INTRODUCTION
Q fever is a zoonosis caused by the intracellular bacterium Coxiella burnetii which has an almost worldwide occurrence [Reference Raoult, Marrie and Mege1]. Human infection is caused by transmission from infected animals, in particular small ruminants. Animals shed C. burnetii in milk, urine, faeces and especially in birth products [Reference Parker, Barralet and Bell2]. Infection is asymptomatic in almost 60% of cases. In the 40% of patients with clinical symptoms, there is usually an acute onset with fever, headache, fatigue and frequently an atypical pneumonia. Hepatitis may also occur [Reference Maurin and Raoult3]. An estimated 1% of infected individuals develop chronic Q fever months to years later with endocarditis as the most frequent presentation. In particular, pregnant women, patients with immune deficiencies or patients with cardiac or vascular grafts are at risk for developing chronic Q fever [Reference Parker, Barralet and Bell2]. In The Netherlands, acute Q fever in humans is a notifiable disease. Up to 2007, 10–20 cases were notified annually [Reference van Steenbergen4]. However, over the past 3 years large epidemics of Q fever occurred in the south of The Netherlands with 168, 1000 and >2300 notifications nationwide in 2007, 2008, and 2009, respectively. Currently, the recommended treatment for acute Q fever is doxycycline (200 mg/day) for 2–3 weeks. Preferably treatment is initiated within 3 days after the onset of symptoms [Reference Parker, Barralet and Bell2, Reference Nabuurs-Franssen5]. Moxifloxacin is recommended in The Netherlands as treatment of second choice, e.g. if doxycycline is not tolerated, and should be given at a dose of 400 mg/day orally for 2–3 weeks [Reference Nabuurs-Franssen5]. For general practitioners (GPs) in The Netherlands, doxycycline (100 mg/day) for 7 days is among the recommended treatments for community-acquired pneumonia of unknown cause, as are β-lactam antibiotics. The latter are not considered to be effective against C. burnetii because of their poor cell-membrane permeability.
There are very few clinical studies investigating the effectiveness of different antibiotic regimens for the treatment of acute Q fever. For a number of antibiotics, only retrospective analyses, case reports or in vitro data are available [Reference Gikas6–Reference Boulos14]. Some of these studies show contradictory results. In both a randomized controlled trial [Reference Sobradillo15] and a small retrospective study [Reference Gikas6], doxycycline outperformed other antibiotics including macrolides like erythromycin and clarithromycin, i.e. a shorter time to defervescence for patients treated with doxycycline. Another retrospective study found a shorter, but not statistically different, time to defervescence in favour of clarithromycin and moxifloxacin over doxycycline [Reference Morovic10].
The first aim of this study was to analyse the antibiotic treatment regimens of notified acute Q fever patients in 2007 and 2008 in The Netherlands (type, dose, and duration of the antibiotic therapy and treatment delay) and to compare the treatment regimens between the first year of the epidemic in The Netherlands (2007) with the second year (2008). This comparison was made because we hypothesized that physicians became much more aware of Q fever and its recommended treatment after the first year of the epidemic because of widespread targeted information from the local health authorities and the attention in the medical and national press. A subsequent aim was to assess whether hospitalization, as an indicator of severe illness, was related to the initial antibiotic therapy.
PATIENTS AND METHODS
Patients
In the most affected region of The Netherlands, the Municipal Health Services (MHS) ‘Hart voor Brabant’ and ‘Brabant-Southeast’ approached the 864 notified Q fever patients with a date of disease onset in 2007 and 2008 for participation in the present study. Inclusion criteria were compatible clinical signs with laboratory-confirmed acute Q fever and a known month of disease onset in 2007 or 2008. Q fever patients that were reported to other MHS and patients with an unknown month of disease onset were excluded. Patient consent was obtained between February 2009 and April 2009. According to Dutch law for research involving human subjects there was no need for approval by a medical ethics committee.
Data collection
GPs of the consenting patients were asked to complete a questionnaire for each study patient on the basis of information from their medical records. The questionnaire included personal data, the number of consultations to the GP during the disease course, whether or not a specialist had been consulted and details on the antibiotic regimen that was prescribed. In addition, GPs were contacted by telephone in order to collect data about the date of hospital admission and discharge. Data about hospitalization (yes/no), date of disease onset, year of birth and gender had been previously collected by the MHS as part of the routine information collected for each notification. Data on comorbidity, smoking behaviour and the number of complaints and complications arising from Q fever were self-reported by patients on a postal questionnaire administered by the MHS.
