Introduction
Large macroalgae, such as kelp species, are dominant foundation organisms along much of the global coastline, where they provide complex biogenic habitat for a high diversity of associated flora and fauna (Steneck et al., Reference Steneck, Graham, Bourque, Corbett, Erlandson, Estes and Tegner2002; Teagle et al., Reference Teagle, Hawkins, Moore and Smale2017). However, fine-scale high-resolution information on levels of biodiversity supported by many species in many regions is lacking and, as such, the ecological importance and wider value of habitat-forming macroalgae is often unknown. In particular, patterns of spatiotemporal variability in macroalgal-associated assemblages, and the nature of facilitative interactions between habitat-formers and allied fauna, are poorly resolved in many regions (Smale et al., Reference Smale, Burrows, Moore, O'Connor and Hawkins2013; Hurd et al., Reference Hurd, Harrison, Bischof and Lobban2014).
Saccorhiza polyschides (Lightfoot) Batters 1902 is a ‘pseudo-kelp’ species found within several ecoregions (Spalding et al., Reference Spalding, Fox, Allen, Davidson, Ferdaña, Finlayson, Halpern, Jorge, Lombana, Lourie, Martin, Mcmanus, Molnar, Recchia and Robertson2007) in the wider North-east Atlantic, ranging from the Mediterranean and Morocco, to the UK, polewards to Norway and the Faroes (Norton, Reference Norton1977; Smale et al., Reference Smale, Burrows, Moore, O'Connor and Hawkins2013). Saccorhiza polyschides is thought to be expanding its leading range edge polewards and is predicted to proliferate with continued ocean warming (Assis et al., Reference Assis, Araújo and Serrão2018), whereas population declines and range contractions at the warm trailing edge have been reported (Smale, Reference Smale2020 and references therein). Anecdotal evidence suggests that S. polyschides populations have proliferated in recent decades in the south-west of the UK (Birchenough & Bremner, Reference Birchenough and Bremner2010; Smale et al., Reference Smale, Burrows, Moore, O'Connor and Hawkins2013), which is the focal region of this study.
Saccorhiza polyschides belongs to the order Tilopteridales but is generally considered ‘kelp-like’ as it serves a similar ecological role as a large brown macroalgal foundation species and canopy-former (Biskup et al., Reference Biskup, Bertocci, Arenas and Tuya2014; Teagle et al., Reference Teagle, Hawkins, Moore and Smale2017). Unlike most kelp species, which are multi-year perennials, S. polyschides is a pseudo-annual species as the macroscopic sporophyte persists for a maximum period of 12–18 months (Norton & Burrows, Reference Norton and Burrows1969). In general, sporophyte recruits appear in early spring and exhibit rapid growth rates through summer and autumn before the onset of senescence, although some individuals may recruit later, overwinter and persist into the following year (Norton & Burrows, Reference Norton and Burrows1969). As S. polyschides can rapidly colonize and expand into disturbed areas but is generally outcompeted by the slower-growing perennial kelp species (e.g. Laminaria spp.), it is considered an opportunistic pioneer species (Smale et al., Reference Smale, Burrows, Moore, O'Connor and Hawkins2013; Arnold et al., Reference Arnold, Teagle, Brown and Smale2016). However, in the absence of Laminaria (due to high temperatures or physical disturbance, for example), S. polyschides can serve as the dominant species and form monospecific stands (Hawkins & Harkin, Reference Hawkins and Harkin1985).
Like other kelp species, sporophytes of S. polyschides are comprised of the blade (i.e. lamina), stipe and holdfast (Figure 1A). Unlike typical Laminarian kelps, however, S. polyschides forms a distinctive hollow bulbous holdfast, which offers internal living space for fauna (Teagle et al., Reference Teagle, Hawkins, Moore and Smale2017, see Norton & Burrows, Reference Norton and Burrows1969 for details of morphology and ecology, and McKenzie & Moore, Reference McKenzie and Moore1981 for holdfast components). Even after the blade and majority of the stipe have senesced, remnant holdfasts may persist attached to the substratum for weeks or even months (Figure 1B), until eventual decay or dislodgement by wave action. However, the value of this microhabitat for sustaining local biodiversity, and general patterns of faunal colonization, are poorly resolved.
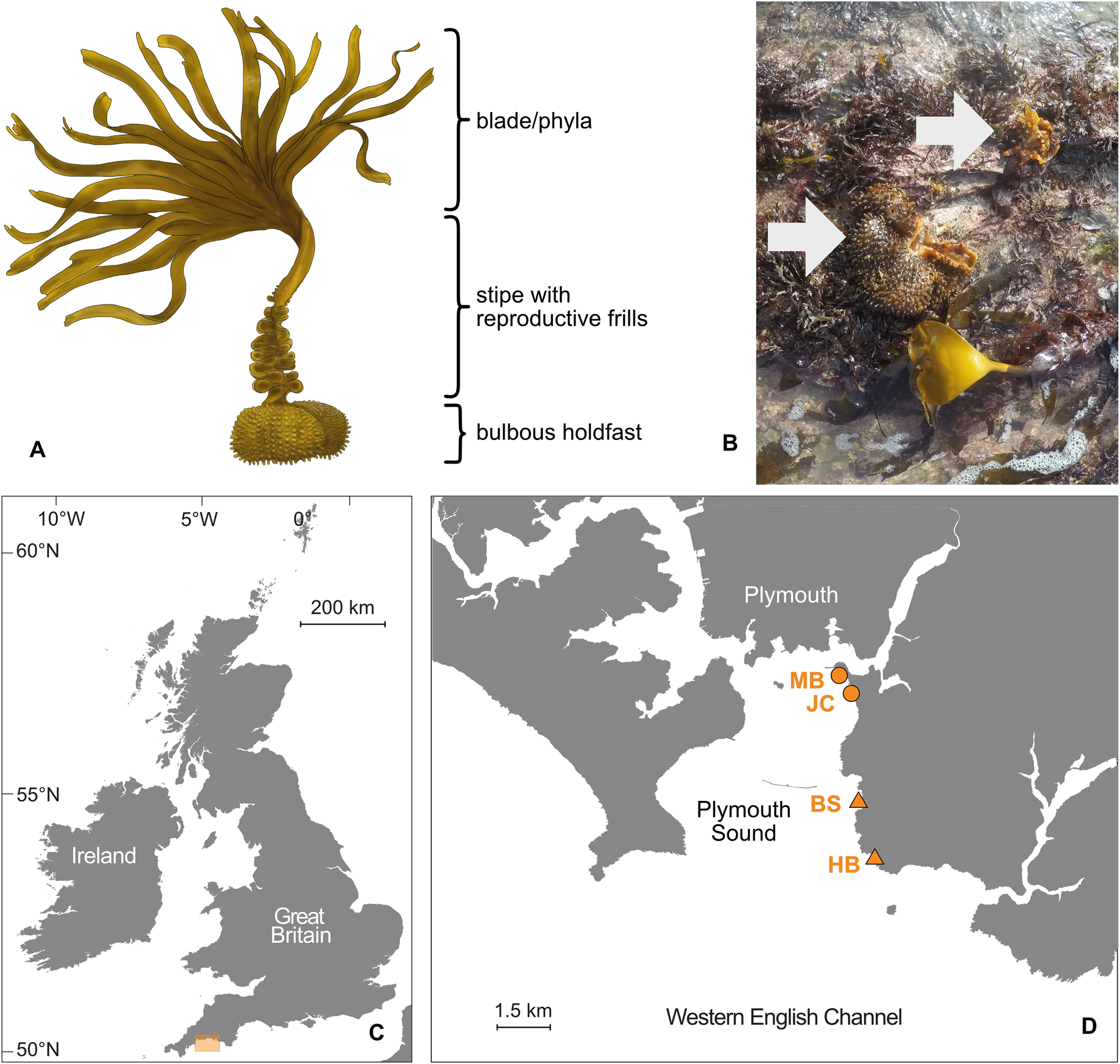
Fig. 1. Study organism and sampling region. (A) Illustration of S. polyschides indicating typical morphology. (B) Representative image of relict decaying holdfasts observed at the study sites (arrows; photographed in March 2020, Bovisand). (C) Map of the UK indicating study region (orange box) in south-west England. (D) Detailed map of Plymouth Sound showing location of sampling sites; moderately wave-exposed sites are marked with a circle (MB, Mount Batten; JC, Jennycliff) and fully wave-exposed sites are denoted with a triangle (BS, Bovisand; HB, Heybrook).
