Infancy is a critical period of dramatic dietary transition, from an entirely milk-based diet to a diet based on solid foods with a variety of family foods from all food groups. Adequate nutrition in infancy not only affects short- and long-term health status( Reference Robinson and Fall 1 , Reference Przyrembel 2 ), but is also known to influence the formation of the child’s dietary habits and food preferences( Reference Mennella and Trabulsi 3 , Reference Schwartz, Scholtens and Lalanne 4 ). It is evident that a diet high in fruit and vegetables in early childhood may lead to lifelong high intake of fruit and vegetables, which has many beneficial effects on weight control and other health outcomes in later life( Reference Devine, Connors and Bisogni 5 – Reference Mikkilä, Räsänen and Raitakari 8 ). However, the proportion of young children who consume less than the recommended amounts of fruit and vegetables is increasing and is becoming a worldwide public health issue( Reference Manios, Kourlaba and Kondaki 9 – Reference Krebs-Smith, Guenther and Subar 11 ). In Japan, 62·7 % and 35·0 % of children aged 1–4 years consumed fruit and vegetables less than once daily, respectively( 12 ). Understanding the modifiable factors involved in determining whether a child’s early-life diet is high in fruit and vegetables and identifying children with unhealthy dietary habits are therefore particularly important steps in preventing the onset of diet-related disease in later life.
The influence of variation in infant nutrition on later dietary habits and food preferences is beginning to receive considerable attention. In particular, breast-feeding is one of a child’s initial opportunities for early exposure to different flavours. Some observational studies have found associations between longer duration of breast-feeding and higher intake levels of fruit and/or vegetables in later childhood( Reference Cooke, Wardle and Gibson 13 – Reference Perrine, Galuska and Thompson 17 ). In addition, some researchers have suggested that there is a ‘sensitive’ period for the introduction of tastes between 4 and 6 months of age in which infants more readily accept a wide range of tastes( Reference Mennella, Lukasewycz and Castor 18 ). This implies that the age at which new tastes are introduced could be important in determining the child’s subsequent acceptance of new foods and in shaping dietary habits. There is some evidence showing that an early introduction to fruit and vegetables is associated with an increased intake of fruit and vegetables in later childhood( Reference de Lauzon-Guillain, Jones and Oliveira 16 ), but the significance of this relationship is inconsistent across studies( Reference Burnier, Dubois and Girard 14 , Reference Möller, de Hoog and van Eijsden 15 ). It should be noted that the previous studies on this topic have been conducted predominantly in Western countries; in fact, we are unaware of any comparable research reported in Asian countries including Japan, although the food cultures of Asian and Western peoples are quite different. It will be necessary to accumulate studies of this kind from various regions with different social and cultural backgrounds in order to obtain reliable scientific evidence on the relationship between infant nutrition and children’s later diets.
In the present study we examine whether infant nutrition (i.e. duration of breast-feeding and age at introduction of solid foods) influences later intake of fruit and vegetables in a group of Japanese toddlers aged 16–24 months. We considered the effects of several potential factors that have been suggested to influence children’s diets, including maternal socio-economic status and maternal fruit and vegetable intake( Reference Cooke, Wardle and Gibson 13 – Reference Möller, de Hoog and van Eijsden 15 ).
Materials and methods
Study population and procedure
The Osaka Maternal and Child Health Study (OMCHS) is a prospective cohort study that investigates preventive and risk factors for maternal and child health problems. The principal objective of the OMCHS is to clarify risk factors for childhood allergic disorders. A detailed description of the rationale, study design and protocol has been published elsewhere( Reference Miyake, Miyamoto and Ohya 19 , Reference Miyake, Sasaki and Tanaka 20 ). Briefly, between November 2001 and March 2003, all pregnant women in Neyagawa City, Osaka Prefecture, Japan were asked to participate. Of 3639 pregnant women, 627 (17·2 %) took part in the study. An additional 375 pregnant women living in other municipalities were enrolled between December 2001 and November 2003. Baseline assessment of the OMCHS was primarily conducted using a set of two self-administered questionnaires on dietary habits and a wide range of lifestyle behaviours. A self-administered questionnaire was also used in each of the second and third surveys, conducted after the birth of the children. The participants returned their completed questionnaires for each survey to the data management centre. Research technicians confirmed missing or illogical data by telephone interview. A total of 1002 pregnant women at 5–39 weeks of gestation completed the baseline survey. Of these 1002 women, 867 and 763 mother–child pairs participated in the second (at 2–9 months postpartum) and third (at 16–24 months postpartum) surveys, respectively. In the case of multiple births (n 7), one child per mother was selected at random to be included in the analyses. Data on 763 mother–child pairs were used for the present analysis.
