Take-Home Points
1. Attempts to halt or reverse the degenerative process in Alzheimer’s dementia have targeted beta amyloid.
2. Although numerous mechanisms to reduce beta amyloid have been successful in engaging this target, none has yet modified the course of Alzheimer’s dementia.
3. While the field sorts out whether to target beta amyloid, tau, or other potential neurodegenerative mechanisms, the epidemic of Alzheimer’s dementia rages on, with an exploding population of patients who not only have progressive neurodegenerative dementia with cognitive and memory problems, but also common and severe behavioral symptoms including agitation and psychosis.
4. Several novel therapeutics for Alzheimer’s dementia are now focusing on symptomatic treatments for agitation and psychosis while the search for disease modifying treatments retrenches and looks for new targets.
Introduction
The bad news is that without disease modifying treatments, the incidence of dementia in the United States is projected to skyrocket from about 5 million individuals affected today to almost 14 million by 2030, all of whom will have disabling behavioral symptoms, from memory disturbances to agitation to psychosis. 1 The further bad news is that setbacks in testing amyloid modifying treatments that successfully target beta amyloid do not yet appear to be disease modifying for Alzheimer’s dementia,Reference Godyń, Jończyk and Panek 2 , Reference Panza, Seripa and Solfrizzi 3 which means that the explosion of cases will continue unabated. The good news is that new pharmacologic approaches to treating the behavioral symptoms of dementia, which will accompany those who have dementia until disease modifying treatments are discovered, have shown good promise, particularly in the area of agitation and psychosis; these will be reviewed here.
Although treatment options limited to symptomatic presentations of the behavioral and neuropsychiatric symptoms of dementia do not arrest or reverse the course of dementia, they are nevertheless useful and strive to relieve patients and caregivers of some of the behavioral and psychiatric sequelae that can severely impact their quality of life. In fact, neuropsychiatric symptoms of dementia affect virtually all patients with dementia at some time during the disease course and are associated with earlier institutionalization, increased caregiving costs (both financial and otherwise), and worsened disease progression.Reference Stahl and Morrissette 4 Unfortunately, pharmacological measures acting on neurocircuitry that may be damaged in the brains of patients with dementia as well as attempts to utilize nonpharmacological, cognition-acting strategies in cognitively impaired patients with dementia may not be as effective as they are for younger, non-demented patients. It is also important to note that improvements in behavioral symptoms may not be due solely to a prescribed psychotropic agent but may also be a result of the extra social contact with the treatment team that medication warrants. In fact, current first-line treatment of any behavioral symptom associated with dementia is nonpharmacological.Reference Stahl and Morrissette 4 However, nonpharmacological interventions are frequently inadequate and have spurred the search for symptomatic treatments while the search for disease-modifying therapies retrenches.
What Are the Behavioral Symptoms of Dementia, and How Are They Currently Treated?
There are many behavioral symptoms associated with dementia (Figure 1),Reference Stahl and Morrissette 4 and the Neuropsychiatric Inventory Questionnaire (NPI) is an excellent resource for evaluating not only the range and severity of a variety of secondary behavioral symptoms associated with dementia but also the impact that such behaviors have on the caregiver.Reference Lanctôt, Amatniek and Ancoli-Isreal 5 , 6 For each behavior, severity is rated on a scale of 1–3 (with 1 being mild), and caregiver distress is rated on a scale of 0–5 (with 0 being not distressing at all).Reference Lanctôt, Amatniek and Ancoli-Isreal 5 , 6 To the extent that there are any pharmacological treatments for the behavioral symptoms of dementia, they are all relatively nonspecific and often poorly evidence-based and off-label.Reference Stahl and Morrissette 4 In fact, one of the most powerful pharmacologic treatments of dementia currently is to discontinue drugs causing worsening of behavioral symptoms often through unwanted drug interactions.Reference Stahl and Morrissette 4 It has been estimated that approximately 60% of nursing home residents (undoubtedly many of whom have dementia) are prescribed unnecessary medications, whereas 42% may not be getting potentially beneficial medications.Reference Stahl and Morrissette 4 This failure to properly utilize medications in the elderly population contributes to morbidity, mortality, and poor quality of life. Additionally, many geriatric patients, including those with dementia, are on numerous medications for a variety of physical, neurological, and psychological disorders. It is critical that potential drug interactions are considered before placing an elderly patient with dementia on a new medication, as the consequences of certain drug interactions can be fatal.