Data analysis
Treatment regimens of the study patients were analysed and described with the emphasis on the initial antibiotic treatment. For docycycline and moxifloxacin (treatments recommended as first and second choice, respectively) not only initial treatment regimens were analysed but also treatments prescribed at any (other) time. To compare the treatment regimens between the first year of the epidemic in The Netherlands (2007) with the second year (2008), treatment regimens of patients with disease onsets in 2007 and 2008 were analysed separately.
To analyse whether initial antibiotic treatment was related to clinical course, four different treatment groups were defined based on the initially prescribed antibiotic:
(1) Doxycycline at the appropriate dosage (200 mg/day).
(2) Moxifloxacin.
(3) Other possibly effective antibiotics, including doxycycline at low dosage [doxycycline (100 mg), clarithromycin, erythromycin, levofloxacin, ciprofloxacin, norfloxacin, co-trimoxazole (trimethoprim+sulfamethoxazole)].
(4) Antibiotics not considered to be effective (β-lactam antibiotics, azithromycin).
The first category also included one patient who was treated with tetracycline (250 mg four times a day) as first treatment.
These categories were chosen because of the expected efficacy of different types of antibiotics. As moxifloxacin was recommended as treatment of second choice after doxycycline in The Netherlands, it was analysed as separate group [Reference Nabuurs-Franssen5]. A number of other new fluoroquinolones such as levofloxacin may have had comparable efficacy [Reference Boulos14], but less information on their clinical effcacy was available, as mainly in vitro studies were available. Other fluoroquinolones such as gatifloxacin were not available in The Netherlands. Co-trimoxazole is recommended as treatment during pregnancy [Reference Carcopino12]. For azithromycin, an in vitro study suggested an unfavourable minimum inhibitory concentration [Reference Lever13].
First, we analysed (univariate) associations between treatment group and the following clinical course characteristics: hospitalization after at least 2 days of antibiotic treatment, hospitalization in general, number of GP consultations, period between first and last GP consultation, duration of hospitalization, switch between antibiotics, and diagnosis during illness. Second, we assessed (univariate and multivariate) the association between treatment group and hospitalization. The outcome measure in these analyses was defined as hospitalization after antibiotic treatment for at least 2 days (yes/no). Because patients who received their first treatment during or after hospitalization did not have an outcome, they were excluded from these analyses. Patients for whom the date of their first treatment was unknown were excluded from all analyses.
Age, gender, year of disease onset, therapeutic delay, underlying morbidity, number of symptoms in the first week of illness, and smoking were regarded as potential confounding factors in the relationship between treatment group and hospitalization after at least 2 days of antibiotic treatment. Age was categorized in two groups: ⩽50 years and >50 years. Therapeutic delay was defined as the number of days between disease onset and the prescription of the first antibiotic treatment. Underlying morbidity was defined as having reported one or more of the following conditions in the patient questionnaire: diabetes, absence of spleen, chronic kidney or liver disease, heart disease, leukaemia or other malignancies, immunocompromised status, lung disease or rheumatism. The number of self-reported symptoms during the first week of illness was obtained from a list of 23 self-reported symptoms attributed to Q fever (fever, night sweats, malaise, headache, sore throat, neck stiffness, myalgia, joint pain, cough, dyspnoea, chest pain, nausea, diarrhoea, abdominal pain in general, upper abdominal pain, jaundice, confusion, memory/attention deficits, ear problems, eye problems, rash, weight loss, severe fatigue). For all continuous variables, the median was used as cut-off point to dichotomize the determinants that were added in the logistic models.
Statistical analysis
Data were entered in Microsoft Access and were analysed in Microsoft Excel (Microsoft Corp., USA) and SPSS version 17.0 for Windows (SPSS Inc., USA). In univariate analyses variables were compared using χ2 tests. Multivariate logistic regression was used to calculate odds ratios (OR) and corresponding 95% confidence intervals (CI) to assess the association between treatment group and hospitalization after at least 2 days of antibiotic treatment. For the determinants that were regarded as potential confounders univariate logistic regression was performed. Determinants with a univariate P value <0·15 were added in the multivariate model. Age and gender were included in the multivariate model regardless of the univariate P value. A P value <0·05 was considered to indicate statistical significance in the multivariate model.
