himanshu 3 ). These are foods that may be dominant in the diets of food-insecure persons. In addition, food-insecure persons may have limited access to meat and other animal products( Reference Rose and Oliveira 23 ). Meat contains highly bioavailable Zn which competes with Cu for absorption and thus may contribute to the lower serum Cu levels in food-secure persons( Reference Wilson 2 ). Insofar as it may be difficult to discern, it would not be far-fetched to reason that food-insecure persons are less likely to drink purified filtered bottled water than food-secure persons. Municipal tap water is one of the good sources of Cu, which may leach from Cu-based pipes or may be added as a disinfectant during the treatment of drinking-water to prevent algae growth( Reference Wilson 2 , 8 , Reference Cristofar and Basiotis 24 ). Food-insecure persons may have low dietary intake of other essential microminerals( Reference Watts 25 , Reference Bremner 26 ), a condition that could promote Cu absorption and retention( Reference Wilson 2 , Reference Ashish, Neeti and Himanshu 3 , Reference Turnlund, Jacob and Keen 9 ). In addition, excess Cu intake can interact unfavourably with the absorption of minerals such as Mo, Zn, Fe, P and K, and the metabolism of vitamins A, B6, C, folic acid and niacin via increased compartmental displacement of these nutrients or increased requirements at the metabolic level( Reference Ashish, Neeti and Himanshu 3 , Reference Pocino, Baute and Malave 27 ).
The high serum Cu in food-insecure persons warrants concern because Cu is among the minerals that progressively bioaccumulate in the human body if excess intake persists( Reference Wilson 2 , Reference Ashish, Neeti and Himanshu 3 , Reference Turnlund, Jacob and Keen 9 ). Serum Cu increases in parallel with dietary intake and, if intake continues, could unfavourably alter Cu nutritional status( Reference Wilson 2 , Reference Ashish, Neeti and Himanshu 3 , Reference Turnlund, Jacob and Keen 9 ). Reported sites of Cu accumulation are the brain, spleen, kidneys, intestines and connective tissues, which are organs reportedly damaged by Cu imbalance( Reference Wilson 2 – Reference Potera 4 , Reference Turnlund, Jacob and Keen 9 , Reference Whitaker, Phillips and Orzol 28 ). Cu imbalance is associated with reversible and in some cases irreversible damage to the gastrointestinal tract, nervous and reproductive systems( Reference Wilson 2 – Reference Potera 4 , Reference Whitaker, Phillips and Orzol 28 ). Of utmost concern is oxidative damage to brain-cell DNA, the liver and kidneys, as well as erythrocytes and membrane lipids which is associated with haemolytic anaemia( Reference Wilson 2 – Reference Potera 4 , Reference Turnlund, Jacob and Keen 9 , Reference Watts 25 , Reference Whitaker, Phillips and Orzol 28 ). A decrease in detoxification ability of the liver and connective tissue breakdown are common in people with high Cu level( Reference Wilson 2 – Reference Potera 4 ). Cu imbalance resulting from excess Cu is associated with nervous system depression and reduction of several components of the immune system, including a decrease in the following: number of neutrophils, lymphocyte proliferation and antigen-specific antibody production( Reference Turnlund, Jacob and Keen 9 , Reference Whitaker, Phillips and Orzol 28 , Reference Wu and Schimmele 29 ). These effects have untoward adverse consequences on the immune system and, together with the other above-noted effects of Cu imbalance, could contribute to the increased risk of mortality among food-insecure persons( Reference Holben 30 ). It is also noteworthy that most of the clinical symptoms of food-insecure persons are similar to those of people who have abnormal Cu level( Reference Wilson 2 – Reference Potera 4 , Reference Whitaker, Phillips and Orzol 28 , Reference Wu and Schimmele 29 , Reference Vozoris and Tarasuk 31 – Reference Vir, Love and Thompson 34 ).
Similar to reports, the observed average serum Cu of women was higher than men’s( Reference Wilson 2 , Reference Ashish, Neeti and Himanshu 3 ). The higher serum Cu in women is another example of a biological confounding among the two genders, although it is alarming to note that the average serum Cu of women observed in the present study is similar to reported levels that were associated with postpartum depression and ovarian syndrome( Reference Crayton and Walsh 13 , Reference Marinov, Tsachev and Doganov 14 ). Studies have used women controls who have serum Cu in the range of 98–116 µg/dl, which is lower than the average observed in the current study( Reference Crayton and Walsh 13 , Reference Marinov, Tsachev and Doganov 14 , Reference Tarasuk 35 ). It can be inferred that, similar to food-insecure persons, women associate with excessive serum Cu.
A limitation of the present study is that, even though smoking is reported to associate with serum Cu, it could not be controlled in the current analysis because the number of smokers in the sample was small and inadequate for partitioning( Reference Turnlund, Jacob and Keen 9 ). Like other cross-sectional studies, a cause-and-effect relationship is difficult to ascertain so statement about causality should be made with caution. We have been able to illuminate an association between food insecurity and serum Cu while applying food security status as the determinant. However, it may be possible that Cu overload contributes to food insecurity due to its ill effects. Either way, there seems to be a strong evidence for an association between food security status and serum Cu. There are some instances in which serum total Cu concentration may not be suitable for Cu status assessment, as in the case of persons affected by congenital metabolic Cu abnormalities such as Menkes syndrome and Wilson disease( Reference Roman, Ross, Caballero and Cousins 36 ). In Menkes syndrome, there is low intestinal Cu absorption, whereas in Wilson disease, there is Cu accumulation in the blood, brain, liver and eyes( Reference Roman, Ross, Caballero and Cousins 36 ). However, the prevalence of such abnormalities in the population is very low and not expected to affect the outcome of the present study. The strengths of the present study include the relatively large sample size and the control of multiple confounders which have enabled reliable estimates of associations and generalizability of findings.
Conclusion
In conclusion, food-insecure persons associate with higher-than-normal serum Cu and are at greater risk of Cu toxicity. There is need to study the sources of Cu in general, and specifically in food-insecure persons, to facilitate reduction of Cu exposure. Examining Cu contents of the food and water supply could explicate any relationships to food insecurity and Cu intake.
Acknowledgements
Acknowledgements: The authors’ sincerest gratitude goes to the US Centers for Disease Control and Prevention, National Center for Health Statistics, Department of Health and Human Services for making the NHANES data sets publicly available. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: The authors declare no conflict of interest. Authorship: F.T. conceptualized the idea, organized the data, performed data analysis, created the figures and wrote the draft of the manuscript. B.X. and M.T. reviewed the conceptual basis, edited and reviewed the tables of results, and contributed to writing and editing of the draft and final versions of the manuscript, A.D. and J.J. did the literature search, created the tables of results, and reviewed the draft and final versions of the manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Review Board of the National Center for Health Statistics, Centers for Disease Control and Prevention of the US Department of Health and Human Services( 15 ). Written informed consent was obtained from all subjects. Like all NHANES data released for public use, all the data for the present study had been de-identified( 15 ).