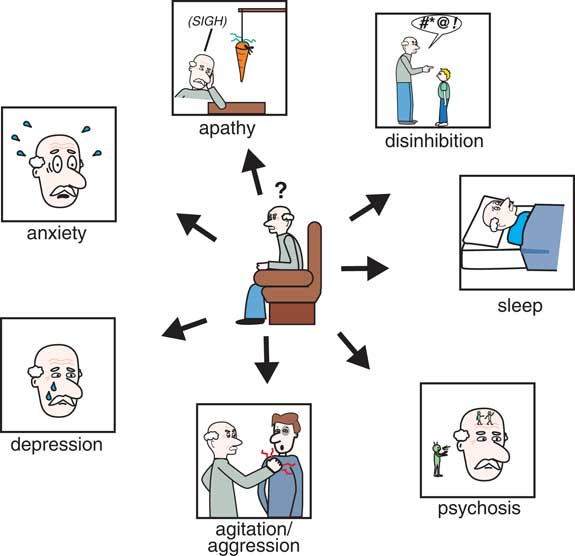
Figure 1 The Neuropsychiatric Inventory Questionnaire (NPI) is an excellent resource for evaluating not only the severity of a variety of secondary behavioral symptoms associated with dementia but also the impact that such behaviors have on the caregiver. For each behavior, severity is rated on a scale of 1–3 (with 1 being mild) and caregiver distress is rated on a scale of 0–5 (with 0 being not distressing at all).
Psychosis and Dementia
Symptoms of psychosis, including hallucinations and delusions, affect many individuals with varying types of dementia, especially in later stages of disease course (Table 1).Reference Stahl and Morrissette 4 Although psychotic symptoms are often episodic and may resolve during the course of dementia, they can be quite troubling for caregivers and may increase the chance of institutionalization. In Alzheimer’s disease, delusions may be more common than hallucinations, whereas, in dementia with Lewy bodies, hallucinations are more common. Psychotic symptoms seem to be related to pathology in the neocortex, and specific symptoms (eg, visual vs auditory hallucinations) likely reflect damage to specific cortical areas.Reference Stahl and Morrissette 4
Table 1 Symptoms of psychosis, including hallucinations and delusions, affect many individuals with varying types of dementia, especially in later stages of disease course4. Although psychotic symptoms are often episodic and may resolve during the course of dementia, they can be quite troubling for caregivers and may increase the chance of institutionalization.

Conventional, first-generation antipsychotics (such as haloperidol) and the atypical, second-generation antipsychotics risperidone, olanzapine, quetiapine, and aripiprazole (all of which block dopamine D2 receptors) are commonly used off label and with considerable controversy to treat psychosis (and agitation) in patients with dementia.Reference Stahl and Morrissette 4 However, these agents may have limited efficacy and come with a wealth of side-effect risks including weight gain and other cardiometabolic issues, anticholinergic-associated worsening of cognition, and sedation, as well as inducing or worsening movement disorders via antagonism at dopamine D2 receptors in the dorsal striatum—which may worsen motor symptoms in patients with Parkinson’s disease and other dementia types in which motor symptoms prevail. Importantly, the use of antipsychotics in elderly patients with dementia has been associated with an increased risk of mortality, warranting an FDA black box warning against their use in this population. For these reasons, it is recommended that current antipsychotics only be used in cases where psychotic behavior is severe, potentially dangerous, and at the lowest dose possible for the shortest duration feasible.Reference Stahl and Morrissette 4 So, how can a novel treatment reduce psychosis in dementia without D2 blockade and without these risks?
The answer may be to target something other than D2 dopamine receptors in psychosis associated with dementia. We have recently reviewed the 3 major theories of psychosis,Reference Stahl 7 which hypothesized to result not just from hyperactive dopamine at D2 receptors in the mesolimbic pathway (Figure 2A), but 2 other theories suggest hyperactive serotonin (5HT, 5-hydroxytryptamine) at 5HT2A receptors in the cortex (Figure 2B) and/or hypoactive glutamate at NMDA (N-methyl-d-aspartate) glutamate receptors in the cortex (Figure 2C).Reference Stahl and Morrissette 4 , Reference Stahl 7 It is entirely possible that more than one of these dysfunctional circuits are the cause of psychosis in patients with dementia, especially as the underlying dementia-related pathology progresses and more brain areas become involved.Reference Stahl and Morrissette 4 Nevertheless, targeting either the glutamate or the serotonin pathways may be helpful in reducing the psychosis associated with dementia. Furthermore, these approaches may avoid the side effects and risks associated with D2 dopamine antagonists for psychosis.