RESULTS
Of the 864 patients who were approached by the MHS, 739 patients responded of whom 594 (68·8% of the patients approached) gave permission to contact their GP for further information (see Fig. 1). The GPs of 36 patients could not be contacted because the correct name of the GP was not available. The remaining 558 patients were registered at a total of 175 GP practices. In total, 136 (77·7%) of the GPs returned 452 completed questionnaires, giving an overall response rate of 52·3%. For 14 cases information on the first treatment was not available, resulting in 438 cases that could be used for the analysis (final participation rate 50·7%). Of the 438 Q fever patients that were included, 68 patients had a date of onset in 2007 and 370 a date of onset in 2008. Age and gender of the patients included in the analysis were approximately comparable with the total group of approached patients from whom data was known. However, percentage hospitalization in included patients (18·0%) was somewhat lower than in the total group of approached patients from whom data was known (23·9%).
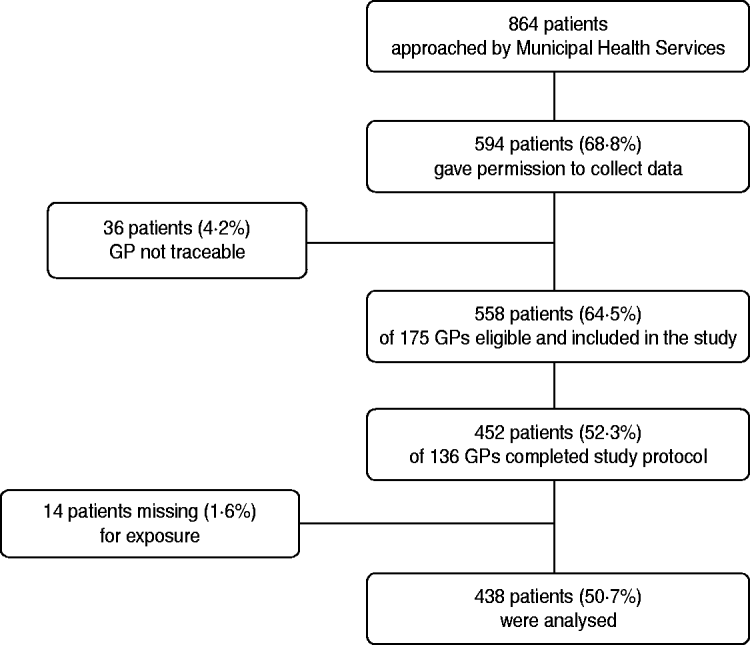
Fig. 1. Flowchart of patients and general practitioners (GPs) included in the study.
Initial antibiotic treatments
In total, 704 antibiotic treatment regimens were prescribed to the 438 patients. The first antibiotic treatment of 356 patients (81·3%) was prescribed by a GP, while 48 patients (11·0%) had their first antibiotic therapy prescribed by a medical specialist. Of 34 patients (7·8%), the prescribing physician was unknown.
Doxycycline was the most commonly prescribed initial antibiotic in 2007 as well as in 2008 (Table 1). Of the patients receiving doxycycline as initial antibiotic, the proportion receiving the recommended dosage went up from 54·5% in 2007 to 86·4% in 2008. However, in 2007 there was a very long therapeutic delay for doxycycline (200 mg dose) as initial antibiotic [median 111 days, interquartile range (IQR) 96·5–209·0, based on nine cases for whom therapeutic delay was known]. Overall there was a reduction in median therapeutic delay until a first antibiotic treatment was prescribed from 10·0 days (IQR 4·8–70·3) in 2007 to 6·0 days (IQR 3·0–18·0) in 2008 (P=0·014 with Mann–Whitney U test). Moxifloxacin was the second most commonly prescribed initial antibiotic in 2007 but in 2008 very few patients received this antibiotic. Of the antibiotics in treatment group 3, erythromycin and levofloxacin were not prescribed as initial antibiotic to any Q fever patients in the study. β-Lactam antibiotics and azithromycin (treatment group 4), considered as ineffective in the treatment of Q fever, were prescribed as initial antibiotic to a significant number of patients in 2007 as well as 2008. β-Lactam antibiotics prescribed as initial antibiotic were penicillin, amoxicillin, feneticillin, and cefuroxim.