The OMCHS was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethics committee of the Osaka City University School of Medicine. Written informed consent was obtained from all participating women.
Maternal data
At the baseline survey during pregnancy, we collected information on maternal age, gestational age at enrolment (weeks), education (<13 years (high school or less), 13–14 years (technical or professional school) or ≥15 years (university or more)) and annual household income (<4 000 000, 4 000 000–5 999 999 or ≥6 000 000 Japanese yen/year). Diet during the preceding month was assessed using a validated, self-administered dietary history questionnaire (DHQ)( Reference Sasaki, Yanagibori and Amano 21 – Reference Shiraishi, Haruna and Matsuzaki 27 ). Estimates of daily intake for 150 food and beverage items as well as for energy, nutrients and alcohol were calculated using an ad hoc computer algorithm for the DHQ, which was based on the Standard Tables of Food Composition in Japan( 28 ). Validity of the DHQ with respect to commonly studied nutritional factors has been investigated in several previous studies, using a dietary record( Reference Sasaki, Yanagibori and Amano 21 , Reference Kobayashi, Murakami and Sasaki 24 , Reference Kobayashi, Honda and Murakami 25 ), 24 h urine excretion( Reference Sasaki, Yanagibori and Amano 22 ) and serum biomarkers( Reference Sasaki, Ushio and Amano 23 , Reference Shiraishi, Haruna and Matsuzaki 26 , Reference Shiraishi, Haruna and Matsuzaki 27 ). In a previous study of ninety-two Japanese women aged 31–69 years, the Pearson correlation coefficients between the DHQ and 16 d semi-weighed dietary record were 0·27 to 0·87 for energy-providing nutrients, 0·39 to 0·71 for other nutrients( Reference Kobayashi, Honda and Murakami 25 ), 0·40 for fruit and 0·56 for total vegetables( Reference Kobayashi, Murakami and Sasaki 24 ). In other studies of Japanese pregnant women, Spearman correlation coefficients between the DHQ-assessed intakes and serum concentrations were 0·29 for folate, 0·22 for vitamin B12 Reference Shiraishi, Haruna and Matsuzaki (26 ), 0·25 for β-carotene and 0·32 for vitamin C( Reference Shiraishi, Haruna and Matsuzaki 27 ). These data suggest the satisfactory validity of the DHQ in terms of these dietary variables. However, its validity with regard to other dietary variables has not been investigated among Japanese pregnant women. Self-reported body height (in centimetres) and weight (in kilograms) were also obtained from the DHQ, from which BMI was calculated as body weight divided by the square of body height (kg/m2). In the second survey, completed after delivery, information on parity and smoking status in pregnancy (never smoked, stopped smoking in pregnancy or smoked throughout pregnancy) was obtained.
Children’s data
Information on baby’s sex, birth weight (in grams) and gestational age at birth was obtained by the self-administered questionnaire composing the second survey( Reference Miyake, Sasaki and Tanaka 20 ). In the third survey, conducted at 16–24 months postpartum, we obtained details of feeding practices in early life (i.e. breast-feeding duration and the age in months at which solid foods were introduced). The duration of breast-feeding was defined as the period during which children had received breast milk, irrespective of exclusiveness, and categorized into four groups: <6, 6–11, 12–17 or ≥18 months. These categories were chosen based on the 25th, 50th and 75th percentiles of breast-feeding duration in the study sample because no clear recommendation on breast-feeding duration exists in Japan. The guideline of weaning for infants in Japan recommends gradual introduction of solid foods from the age of 5 to 6 months( 29 ). Based on this guideline, the ages of the children when solid foods were introduced were categorized into three groups: <5, 5–5·9 or ≥6 months. Information on the child’s dietary habits during the preceding month was obtained at the third survey from the mother in terms of the consumption frequency of twenty-one selected food and beverage items without specification of portion size( Reference Okubo, Miyake and Sasaki 30 ). Eight predefined frequency categories ranging from ‘less than once per month’ to ‘two or more times per day’ were used. For predefined frequency categories, the midpoint of each category was assumed to be the most likely consumption (e.g. reported consumption of ‘4–6 times/week’ was calculated as ‘5 times/week’). All reported frequency categories for each item were then converted to a daily consumption value. Of the foods listed on the questionnaire, three items were specifically related to fruit and vegetable intake: fruit, vegetables and vegetables from commercial baby foods. Daily intake of vegetables, as the total consumption frequency (in times per day), was calculated as the sum of vegetables and vegetables from commercial baby foods.