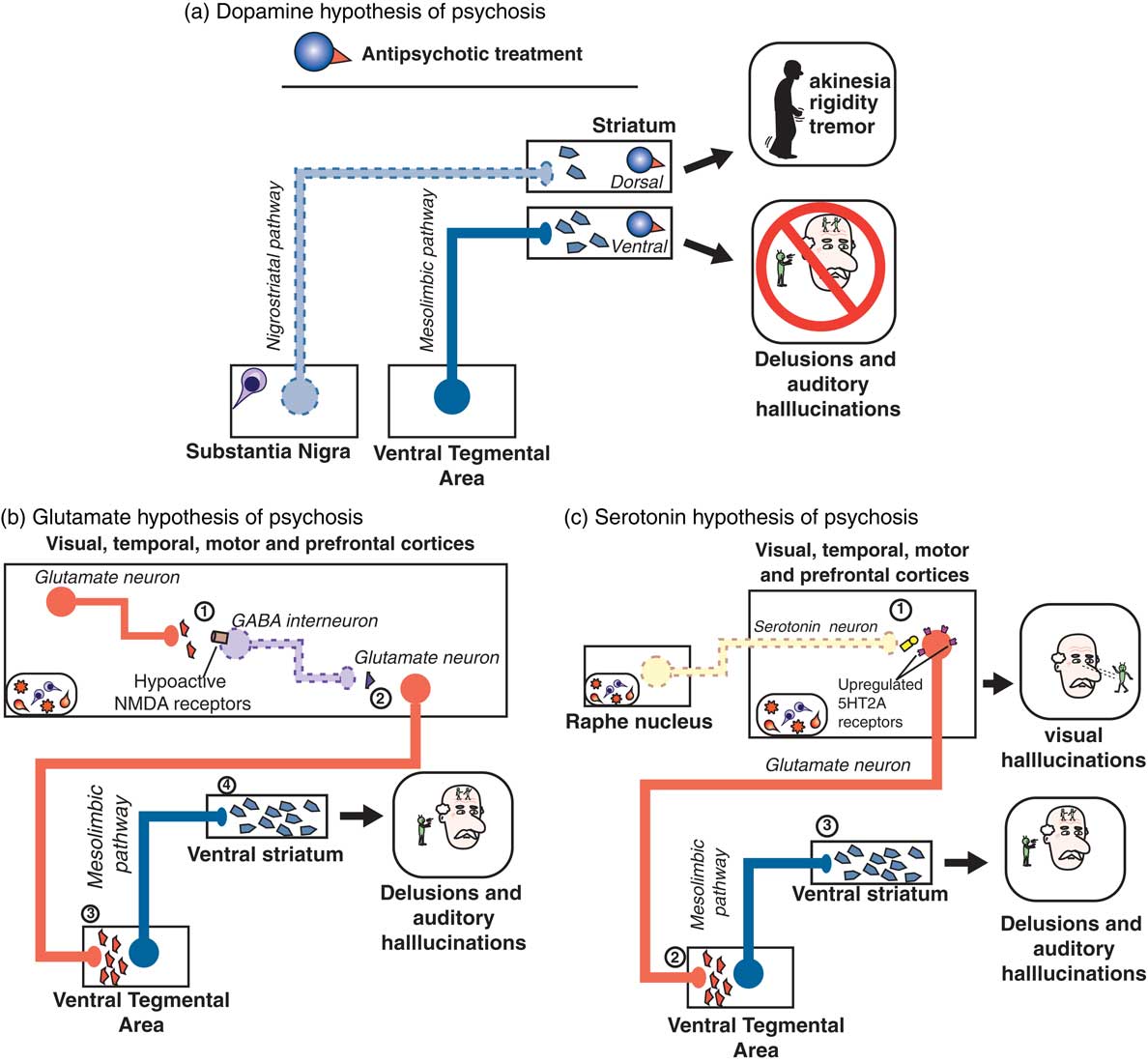
Figure 2 (A) According to the dopamine D2 hypothesis of psychosis, dopaminergic projections along the mesolimbic pathway (blue; from the ventral tegmental area [VTA] to the ventral striatum) are hyperactive, leading to excess dopaminergic neurotransmission in the ventral striatum and resulting in psychosis, particularly delusions and auditory hallucinations. On the other hand, as neuropathology such as Lewy bodies leads to death of dopaminergic neurons of the nigrostriatal pathway, there is a paucity of dopaminergic input to the dorsal striatum, resulting in movement symptoms. Treatment with a dopamine D2 receptor antagonist such as an antipsychotic can act in the ventral striatum to reduce dopaminergic input, thereby ameliorating symptoms of psychosis. However, the antipsychotic blockade of dopamine D2 receptors in the dorsal striatum may actually further reduce the already limited dopaminergic input from the substantia nigra, leading to worsening of motor symptoms. (B) According to the glutamatergic NMDA hypothesis of psychosis, within the cerebral cortices (where amyloid, tau, or Lewy body neuropathology may be prevalent), (1) glutamatergic input to GABA interneurons is reduced due to hypoactive NMDA receptors located on GABA neurons. These GABA neurons are therefore not activated, leading to (2) reduced GABAergic input on downstream glutamatergic neurons, i.e. disinhibition of (3) glutamatergic input onto, and thus activation of, dopaminergic neurons in the VTA occurs, leading to (4) excessive dopaminergic input to the ventral striatum and psychosis. (C) The serotonin 5HT2A hypothesis of psychosis posits that loss of serotonergic neurons in the raphe nuclei (due to Lewy body, amyloid, and/or tau pathology) leads to (1) hypoactive serotonergic neurotransmission in the cerebral cortices, which hypothetically causes upregulation of serotonin 5HT2A receptors located on glutamatergic neurons. This excessive glutamate, particularly in visual cortices, is thought to be the neurobiological substrate for visual hallucinations as are often seen in Parkinson’s disease psychosis. (2) Glutamatergic input onto, and thus activation of, dopaminergic neurons in the VTA also occurs, leading to (3) excessive dopaminergic input to the ventral striatum and psychosis.