Table 1. First prescribed antibiotic and therapeutic delayFootnote a in 2007 and 2008
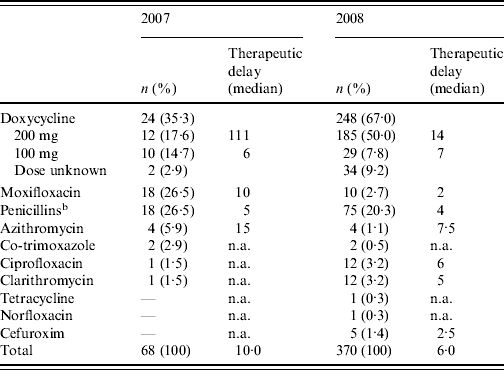
n.a., Not applicable.
a Period between disease onset and first antibiotic treatment prescribed (days).
b Penicillin, amoxicillin, feneticillin.
Baseline characteristics of the patients stratified by initial treatment group are shown in Table 2. Significant associations were found between treatment group and year of disease onset, the prescribing physician (medical specialist or GP), and consulting a medical specialist (lung specialist, internist, cardiologist).
Table 2. Baseline characteristics by initial treatment group
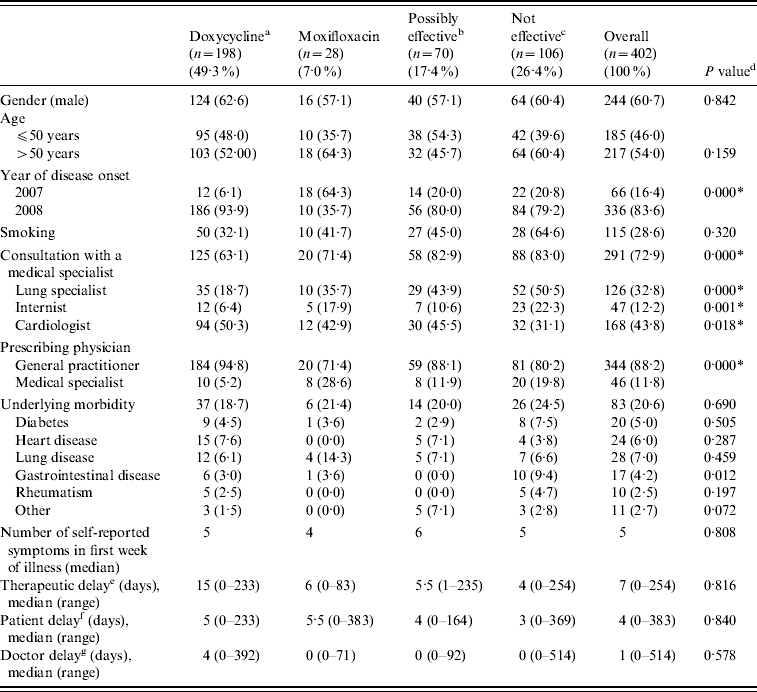
a Doxycycline at the appropriate dosage (200 mg doxycycline).
b Other possibly effective antibiotics, including doxycycline at low dosage [doxycycline (100 mg), clarithromycin, ciprofloxacin, norfloxacin, and co-trimoxazole (trimethoprim+sulfamethoxazole)].
c Antibiotics that are not considered to be effective: β-lactam antibiotics and azithromycin.
d P values of χ2 tests for the association between treatment group and baseline characteristics.
e Period between disease onset and first antibiotic treatment prescribed.
f Period between disease onset and first GP consultation.
g Period between first GP consultation and first antibiotic treatment prescribed.
* P<0·05.
Doxycycline and moxifloxacin at any time during treatment
In 2007, 41 (60·3%) of the 68 patients that received a known antibiotic therapy received an antibiotic of first or second choice (200 mg doxycycline or moxifloxacin) at any time during the Q fever treatment course. In 2008 that percentage was higher with 267 (72·2%) of the 370 patients receiving doxycycline (200 mg) or moxifloxacin. The median therapeutic delay until an antibiotic of first or second choice (200 mg doxycycline or moxifloxacin) was prescribed was 45 days in 2007 vs. 15 days in 2008. In 2007, the mean duration of 100 mg/day doxycycline treatment was 9·1 days (range 7–27), whereas for 200 mg/day doxycycline treatment the mean duration was 16 days (range 3–30). In 2008, the mean duration for doxycycline treatment was 7·6 days (range 7–14) and 14·7 days (range 7–28), respectively, for a dose of 100 mg and 200 mg/day. The mean duration of moxifloxacin treatment was 8·1 days (range 5–14 days) in 2007 vs. 9·6 days (range 5–14 days) 2008.