Despite anecdotal evidence that S. polyschides has proliferated in recent decades along southern coastlines of the UK, perhaps in response to increased disturbance to Laminaria canopies and higher sea temperatures offering favourable conditions (Birchenough & Bremner, Reference Birchenough and Bremner2010; Yesson et al., Reference Yesson, Bush, Davies, Maggs and Brodie2015), very little is known about the structure of S. polyschides populations and associated assemblages in the UK. To date, just two taxonomic studies have examined holdfast assemblages associated with S. polyschides populations in the UK, both of which were conducted more than 40 years ago (Norton, Reference Norton1971; McKenzie & Moore, Reference McKenzie and Moore1981). Moreover, these studies were conducted in the cooler, more northerly distribution range of S. polyschides (NW England and Scotland), and information on population structure and associated diversity is lacking in warmer, more southerly areas where it may be proliferating. It is clear, however, that like other large habitat-forming brown macroalgae, the population structure and morphology of S. polyschides is strongly mediated by environmental conditions. In addition to temperature and light, variability in exposure to wave action may influence S. polyschides populations (Norton, Reference Norton1969; Burrows, Reference Burrows2012), as has been observed for other kelp species (Wernberg & Thomsen, Reference Wernberg and Thomsen2005; Bekkby et al., Reference Bekkby, Rinde, Gundersen, Norderhaug, Gitmark and Christie2014; Smale et al., Reference Smale, Burrows, Evans, King, Sayer, Yunnie and Moore2016).
Here, we quantified the structure (density and biomass) of ‘overwintering’ sporophytes at four sites within Plymouth Sound (south-west England, UK), examined their assemblages and report novel observations of faunal diversity supported by S. polyschides. Our a priori expectation was that population structure and sporophyte morphology, and consequently associated faunal assemblage structure, would vary between sites with differing levels of wave exposure.
Materials and methods
Samples were collected from the rocky shore on spring low tides in February 2020 at four sites within Plymouth Sound (50°N). The northern fringes of Plymouth Sound towards the city are partially sheltered from wave action through the natural harbour of the Sound and by the artificial breakwater, built in 1841 (Davies, Reference Davies and Hiscock1998; Knights et al., Reference Knights, Firth, Thompson, Yunnie, Hiscock and Hawkins2016), whereas the southern fringes are mostly exposed and open towards the western English Channel (Figure 1C, D). Four survey sites were chosen (from north to south: MB – Mount Batten; JC – Jennycliff; BS – Bovisand; HB – Heybrook; Figure 1D), two of which were moderately exposed to wave action (MB, JC) and two of which were fully exposed (BS, HB). Sites were typical intertidal rocky shores deemed representative of the wider region and were without obvious local anthropogenic impacts.
The density of S. polyschides sporophytes was recorded within 10 haphazardly placed 1 m2 survey quadrats at each site. Quadrat samples were stratified for stable rocky substrate (i.e. bedrock, large boulders) and placed at least 2 m apart from one another. Additionally, 10 sporophytes were randomly collected at each site. Each individual was carefully removed from the substratum and directly transferred in a moist cotton bag to prevent loss of mobile fauna, which was then sealed and transported to the laboratory for further analysis. Quadrat samples and sporophyte collections were conducted at a tidal height of +0.5–0.8 m (Chart datum). On return to the laboratory, measurements of length, fresh weight biomass and internal holdfast volume (i.e. living space) were obtained for each sample. Internal living space was quantified by first measuring the volume, through water displacement, of the holdfast wrapped in cling film and then again with unwrapped fragments of holdfast, and calculating the difference between the two (see Teagle et al., Reference Teagle, Moore, Jenkins and Smale2018). All organisms associated with sporophyte samples (i.e. within holdfasts and on holdfast/stipe/blade) were carefully removed, sorted to coarse taxonomic groups and enumerated. For sessile colonial taxa (e.g. Porifera and Bryozoa) each cohesive unit of organisms was recorded as one individual. The total abundance of all fauna, abundance of taxonomic groups, richness and diversity (Shannon–Wiener index) of taxonomic groups were calculated for each sample. Due to time and logistical constraints it was not practicable to identity all fauna to a fine taxonomic resolution. However, to examine faunal diversity in more detail, one sample from Bovisand (BS) was analysed to a finer taxonomic level. For this sample, all fauna retained on a 500 μm sieve were preserved in 70% ethanol and later identified to the finest taxonomic resolution practicable, enumerated and weighed (tissue dried) for biomass. For associated vertebrates (i.e. fishes) any eggs present were counted and examined under a dissection microscope to estimate developmental stage.
Between-site and exposure variation in response variables was tested statistically with Kruskal–Wallis tests and subsequent post-hoc analysis, conducted with R version 4.0.0 (R Core Team, 2020) and R Studio. Correlations between holdfast volume and faunal assemblage variables were tested with Spearman's rank correlation. Between-site and exposure variability in multivariate assemblage structure were examined with metric Multidimensional Scaling Ordination (mMDS), and tested statistically with one-way PERMANOVA. Where significant differences between sites were detected, SIMPER analysis was conducted to determine which phyla contributed most to the observed dissimilarity. Multivariate analyses were conducted with PRIMER version 7.0 with the PERMANOVA add-on (Anderson et al., Reference Anderson, Gorley and Clarke2008; Clarke et al., Reference Clarke, Gorley, Somerfield and Warwick2014).