Evidence that this approach may indeed be useful for a novel treatment of psychotic behavioral symptoms of dementia is the finding that the first non-D2 antagonist approved for the treatment of psychosis targets 5HT2A receptors.Reference Stahl 8 That is, the selective 5HT2A/2C antagonist pimavanserin (Figure 3)—which does not have D2 antagonist properties—improves psychosis associated with Parkinson’s disease.Reference Stahl 8 Pimavanserin may be particularly effective in reducing visual hallucinations without exacerbating motor effects. Side effects may include peripheral edema, confusion, nausea, and potential QTc prolongation.Reference Stahl 8
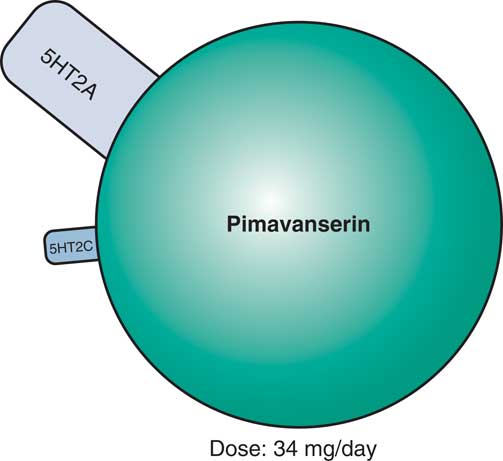
Figure 3 Pimavanserin, a novel serotonin 5HT2A and 5HT2C antagonist with no dopamine D2 binding affinity, is now approved for the treatment of psychosis in Parkinson’s disease and appears to be effective in reducing visual hallucinations, without exacerbating motor effects. Side effects may include peripheral edema, confusion, nausea, and potential QTc prolongation. Pimavanserin is currently in testing for psychosis associated with other forms of dementia, such as Alzheimer’s disease or all-cause dementia.
More recently, a Phase II trial reports that pimavanserin also improves the psychosis associated with Alzheimer’s disease.Reference Ballard 9 Also, a Phase III study of psychosis from all causes of dementia is also now underway to determine whether pimavanserin can reduce psychotic symptoms and maintain this improvement in these patients. If so, pimavanserin would become not only the first non-D2 antagonist for the treatment of psychosis in dementia, but also in fact the first and only approved treatment of any kind for psychosis in dementia.