Association between initial antibiotic treatment and various clinical course characteristics
Clinical course characteristics stratified by initial treatment group are shown in Table 3. Significant associations were found between treatment group and hospitalization, period between first and last GP consultation, a switch between antibiotics (i.e. treatment with another type of antibiotic after the initial antibiotic therapy), and a diagnosis of pneumonia or an unknown diagnosis during the illness.
Table 3. Clinical course characteristics by initial treatment group
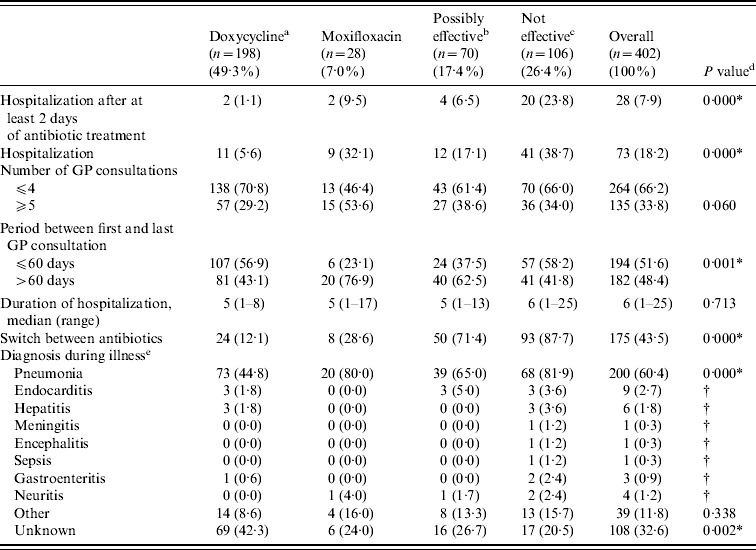
a Doxycycline at the appropriate dosage (200 mg doxycycline).
b Other possibly effective antibiotics, including doxycycline at low dosage [doxycycline (100 mg), clarithromycin, ciprofloxacin, norfloxacin, and co-trimoxazole (trimethoprim+sulfamethoxazole)].
c Antibiotics that are not considered to be effective β-lactam antibiotics and azithromycin.
d P values of χ2 tests for the association between treatment group and baseline characteristics.
e More than one diagnosis possible. Information only known from patients who completed the postal questionnaire.
* P<0·05.
† P value could not be calculated because of insufficient numbers.
Association between initial antibiotic treatment and hospitalization
In the univariate logistic analysis, hospitalization after antibiotic treatment for at least 2 days after prescription was significantly associated with age, year of disease onset and therapeutic delay. In the multivariate analysis, with the same outcome measure, a total of 388 cases were included. All treatment groups showed significantly lower risks for hospitalization in Q fever patients compared to the group of potentially ineffective antibiotics such as penicillins (Table 4). Doxycycline at the appropriate dose (200 mg/day) showed the lowest risk and was the most significant. A long therapeutic delay (>7 days) was also significantly associated with a lower risk for hospitalization. A disease onset in 2007 was significantly associated with a higher risk for hospitalization.
Table 4. Results of multivariate analysis: odds ratios for hospitalization after at least 2 days of antibiotic treatment (n=388)
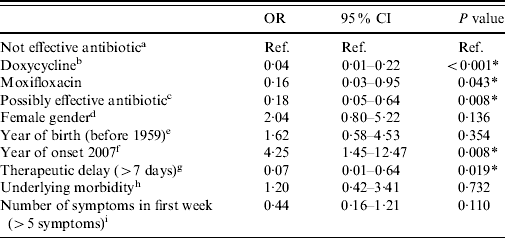
OR, Odds ratio, CI, confidence interval.
a Antibiotics that are not considered to be effective: β-lactam antibiotics and azithromycin.
b Doxycycline at the appropriate dosage (200 mg/day).
c Other possibly effective antibiotics, including doxycycline at low dosage [doxycycline (100 mg/day), clarithromycin, ciprofloxacin, norfloxacin, and co-trimoxazole (trimethoprim+sulfamethoxazole)].
d Reference=male.
e Reference=1959 or later.
f Reference=2008.
g Reference=⩽7 days.
h Reference=no underlying morbidity.
i Reference=⩽5 symptoms.
* P<0·05.