Results
Density, biomass and morphology of S. polyschides
The density of S. polyschides sporophytes varied considerably both between and within sites (Figure 2A), with sample-level density ranging from 0–5 individuals per m2. The density of sporophytes varied significantly between sites (Kruskal–Wallis test; P < 0.05), with densities at MB greater than those recorded at other sites. Sporophyte density did not differ significantly between moderately and fully wave-exposed sites (P > 0.05). Only ‘old’ sporophytes, presumably recruits from the previous year, were recorded, most of which were comprised only of a decaying holdfast. Total sporophyte length was also highly variable between and within sites (Figure 2B). For example, at JC individuals’ length ranged from 1.8 to 69.0 cm, whereas mean values ranged across sites from 3.74 ± 1.25 cm at BS to 16.76 ± 22.9 cm at JC. Although total wet weight was less variable between sites (Figure 2C), mean values at MB were still twice that at BS, and individual sporophyte weight ranged from 15–282 g across the study. Finally, mean values for the volume of internal living space were more comparable across sites (Figure 2D), although individual sporophytes were highly variable, ranging from 19–675 cm3 at BS alone. No significant differences in sporophyte metrics between sites nor exposure were recorded.
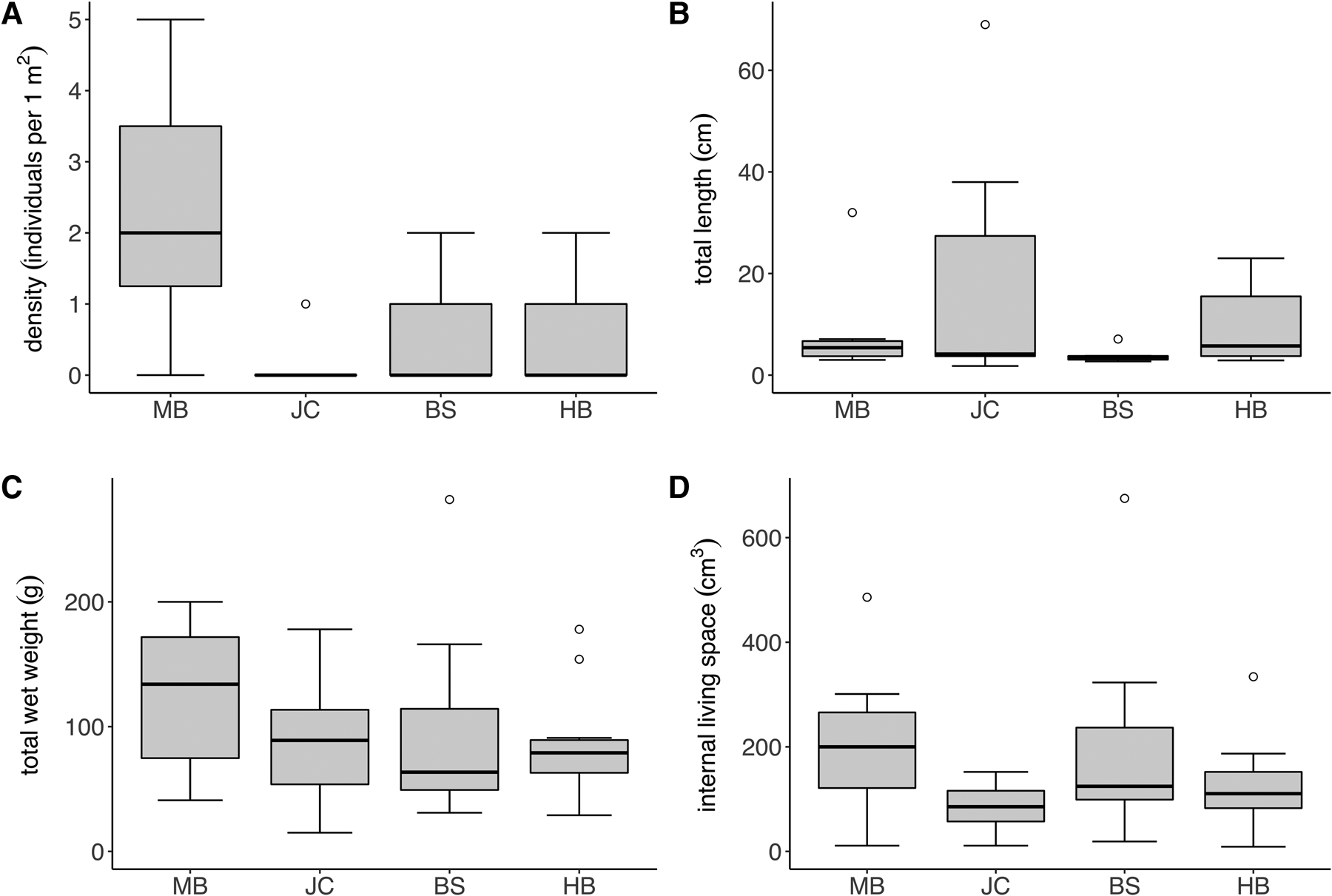
Fig. 2. Density and morphology of S. polyschides at the study sites; box and whisker plots indicate interquartile range (median, whiskers incl. outliers). (A) Density, (B) total length, (C) total weight and (D) holdfast internal living space of S. polyschides at the study sites (MB, JC, BS, HB) in Plymouth Sound (N = 10 per site).
Faunal assemblages
Fauna representing eight different taxonomic phyla were observed in association with S. polyschides. Faunal abundances varied markedly between samples, ranging from 0–593 individuals, and mean abundance values varied between sites, ranging from 1.2 ± 1.03 at JC to 68.6 ± 184.33 at BS (Figure 3A). Statistically, faunal abundance varied between sites (Kruskal–Wallis test; P < 0.05), with values at JC lower than abundances at BS and HB, and between wave exposure levels (P < 0.05). In terms of contribution to total abundance, the phyla Arthropoda, Annelida, Mollusca and Bryozoa were present at all four sites. While Mollusca were consistently most abundant, Arthropoda were also relatively abundant at all sites, and Echinodermata and Cnidaria were common at BS and HB (Figure 3B).