Agitation and Dementia
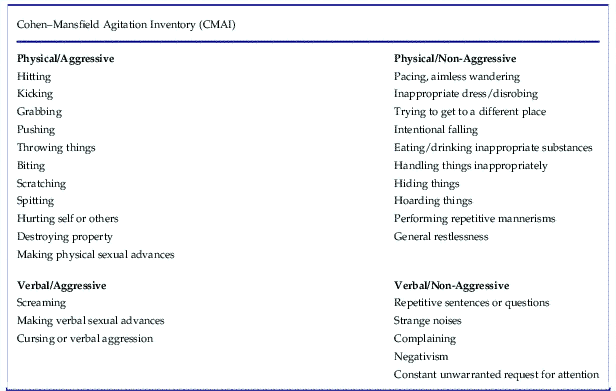
Agitation (encompassing emotional stress, excessive psychomotor activity, aggression, irritability, and disinhibition) is quite common in patients with dementia, affecting 50% or more of patients with Alzheimer’s disease.Reference Stahl and Morrissette 4 First-line treatment involves addressing potential unmet needs that may be causing agitation, such as pain, hunger, or thirst.Reference Stahl and Morrissette 4 Agitation can be conceptualized as physical and verbal behaviors that are excessive, inappropriate, repetitive, nonspecific, and observable. The Cohen–Mansfield Agitation Inventory is a 29-item, clinician-rated, 7-point scale that can be used to assess both aggressive and non-aggressive verbal and physical behaviors indicating agitation (Table 2).Reference Stahl and Morrissette 4
Table 2 Agitation can be conceptualized as physical and verbal behaviors that are excessive, inappropriate, repetitive, nonspecific, and observable. The Cohen–Mansfield Agitation Inventory is a 29-item, clinician-rated, 7-point scale that can be used to assess both aggressive and non-aggressive verbal and physical behaviors indicating agitation.
Agitation in patients with dementia may hypothetically stem from either impaired top-down cortical control of impulsive behaviors, irregular bottom-up drive from limbic regions, or as a result of psychosis.Reference Stahl 10 , Reference Stahl and Morrissette 11 The neurotransmitters dopamine (DA), norepinephrine (NE), serotonin (5HT), acetylcholine (ACh), glutamate (Glu), and gamma-aminobutyric acid (GABA) and possibly sigma receptors are all thought to be involved in aggressive or agitated behaviors in the prefrontal cortex (PFC) of aggressive/agitated patients.Reference Stahl 10 , Reference Stahl and Morrissette 11
According to published expert consensus guidelines for the pharmacological treatment of violence in association with cognitive impairment, treatment of agitation and aggression in patients with dementia, targeting neurotransmitters to hypothetically enhance top-down cortical control, reduce bottom-up limbic drives, or both, first involves non-antipsychotic-based therapies (eg, anticonvulsants, adrenergic agents, antidepressants), with next-line measures including antipsychotic treatment avoiding antipsychotics with a greater propensity for motor side effects in patients with Parkinson’s disease dementia or other dementia types in which movement symptoms prevail.Reference Stahl, Morrissette and Cummings 12
New developments in the treatment of agitation in Alzheimer’s dementia include evaluation of agents with novel therapeutic mechanisms in clinical trials utilizing well-defined and well-rated agitation (Table 2) in patients with Alzheimer’s dementia. The novel dopamine 2 partial agonist brexpiprazole with multiple 5HT and alpha adrenergic receptor binding properties (Figure 4)Reference Stahl 13 has reported evidence of efficacy in reducing agitation in Alzheimer’s disease.Reference Grossberg, Kohegyi and Mergel 14 , Reference Cummings, Kohegyi and Mergel 15 The actions of brexpiprazole, although classified as an antipsychotic because it exhibits D2 properties, may be due to the non D2 binding properties of this agent (Figure 4), and the safety profile for cardiovascular, metabolic, and movement risk may be more acceptable than other agents in this class.Reference Grossberg, Kohegyi and Mergel 14 , Reference Cummings, Kohegyi and Mergel 15 Further studies will determine whether this agent will be heading for formal FDA approval for the treatment of agitation in Alzheimer’s dementia.