Statistical analysis
Descriptive data are presented as mean values and standard deviations for continuous variables and as percentages of the participants for categorical variables. Differences in group characteristics according to categories of feeding practices in early life were examined with the χ 2 test for categorical data or ANOVA for continuous data. Univariate and multiple logistic regression analyses were used to examine the relationships between feeding practices in early life and later intake of fruit and vegetables. In the present study, we calculated odds ratios and 95 % confidence intervals of low intake frequency of fruit and vegetables (less than once daily) for each category of infant feeding practices to clarify how these practices affect the risk of forming unhealthy dietary habits in early childhood. Because no dietary recommendation on fruit and vegetable intake for young children exists in Japan, the cut-off point was chosen based on a literature review of the earlier studies( Reference Burnier, Dubois and Girard 14 , Reference de Lauzon-Guillain, Jones and Oliveira 16 , Reference Perrine, Galuska and Thompson 17 ). The lowest category of each variable of infant feeding practices served as the reference group.
For the logistic regression analysis, we controlled for the effects of the following potential confounding factors: maternal factors were age at enrolment, BMI, education, annual household income, smoking status during pregnancy, and fruit and vegetable intake during pregnancy; child factors were sex, birth weight and age at the third survey. Because the two early-life feeding practices studied here (breast-feeding duration and the age of introduction of solid foods) are strongly correlated, they were entered simultaneously in the regression models. Tests for trend associations were based on continuously distributed variables and adjusted for potential confounders. All statistical analyses were performed using the SAS statistical software package version 9·4. All reported P values are two-tailed and P<0·05 was considered to be statistically significant.
Results
Characteristics of the study population
Characteristics of the 763 mother–child pairs are shown in Table 1. Compared with other mothers in the OMCHS cohort (n 239), those included in the analyses were slightly older at enrolment (P<0·001); tended to have a younger gestational age (P=0·02), a higher level of education (P<0·001) and a higher annual household income (P<0·001); and were more likely to be primiparous (P<0·001) and less likely to smoke at baseline (P<0·001). There were no differences in maternal height, BMI, energy intake, or fruit and vegetable intake during pregnancy between the mothers studied and the remaining mothers. Slightly fewer than 25 % of children were breast-fed for <6 months, while another 24 % were breast-fed for ≥18 months. The introduction of solid foods usually occurred at 5 months of age (43·5 %) in the present sample. At 18–24 months of age, 52·0 % and 12·3 % of the children in the present study were at risk of consuming less than one serving daily of fruit and vegetables, respectively.
Table 1 Characteristics of the mother-child pairs in OMCHS (n 763)Footnote *
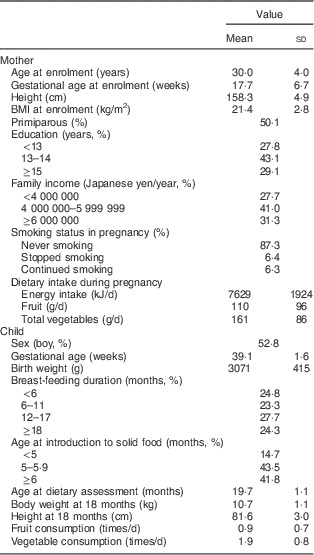
OMCHS, Osaka Maternal and Child Health Study.
* Values are means and standard deviations or percentages.
Associations between feeding practices in early life and participant characteristics
Characteristics of the mother–child pairs according to four categories of breast-feeding duration and three categories of age at introduction of solid foods are shown in the online supplementary material, Supplemental Tables 1 and 2, respectively. Compared with mothers who breast-fed for <6 months, those who breast-fed for ≥18 months were older, tended to have a higher level of education and a higher annual household income, were less likely to smoke during pregnancy and introduced solid foods later (Supplemental Table 1, all P<0·05). A significant difference was observed between breast-feeding duration and child’s vegetable intake at 18 months of age: children who were breast-fed for 6–11 months consumed more vegetables than did those who were breast-fed for <6 months (P<0·05). The mothers who introduced solid foods to their children after 6 months of age were older and breast-fed for longer than those who introduced solid foods before 5 months of age (all P<0·05 in Supplemental Table 2). The children who began eating solid foods at 5 months of age consumed significantly more vegetables at 16–24 months of age than did those who began eating solid foods before 5 months of age (P<0·05).