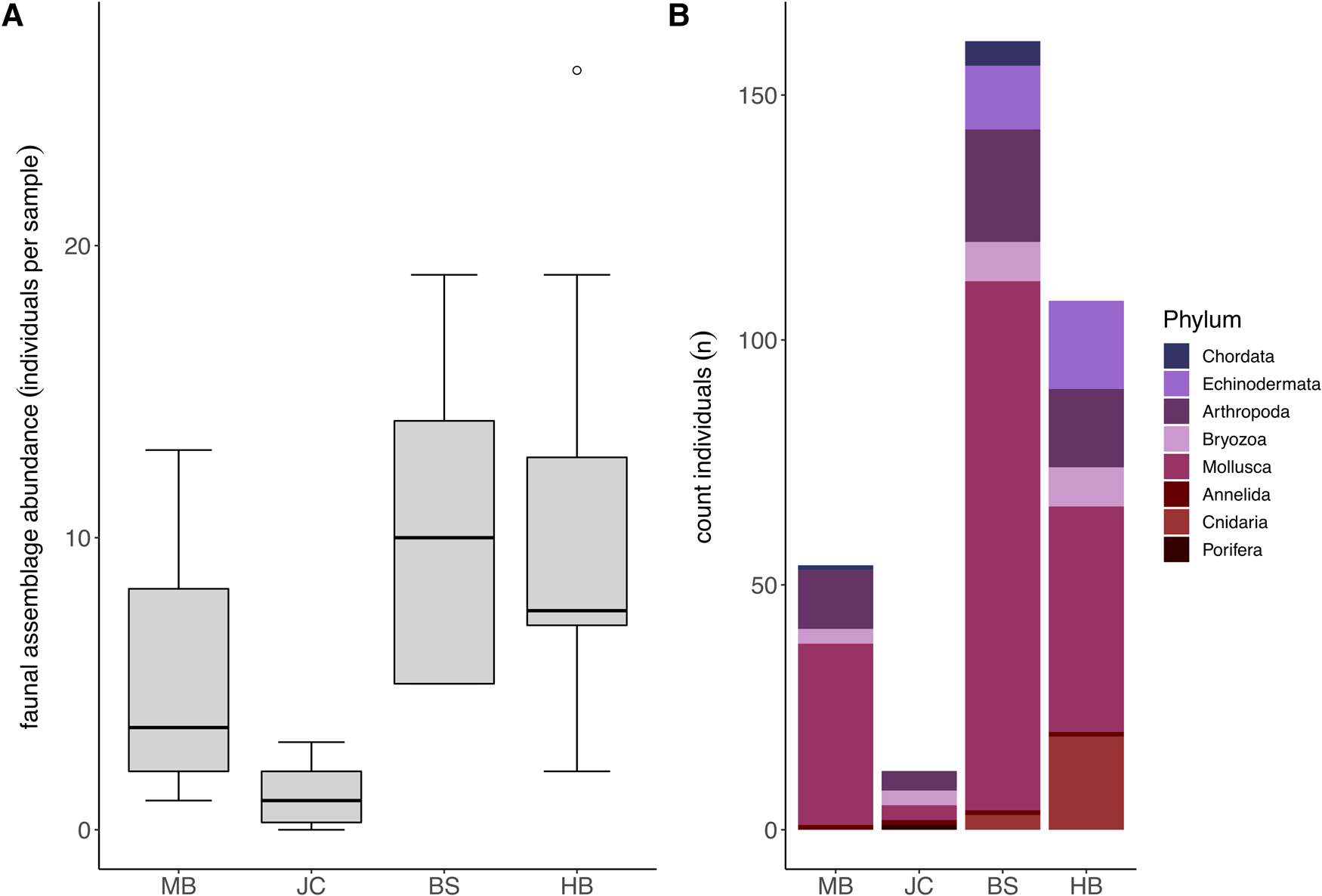
Fig. 3. Faunal assemblages associated with S. polyschides sporophytes at each study site. (A) Mean total faunal abundance per holdfast at each study site (note that plot excludes maximum outlier at BS with an abundance of 593). (B) Combined total abundance per site of each coarse taxonomic grouping.
Taxon richness and Shannon diversity was generally higher in fully wave-exposed sites (BS, HB) than the moderately exposed sites (MB, JC) (Kruskal–Wallis test; P < 0.05). Taxon richness values (Figure 4A) were significantly lower at JC in comparison with BS and HB, whilst Shannon diversity (Figure 4B) was lower at MB compared with both BS and HB, and lower at JC compared with HB (multi comparison analysis after significant Kruskal–Wallis test; P < 0.05).
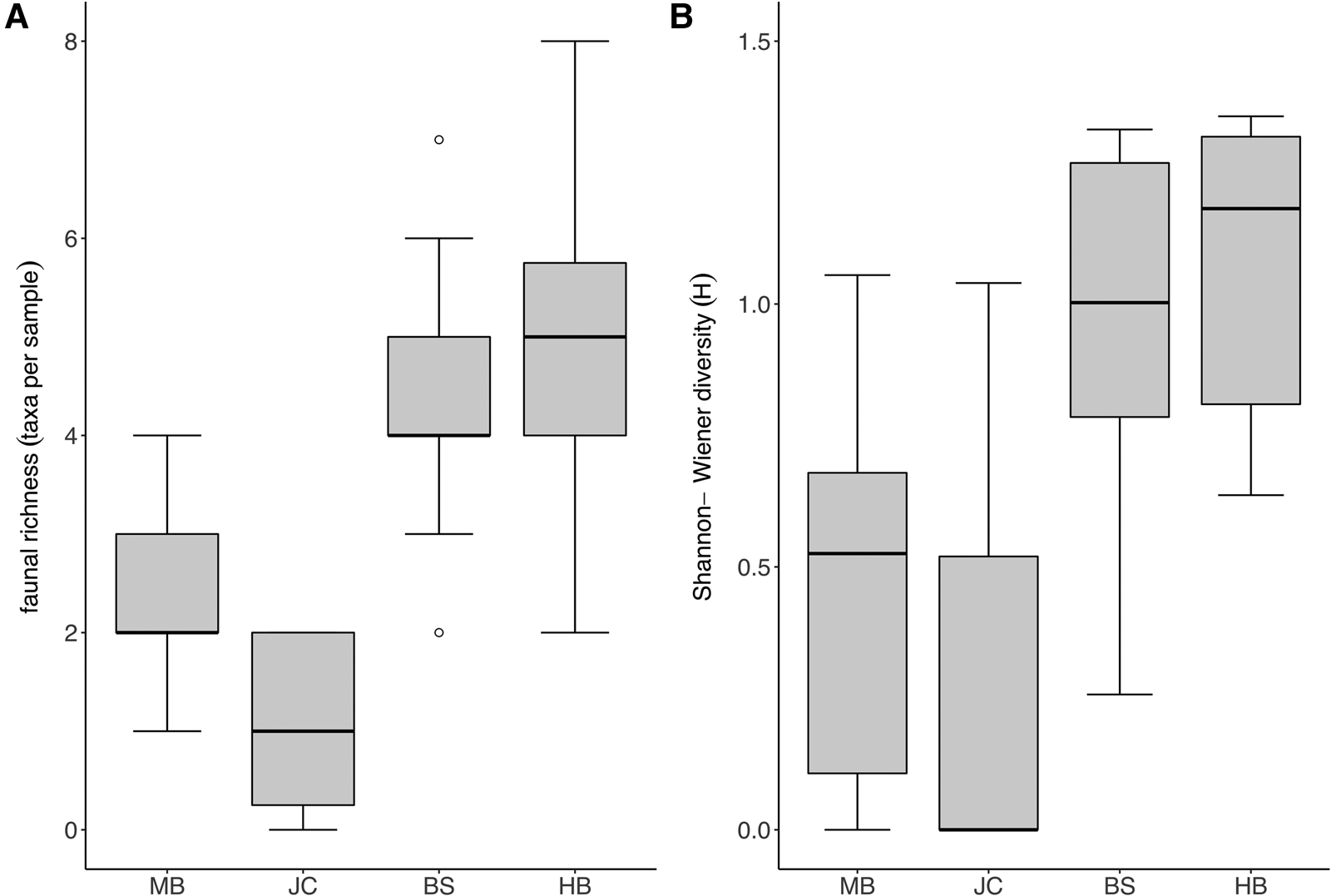
Fig. 4. Diversity of faunal assemblage associated with S. polyschides sporophytes at each study site. (A) Mean taxon richness (coarse taxonomic groups) per holdfast at each study site (note that plot excludes maximum outlier at BS with a diversity of 27 taxa). (B) Mean Shannon-Wiener diversity (H) for each site, based on coarse taxonomic groupings.