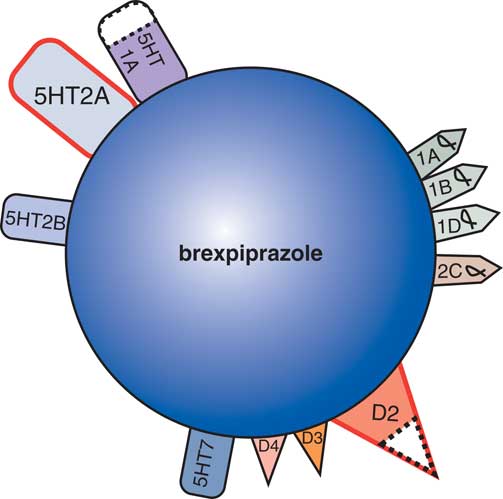
Figure 4 As a dopamine D2 receptor partial agonist, with dopamine D3, serotonin 5HT1A, serotonin 5HT2A, and adrenergic alpha-1 receptors binding properties, the atypical antipsychotic brexpiprazole shares many properties with aripiprazole. Brexpiprazole is currently being tested for its efficacy and safety in the treatment of agitation in patients with Alzheimer’s disease.
Another set of developments are trials of the sigma and glutamate active agent dextromethorphan (combined with a CYP450 2D6 inhibitor) (Figure 5). Dextromethorphan (DXM) is a sigma-1 and mu opioid receptor agonist with antagonist properties at NMDA and nicotinic α3β4 receptors and inhibition of both serotonin and norepinephrine reuptake (SERT and NET, respectively).Reference Stahl 16 Dextromethorphan combined with quinidine (Q), which inhibits the cytochrome P450 2D6 (CYP 2D6) enzyme that metabolizes DXM, thereby increasing the bioavailability of DXM 20-fold, is FDA-approved for the treatment of pseudobulbar affect.Reference Stahl 16 The rationale for possible efficacy in agitation of Alzheimer’s disease derives from the finding that pseudobulbar affect is a condition that has been linked to the emotional instability of agitation in Alzheimer’s disease.Reference Stahl 17 Recent data have indeed indicated that dextromethorphan-quinidine may significantly reduce agitation in patients with Alzheimer’s disease with relatively good tolerability.Reference Porsteinsson and Antonsdottir 18 , Reference Cummings, Lyketsos and Tariot 19 Also, AVP-786, a deuterated version of DXM combined with a low dose of quinidine, is undergoing similar clinical testing. Deuteration of dextromethorphan reduces first-pass liver metabolism, slowing the rate of metabolism of dextromethorphan and allowing for an even lower dose of quinidine. Yet another dextromethorphan formulation is in testing for agitation in Alzheimer’s dementia—a combination of dextromethorphan with the antidepressant bupropion, a norepinephrine and dopamine reuptake inhibitor and nicotinic acetylcholine receptor antagonist that has CYP 2D6-blocking capabilities and thus inhibits metabolism of dextromethorphan and known as AXS-05. It will be interesting to see if either or both of these agents containing dextromethorphan gains FDA approval for agitation in Alzheimer’s dementia, and whether the additional pharmacologic properties of bupropion are a positive factor in clinical outcome, or eventually, whether a combination of the different pharmacologic mechanisms of dextromethorphan/2D6 inhibition added to those of brexpiprazole would be even more robust in reducing agitation in dementia.

Figure 5 Dextromethorphan (DXM) is a sigma-1 and mu opiate receptor agonist with antagonist properties at NMDA and nicotinic α3β4 receptors and inhibition of both serotonin and norepinephrine reuptake inhibitors (SERT and NET, respectively). Dextromethorphan combined with quinidine (Q), which inhibits the cytochrome P450 2D6 (CYP 2D6) enzyme that metabolizes DXM, thereby increasing the bioavailability of DXM 20-fold, is FDA-approved for the treatment of pseudobulbar affect. Recent data have indicated that dextromethorphan-quinidine may reduce agitation in patients with Alzheimer’s disease with relatively good tolerability. AVP-786, a deuterated version of DXM combined with a low dose of quinidine, is now in testing for agitation in Alzheimer dementia. Deuteration of dextromethorphan reduces first pass liver metabolism, slowing the rate of metabolism of dextromethorphan and allowing for an even lower dose of quinidine. Another combination of dextromethorphan with the antidepressant bupropion, a norepinephrine and dopamine reuptake inhibitor and nicotinic acetylcholine receptor antagonist that has CYP 2D6-blocking capabilities and thus inhibits metabolism of dextromethorphan, called AXS-05, is also being tested in agitation in Alzheimer’s dementia.