Table 2 Association between breast-feeding duration and low consumption frequency of fruit and vegetables (<1 time/d) at 18–24 months of age among children who participated in the OMCHS (n 763)
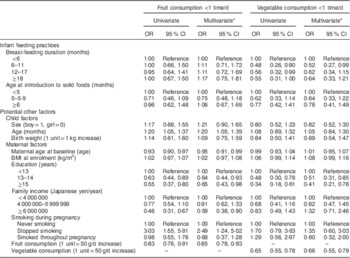
OMCHS, Osaka Maternal and Child Health Study.
* Adjusted for all other variables shown in the table.
Associations of feeding practices in early life with later intake of fruit and vegetables
Table 2 shows the association of breast-feeding duration, the timing of introduction of solid foods and potential confounders with low intake (less than once daily) of fruit and vegetables at 16–24 months of age. Neither breast-feeding duration nor age at introduction of solid foods was associated with the risk of low intake frequency of fruit at 16–24 months of age. In contrast, children who were breast-fed for ≥6 months had significantly lower odds of consuming less than one serving daily of vegetables than were those who were breast-fed for <6 months, but a linear trend was not observed (P for trend=0·13). The lower risks among children breast-fed for 12–17 or ≥18 months were no longer observed after adjustment for potential maternal and child confounders. The decreased risk of low vegetable intake remained only among children who were breast-fed for 6–11 months. When we categorized breast-feeding duration into two groups using 6 months as the cut-off, breast-feeding for ≥6 months was significantly associated with a decreased risk of low intake of vegetables at 16–24 months of age (crude OR=0·53; 95 % CI 0·34, 0·84). Adjustment for potential confounders did not modify the results (adjusted OR=0·59; 95 % CI 0·36, 0·97). No association was observed between age at introduction of solid foods and later intake of vegetables.
Associations of other potential confounders with later intake of fruit and vegetables
Among the potential confounders examined, maternal education and maternal fruit and vegetable intake during pregnancy were common and strong risk factors related to children’s fruit and vegetable intake at 18–24 months of age. Children who were older at dietary assessment and whose mothers had stopped smoking during pregnancy had an increased risk of low intake of fruit, whereas children whose mothers had a higher level of education, higher annual family income and higher fruit intake during pregnancy had a reduced risk of low intake of fruit. On the other hand, higher level of education and vegetable intake during pregnancy were directly associated with a reduced risk of low vegetable intake by children at 16–24 months of age.
Discussion
The main finding of the current study was that breast-feeding duration may influence later vegetable intake among Japanese toddlers, as observed in the previous Western studies, whereas age at introduction of solid foods did not have an obvious effect on toddlers’ fruit or vegetable intake. We found that children who were breast-fed for ≥6 months had a significantly reduced risk of low intake frequency of vegetables than those who were breast-fed for <6 months, suggesting a threshold effect rather than a monotonic trend.
A growing number of epidemiological studies have been published showing the association of infant feeding practices with later intake of fruit and vegetables( Reference Cooke, Wardle and Gibson 13 – Reference Perrine, Galuska and Thompson 17 ). To our knowledge, however, this association has not previously been described in non-Western populations. Our finding that breast-feeding for ≥6 months may reduce the risk of low intake frequency of vegetables in early childhood is consistent with the findings of previous Western studies( Reference Cooke, Wardle and Gibson 13 – Reference Perrine, Galuska and Thompson 17 ). A recent analysis of four European cohorts, from the UK, France, Greece and Portugal, found that longer breast-feeding duration, regardless of exclusiveness, was consistently related to higher fruit and vegetable intake when the children were between 2 and 4 years of age( Reference de Lauzon-Guillain, Jones and Oliveira 16 ). Positive associations between longer exclusive breast-feeding duration and later intake of vegetables have also been observed in studies from Quebec( Reference Burnier, Dubois and Girard 14 ), Amsterdam( Reference Möller, de Hoog and van Eijsden 15 ) and the USA( Reference Perrine, Galuska and Thompson 17 ). In particular, the result of the Quebec study suggests a threshold effect rather than a linear trend effect, with a cut-off at 3 months of exclusive breast-feeding in relation to later vegetable intake( Reference Burnier, Dubois and Girard 14 ). This is similar to the apparent threshold effect at 6 months of total breast-feeding that we observed in the present study. It remains unclear whether the effect of breast-feeding on later intake of vegetables is truly a threshold effect rather than a linear trend effect because the type of breast-feeding (i.e. whether exclusive or combined with solid food) and breast-feeding duration were not categorized consistently across the available studies( Reference Burnier, Dubois and Girard 14 – Reference de Lauzon-Guillain, Jones and Oliveira 16 ). Nevertheless, it is clear that a longer duration of breast-feeding is consistently linked to higher vegetable intake later in childhood regardless of country.