We found positive correlations between holdfast volume and faunal abundance (Spearman's rank correlation: P = 0.029, ρ = 0.34), and between holdfast volume and taxon richness (P = 0.036, ρ = 0.33), respectively. In contrast, holdfast volume and Shannon diversity were not significantly correlated (P > 0.05).
Metric MDS showed that faunal assemblages (described at the phyla level) were highly variable both within and between sites (Figure 5). Even so, clear separation between most sites was evident. Results of a one-way PERMANOVA (4999 permutations, unrestricted) shows significant differences of phyla composition between sites (df = 3, F = 7.49, P < 0.05). Post-hoc pairwise tests showed that all sites differed from one another (P < 0.05; Table 1) with the exception of BS and HB (P > 0.05). SIMPER analysis determined that the Mollusca taxonomic grouping (30–44% contribution) was the main driver of dissimilarity in assemblage structure in all site comparisons (Table 1). The Mollusca grouping comprised primarily of Steromphala umbilicalis, although Patella pellucida and Nudibranchia were also common at some sites.
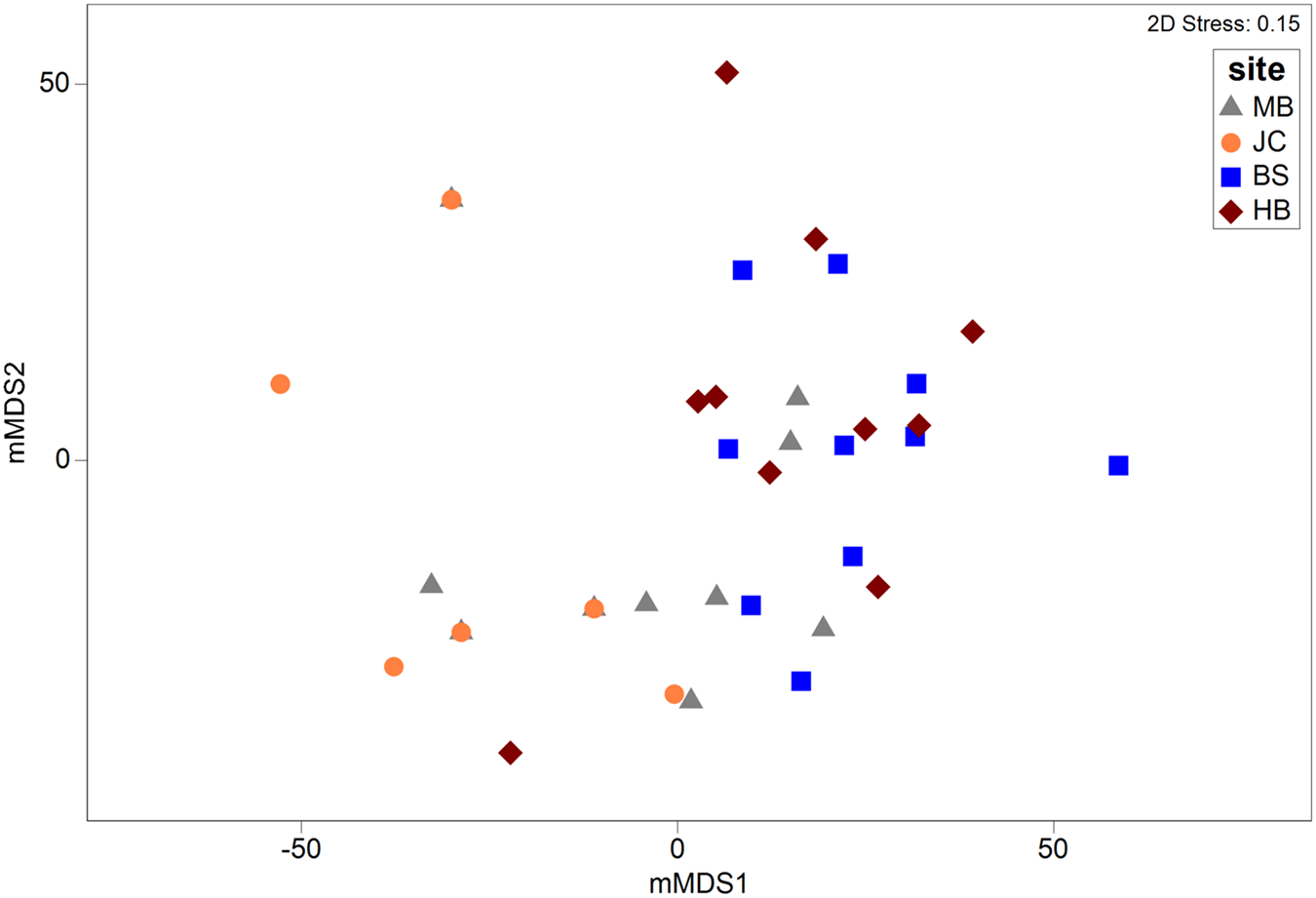
Fig. 5. Metric MDS plot depicting multivariate assemblages across sites. Ordination is based on a Bray–Curtis similarity matrix (with dummy variable = 1) generated from square root transformed abundance data at a coarse taxonomic level (i.e. phyla).
Table 1. Results of post-hoc pairwise tests to determine differences between sites and, where significant differences were found, results of SIMPER analysis to determine phyla contributing most to the observed differences between sites
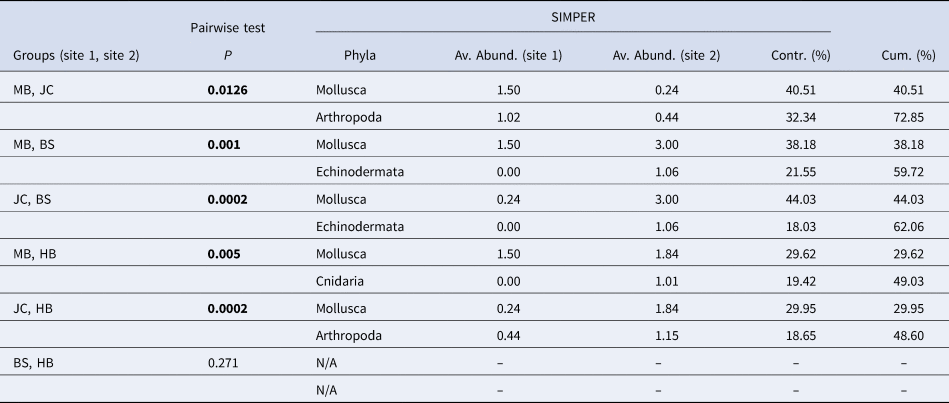
‘Av. Abund.’ is the average abundance of each phyla at each site in the comparison; ‘Contr. (%)’ is contribution of each phyla to the overall dissimilarity between sites; and ‘Cum. (%)’ is a running total percentage of the contribution to the observed dissimilarity (cumulative contribution). Significant P values in bold.
Most organisms were found within holdfasts, although some individuals (e.g. the molluscs Steromphala umbilicalis and Patella pellucida) were associated with the decaying stipe and blade. Across the study, we found no evidence of strong or significant correlations between habitat volume (i.e. holdfast internal living space) and either faunal abundance or richness.
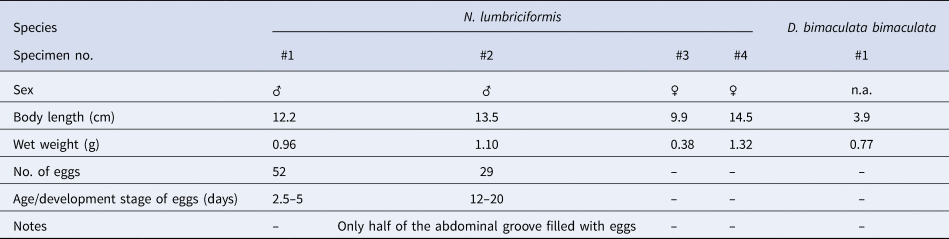
One sample from BS was analysed to a finer taxonomic resolution. Five fish species were recorded within the holdfast, namely one individual of the two-spotted clingfish Diplecogaster bimaculata bimaculata (Bonnaterre, 1788) and four individuals of the worm pipefish Nerophis lumbriciformis (Jenyns, 1835) (Figure 6A). The fishes were measured and weighed, and sexes were assigned for N. lumbriciformis (Table 2). Interestingly, two of the male pipe fishes had eggs attached to the abdominal breeding groove (exclusively paternal brood care in Syngnathidae; see Wheeler, Reference Wheeler1969; Monteiro et al., Reference Monteiro, Almada, Santos and Vieira2001), which showed different developmental stages of the 30 day brooding period (Figure 6B, C). Based on a previous developmental study (Monteiro et al., Reference Monteiro, Almada and Vieira2003) we estimated that eggs brooded by one male were 2.5–5 days post-fertilization (see Figure 6B with visible embryonic shield), and the other at least 12 days post-fertilization (≥12, but <20 days with clearly visible eyes of the fish larvae, see Figure 6C).
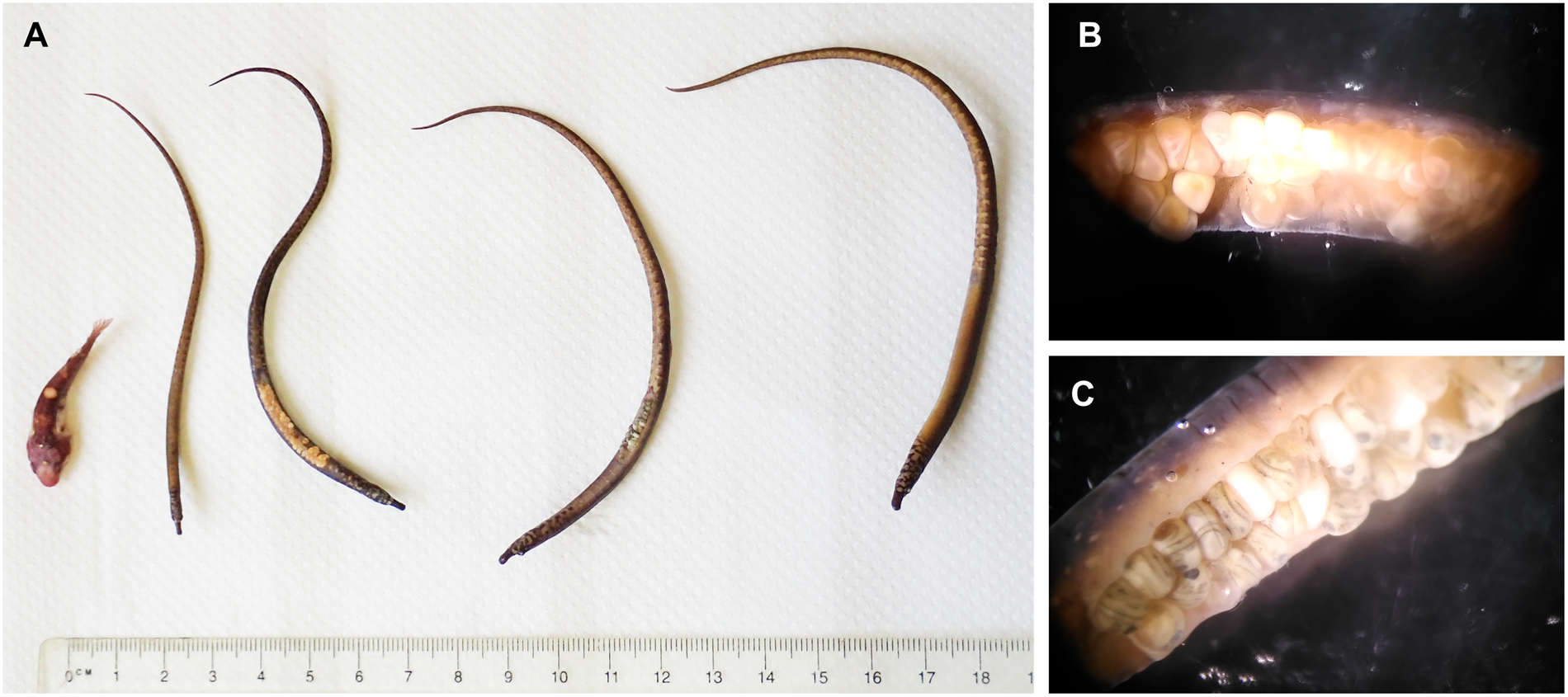
Fig. 6. Fishes found in one of the S. polyschides holdfasts collected at Bovisand (BS). (A) Fishes from left to right: one Diplecogaster bimaculata bimaculata (two-spotted clingfish), four Nerophis lumbriciformis (worm pipefish). (B) Abdominal groove of N. lumbriciformis male no. 1 with 52 eggs, 2.5–5 days after fertilization. (C) Abdominal groove of N. lumbriciformis male no. 2 with 29 eggs, 12–20 days after fertilization (estimated development stages of eggs according to Monteiro et al., Reference Monteiro, Almada and Vieira2003).
Table 2. Biological information for fish sampled in one S. polyschides holdfast from Bovisand (BS)
Besides the phylum Chordata (including the named fish species), species from five other phyla were recorded in the S. polyschides sample (Table 3), with the highest number of individuals belonging to the Amphipoda, followed by Polychaeta and the molluscan gastropod Steromphala umbilicalis. In terms of biomass, we recorded the greatest values for the crab Cancer pagurus, the gastropod Steromphala umbilicalis and the pipefish Nerophis lumbriciformis.