In contrast, breast-feeding duration was not related to later fruit intake in the present study; this finding was inconsistent with those of previous papers( Reference Möller, de Hoog and van Eijsden 15 , Reference Perrine, Galuska and Thompson 17 ). The duration of exclusive breast-feeding and that of breast-feeding in combination with solid foods both showed linear associations with fruit intake in the previous Western studies, although these associations were not always significant( Reference Möller, de Hoog and van Eijsden 15 ). The reason for this inconsistency is not clear, but may be related to differences in the food cultures of Western countries and Japan with regard to fruit in the diet. In Japan, fruit is typically considered to belong to a category of desserts with high water content, traditionally called mizu-gashi. Accordingly, fruit might be offered to young children less frequently than other foods including vegetables and rice. As observed in the present study, more than half of the children (52·0 %) did not consume fruit every day. This tendency was also observed in the National Nutrition Survey on Preschool Children in Japan, 2005( 12 ). Further studies will be necessary to determine whether the results seen in this group would also be observed in a more representative sample of the Japanese population.
In the present study, no association was observed between age at introduction to solid foods and later intake of fruit and vegetables, although children who began eating solid foods at 5 months of age consumed significantly more vegetables than did those who began eating solid foods before 5 months of age (P<0·05). In the Avon Longitudinal Study of Pregnancy and Children (ALSPAC), later introduction to fruit appeared to be related to lower fruit intake at 2 years, but not at 3, 4, 7 or 9 years, whereas later introduction to vegetables was related to lower vegetable intake throughout childhood( Reference de Lauzon-Guillain, Jones and Oliveira 16 ). In a study in Amsterdam, introducing solid foods before the age of 4 months was associated with higher fruit intake compared with introducing them at 6 months, but no relationship was observed between age at introduction of solid foods and vegetable intake( Reference Möller, de Hoog and van Eijsden 15 ). Another study from Quebec showed no relationship between age of introduction to vegetables and later vegetable intake( Reference Burnier, Dubois and Girard 14 ). Overall, the evidence regarding the relationship between age at introduction to solid foods, regardless of the types of foods initially introduced, and later intake of fruit and vegetables is currently inconclusive.
In keeping with the growing recognition of the potential effects of mothers’ diets and sociodemographic factors on children’s diets( Reference Cooke, Wardle and Gibson 13 – Reference Möller, de Hoog and van Eijsden 15 , Reference North and Emmett 31 ), we found that maternal education level and fruit and vegetable intake during pregnancy were independently associated with children’s intake of fruit and vegetables. Although it is not clear exactly how higher maternal education level influences children’s diets, it is presumed that more educated mothers are more interested, and able, to acquire information on children’s nutrition. Mothers’ food choices for their children are likely determined based on the maternal knowledge and ability to understand and apply dietary recommendations, which may be connected to their education level. It is also known that maternal dietary habits during pregnancy influence children’s dietary habits( Reference Robinson, Marriott and Poole 32 ). Mothers who choose healthy behaviours for themselves are more likely to choose them for their children. Additionally, maternal diet influences the child’s diet through chemosensory means( Reference Mennella 33 , Reference Beauchamp and Mennella 34 ). Experimental research has revealed that a child’s first experiences of flavour occur in utero, long before he/she is introduced to solid foods in infancy( Reference Mennella 33 , Reference Beauchamp and Mennella 34 ). Flavour volatiles and taste compounds from the maternal diet during pregnancy are transmitted to the amniotic fluid and to human milk, flavouring both( Reference Beauchamp and Mennella 34 ). Thus, breast-fed infants whose mothers consumed a diet rich in fruit and/or vegetables during pregnancy may exhibit greater acceptance of the flavours of vegetables, although the present study shows no evidence that this occurs for fruit as well.