Table 3. The abundance (count) and biomass (wet weight) of fauna recorded in association with a single holdfast sampled at Bovisand (BS)
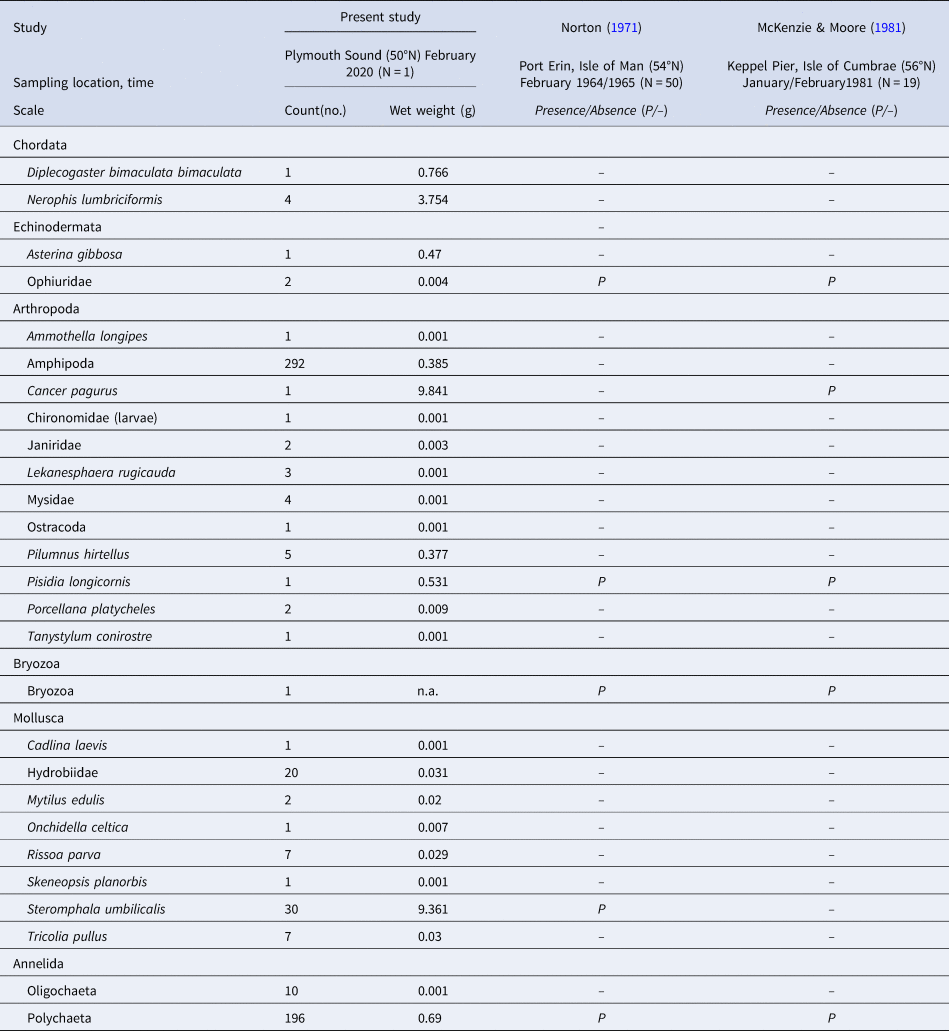
The taxa recorded here are compared with those listed in previous studies by Norton (Reference Norton1971) and McKenzie & Moore (Reference McKenzie and Moore1981), in terms of presence/absence.
Discussion
We observed considerable variability in the density, biomass and morphology of S. polyschides sporophytes between sites. Faunal abundance, taxon richness and Shannon diversity varied between moderately (JC, MB) and fully (BS, HB) wave-exposed sites, whereas sporophyte density, biomass and morphology did not differ between levels of wave exposure. In general, density, internal volume and biomass were greatest at MB, which is the least wave-exposed site and the site most influenced by the estuaries within Plymouth Sound. Given that S. polyschides is an opportunistic species and generally outcompeted by the longer-lived Laminaria species, competitive release at MB where Laminaria species are less abundant (authors pers. obs.) may partly explain this pattern. Interestingly, density and holdfast internal volume were lowest at JC, but total length and (to a lesser extent) biomass were high, indicating that a number of senescing sporophytes retained a partial stipe in addition to the decaying holdfast. Greater wave exposure at BS and HB might induce faster senescence in comparison to the less wave-exposed sites (MB and JC), although timing of sporophyte recruitment may also be important. High levels of between-site variability in population density and morphology have been previously reported for many kelp species, being seemingly a common feature of macroalgal stands, and may be driven by a range of factors including differences in wave exposure, sedimentation rates, light availability, substratum characteristics and grazing pressure (Watanabe & Harrold, Reference Watanabe and Harrold1991; Pedersen et al., Reference Pedersen, Nejrup, Fredriksen, Christie and Norderhaug2012; Smale et al., Reference Smale, Burrows, Evans, King, Sayer, Yunnie and Moore2016; Traiger & Konar, Reference Traiger and Konar2018; Smale et al., Reference Smale, Pessarrodona, King, Burrows, Yunnie, Vance and Moore2020).
Within-site variability in density and morphology (i.e. between quadrats and sporophyte samples) was also high. Previous larger-scale surveys in the UK have shown that S. polyschides generally exhibits a patchy distribution, inhabiting disturbed areas, semi-stable substratum or marginal habitats where Laminaria species are less abundant (Smale & Moore, Reference Smale and Moore2017). Clearly, a better understanding of the distribution, population structure and ecological interactions relating to S. polyschides is needed to predict the effects of continued environmental change.
We recorded marked variability in faunal abundances and coarse-level assemblage diversity between sites, wave exposure and sporophyte samples. Pronounced small-scale spatial variability is a commonly observed pattern in coastal marine ecosystems (Fraschetti et al., Reference Fraschetti, Terlizzi and Benedetti-Cecchi2005), and has been reported for kelp holdfast assemblages previously (Anderson et al., Reference Anderson, Diebel, Blom and Landers2005; Teagle et al., Reference Teagle, Moore, Jenkins and Smale2018). Spatial variability may be underpinned by differences in the biogenic structures themselves (e.g. holdfast size and age), or differences in physical (e.g. reef topography, wave exposure, light) or biological (e.g. food supply, predation, dispersal) factors occurring over similar spatial scales. While the drivers of the spatial variability patterns observed here remain unknown, it is likely that the structure of the biogenic habitat itself is important, given that internal living space, total faunal abundance, and diversity were notably lower at JC compared with other sites. Overall, total faunal abundance and diversity (faunal taxon richness and Shannon–Wiener diversity) were markedly higher at the two more wave-exposed sites (BS, HB) compared with the more sheltered sites (MB, JC), which may in turn relate to differences in food supply, recruitment or sedimentation rates (Smale et al., Reference Smale, Wernberg and Vance2011; Bustamante & Branch, Reference Bustamante and Branch1996; Teagle et al., Reference Teagle, Hawkins, Moore and Smale2017), all of which could influence assemblage structure (Teagle et al., Reference Teagle, Hawkins, Moore and Smale2017, Reference Teagle, Moore, Jenkins and Smale2018).
We also recorded significant positive correlations between holdfast volume and faunal abundance and richness. Positive relationships between the habitat size offered by foundation species and associated faunal communities are commonplace and have been reported previously for several kelp species (Anderson et al., Reference Anderson, Diebel, Blom and Landers2005; Tuya et al., Reference Tuya, Larsen and Platt2011; Teagle et al., Reference Teagle, Moore, Jenkins and Smale2018). Intuitively, a larger holdfast offers more living space and may support a higher number of individuals and taxa, although larger holdfasts may become accessible to predators or more susceptible to wave action (Christie et al., Reference Christie, Jørgensen, Norderhaug and Waage-Nielsen2003). It is unclear whether the larger holdfasts offered favourable refugia during winter storms and whether these relationships are consistent through time. Clearly, further work on the population structure of S. polyschides and its associated assemblages in south-west England is needed to determine patterns and drivers of spatiotemporal variability.
Remnant holdfasts of senescing Saccorhiza polyschides sporophytes provided valuable habitat for a high abundance and diversity of organisms, with >27 distinct taxa (belonging to eight phyla) and up to ~600 individuals recorded within a single holdfast. Perhaps most notably, we recorded five fish individuals within a single holdfast, including four individuals of the worm pipefish Nerophis lumbriciformis. Previous studies on S. polyschides have recorded common small fish species including Montagu's sea snail Liparis montagui (also recorded at JC) and the two-spotted clingfish Diplecogaster bimaculata bimaculata (also recorded at BS) within the ‘bulb interior’ of holdfasts (e.g. Ryland, Reference Ryland1969; Norton, Reference Norton1971; McKenzie & Moore, Reference McKenzie and Moore1981; Gordon, Reference Gordon1983). Yet, to our knowledge, species belonging to the Syngnathidae (family of fish including seahorses, sea dragons, pipefishes) have not previously been observed in association with S. polyschides, demonstrating a previously overlooked interaction.
The worm pipefish Nerophis lumbriciformis is often found in intertidal habitats fringing the North-east Atlantic Ocean, mostly amongst cobbles and stones or in association with macroalgae (Wheeler, Reference Wheeler1969; Dawson, Reference Dawson, Whitehead, Bauchot, Hureau, Nielsen and Tortonese1986; Monteiro et al., Reference Monteiro, Da Natividade Vieira and Almada2002a, Reference Monteiro, Vieira and Almada2002b). While N. lumbriciformis has been commonly observed in association with macroalgal stands (e.g. living in-between Ascophyllum nodosum fronds), it has not been previously found inside a macroalgal holdfast. It seems likely that N. lumbriciformis utilized the bulbous holdfast of S. polyschides as a microhabitat during periods of low tide (to reduce heat or desiccation stress) (Monteiro et al., Reference Monteiro, Vieira and Almada2002b), as foraging ground (Polte & Buschbaum, Reference Polte and Buschbaum2008), and/or as a shelter from heavy wave action during winter storms, although further temporal sampling is needed to determine the seasonality of this behaviour. Given that two of the male N. lumbriciformis were brooding, S. polyschides holdfasts may be important as temporary nursery microhabitats. In coastal waters of the British Isles, Wheeler (Reference Wheeler1969) observed the breeding season of N. lumbriciformis to extend from June until August (exceptionally May–September), although Monteiro et al. (Reference Monteiro, Almada, Santos and Vieira2001) later proposed a more general reproductive window (during periods of water temperatures between 13–16°C) as breeding seasons vary between regions and populations. However, we observed males carrying fertilized eggs in February, when local sea temperatures are typically <10°C (Smyth et al., Reference Smyth, Fishwick, Al-Moosawi, Cummings, Harris, Kitidis, Rees, Martinez-Vicente and Woodward2010; Pessarrodona et al., Reference Pessarrodona, Moore, Sayer and Smale2018), which constitutes an anomalous observation that warrants further work.
In previous studies on typical Laminarian kelp species, observations of fishes and large decapods inhabiting holdfasts are very rare (McKenzie & Moore, Reference McKenzie and Moore1981; Teagle et al., Reference Teagle, Moore, Jenkins and Smale2018). In contrast, both this study and previous research show that large mobile fauna commonly inhabit the bulbous hollow holdfast structure offered by S. polyschides sporophytes (Norton, Reference Norton1971; McKenzie & Moore, Reference McKenzie and Moore1981). It is still unknown whether these holdfasts are used as a temporary refugia or permanent microhabitats, although due to the pseudo-perennial lifecycle of S. polyschides (Norton & Burrows, Reference Norton and Burrows1969), temporary refugia is more likely. Our study was conducted during an unusually stormy winter season, with named storms ‘Ciara’ and ‘Dennis’ occurring around the sampling dates. Both storm events were characterized by high amounts of rainfall, and above-average wind speeds and wave heights (Kendon, Reference Kendon2020a, Reference Kendon2020b; Parry et al., Reference Parry, Barker, Sefton, Hannaford, Turner, Muchan, Matthews and Pennington2020; Galvin, Reference Galvin2021). Maximum hourly wind gust speeds of 250 kn for ‘Ciara’ and 200 kn for ‘Dennis’ were recorded at MB (Galvin, Reference Galvin2021), representing the most sheltered site in this study. It is feasible, therefore, that large mobile fauna utilized the microhabitat provided by S. polyschides holdfasts as temporary shelter.
Further detailed studies are necessary to understand the effects of seasonal changes on S. polyschides population structure, its associated faunal community, and the variability between sites with differing levels of wave exposure.
In summary, the unique biogenic structure provided by S. polyschides supports abundant faunal assemblages, even during periods of senescence when the majority of the sporophyte has decayed and only remnant holdfasts remain. As a habitat former, S. polyschides may function differently to other ‘true’ kelp species, as the bulbous hollow holdfast offers greater internal living space for utilization by large mobile fauna, such as the N. lumbriciformis individuals reported here. However, in contrast to Laminarian kelps which may persist for >10 years and therefore offer long-lived stable habitat (Smale et al., Reference Smale, Burrows, Evans, King, Sayer, Yunnie and Moore2016; Teagle & Smale, Reference Teagle and Smale2018) S. polyschides is short-lived and offers temporary refuge, which may be particularly important during periods of intense wave action. Given that S. polyschides is thought to be proliferating in the cooler northern parts of its range (Birchenough & Bremner, Reference Birchenough and Bremner2010; Yesson et al., Reference Yesson, Bush, Davies, Maggs and Brodie2015), a better understanding of its role in habitat provision and local biodiversity maintenance is needed to predict the wider impacts of environmental change.
Acknowledgements
Both authors thank members of the BEECH group for their support and Jack Sewell for taxonomic assistance and seaweed drawing.
Financial support
NS was supported by the Natural Environmental Research Council (NERC grant number NE/SO07210/1) as part of the INSPIRE DTP. DS was funded by a UKRI Future Leader Fellowship (MR/S032827/1).