The strengths of the current study include its prospective design, the studying of a homogeneous population of Japanese mother–child pairs with similar backgrounds and our consideration of a wide range of confounding influences on the relationship between infant feeding practices and later intake of fruit and vegetables. Several limitations of the study also have to be considered, however. First, the study participants were not a representative sample of Japanese mother–child pairs in the general population. Compared with mothers who did not supply their children’s dietary data, the mothers included in the analysis tended to be older, better educated, less likely to smoke during pregnancy and to have higher annual household income. Thus, the present findings might not be generalized. However, we adjusted for each of these factors in our statistical models, and unless the associations between infant feeding practices and later intake of fruit and vegetables were different in the remainder of the cohort, it is unlikely that selection bias could have been solely responsible for our findings. Second, our sample size was relatively small compared with those of previous studies( Reference Burnier, Dubois and Girard 14 – Reference Perrine, Galuska and Thompson 17 ). Therefore, the statistical power may have been insufficient to allow the detection and stability of results concerning the association between infant feeding practices and later intake of fruit and vegetables. Third, the dietary data of children were obtained from a non-validated questionnaire that was developed for the OMCHS survey. The accuracy of this method for assessing consumption frequency remains a serious concern and incompleteness of the assessment cannot be ruled out. Furthermore, misreporting of self-reported food intake is a source of measurement error. This error is expected to have attenuated associations. In addition, we used a validated dietary assessment questionnaire for the mothers( Reference Sasaki, Yanagibori and Amano 21 – Reference Shiraishi, Haruna and Matsuzaki 27 ), but the validity of the DHQ regarding fruit and vegetables has not been examined among pregnant women and the results should accordingly be interpreted with caution. Fourth, maternal body weight just before pregnancy was not available. We therefore used self-reported body weight collected at the baseline survey between 5 and 39 weeks of gestation. Unfortunately, this variable did not accurately reflect the usual maternal weight status, especially if it was based on maternal weight during the later stages of pregnancy. Finally, a longer duration of breast-feeding may act as a marker for more healthy behaviours of the mothers and/or highly health-conscious family influences, which could potentially confound associations with fruit and vegetable intake of children. While we controlled for a large number of potential confounding factors and other influences on fruit and vegetable intake of children, such as maternal socio-economic status and maternal dietary intake, we cannot rule out unmeasured or residual confounding in an observational study. Moreover, the validity of potential confounders from self-administered questionnaires developed for the present study is questionable because of the lack of a validation study. Therefore, the results should be interpreted with caution.
In conclusion, the present study found that breast-feeding duration of at least 6–11 months may prevent a low intake frequency of vegetables among Japanese toddlers. As dietary habits established in early life may track from childhood to adulthood, additional longitudinal studies are needed to confirm these findings and to establish the long-term effects of early feeding practices on later dietary intake in non-Western, especially Asian, populations.
Acknowledgements
Acknowledgements: The authors thank the Neyagawa City Government, Hirakata City Government, Katano City Government, Shijonawate City Government, Kaizuka City Government, Takaishi City Government, Hannan City Government, Neyagawa City Medical Association, Hirakata City Medical Association and Kadoma City Medical Association for their valuable support. Financial support: This work was supported in part by a Grant-in-Aid (grant numbers 13770206 and 16790351) for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology and Research on Allergic Disease and Immunology from the Ministry of Health, Labour, and Welfare. The funders had no role in the design, analysis or writing of this article. Conflict of interest: Y.M. and K.T. have received funding from Meiji Co., Ltd. The other authors have indicated they have no financial relationships relevant to this article to disclose. Authorship: H.O. conducted the statistical analyses and wrote the manuscript. Y.M. contributed to the planning of the OMCHS and data collection and assisted in manuscript preparation. S.S. assisted in manuscript preparation. K.T. contributed to data collection. Y.H. supervised the design and execution of the OMCHS. All authors contributed to and have approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethics committee of the Osaka City University School of Medicine. Written informed consent was obtained from all participating women.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1368980015001779