DISCUSSION
This study demonstrated that doxycycline was the antibiotic most prescribed to Dutch Q fever patients in 2007 and 2008. This is in accordance with prevailing guidelines [Reference Nabuurs-Franssen5]. Treatment with antibiotics from all three groups with potentially effective antibiotics, i.e. the recommended first and second choice antibiotics (200 mg/day doxycycline and moxifloxacin) and the possibly effective antibiotics (100 mg doxycycline, clarithromycin, ciprofloxacin, norfloxacin, co-trimoxazole), were associated with a reduced risk of hospitalization in Q fever patients after adjustments for known important confounders, compared to treatment with a group of potentially ineffective antibiotics such as penicillins. Doxycycline (200 mg/day) was associated with the greatest reduction in the risk of hospitalization. In our study a diagnosis of hepatitis or endocarditis (a presentation of chronic Q fever) was rare, therefore results are not necessarily applicable to patients presenting with these clinical pictures. Because of the high number of cases during the Q fever epidemics in The Netherlands in 2007–2009, many cases might be at risk for developing chronic Q fever after the study period. It could be speculated that effective treatment in the acute phase of the disease may also have an effect on the prevention of complications like chronic Q fever.
In our multivariate analysis we have excluded patients who received their first antibiotics in hospital, in order to use hospital admission after at least 2 days of antibiotic treatment as the outcome measure. It is possible that patients who received their first antibiotics in hospital had a worse outcome than others. However, we were not able to analyse this.
In the few previous clinical studies on the effectiveness of different antibiotic regimens for the treatment of acute Q fever, defervescence was the main outcome measure. Our findings, although another outcome measure was used, are consistent with the findings of Gikas et al. [Reference Gikas6], who demonstrated in their retrospective study that hospitalized Q fever patients treated with doxycycline (200 mg/day) or macrolides had a significantly shorter time to defervescence than patients treated with β-lactam antibiotics. Comparable to the findings of both Gikas et al. [Reference Gikas6] and Sobradillo et al. [Reference Sobradillo15], doxycycline (200 mg/day) was more effective than macrolides. In contrast, the retrospective study of Morovic [Reference Morovic10] found no statistically significant differences in time to defervescence for doxycycline (200 mg/day) compared to clarithromycin and moxifloxacin, but the number of patients included in their study was relatively low (n=77). It should be noted that both retrospective studies did not correct for possible confounders [Reference Gikas6, Reference Morovic10], reducing the validity of these studies.
In 2007 and 2008 more than a quarter of patients received β-lactam or azithromycin as initial antibiotic treatment, which are not considered to be effective against C. burnetii. No fewer than 24% of the patients who were treated initially with one of these antibiotics was hospitalized after at least 2 days of antibiotic prescription. The therapeutic delay differed considerably between 2007 and 2008; both for delay until the first antibiotic treatment was prescribed and for delay until the first or second choice antibiotics (200 mg/day doxycycline and moxifloxacin) were prescribed. Particularly the long therapeutic delay for doxycycline at the appropriate dose in 2007 is remarkable. This suggests that the recommended treatment in these cases was prescribed only when the serological test, perhaps unexpectedly, showed a positive result. Probably, the difference in therapeutic delay between 2007 and 2008 in general is because in 2007 Q fever was a relatively unknown disease that had not previously occurred in The Netherlands at this scale. Therefore, during the outbreak in 2007, GPs were not familiar with the clinical picture and unaware of existing recommendations for treatment. Increased awareness among GPs and medical specialists, as a consequence of widespread targeted information from the local health authorities and the attention in the medical and national press [Reference Nabuurs-Franssen5, Reference Van Steenbergen16], shortened the diagnostic and treatment delay in 2008. These specific treatment recommendations could also have contributed to the increase in prescriptions for doxycycline and the decrease in moxifloxacin in 2008.
This study has a number of potential limitations that are related to its observational retrospective design. First, the self-reported questionnaire was administered 1 year after disease onset for patients from 2008 and 1 to 2 years after disease onset for patients from 2007. This is a lengthy recall period that may well have affected the reliability of the information about comorbidity, diagnosis during the illness, and the number of complaints arising from Q fever during the first week of illness. Second, the final response rate of the study was relatively low. Included cases were approximately comparable to the total group of patients approached as for age and gender, but hospitalization rate was somewhat lower. Third, the date of disease onset that was reported in the notifications was often unclear, possibly because of the aspecific nature of Q fever signs and symptoms. This might have affected the accuracy of the measurement of therapeutic delay. Finally and most importantly, it is difficult to separate causes and effects in this study. The choice of a particular type of antibiotic may be influenced by factors like age, underlying morbidity, disease severity at initial presentation, and preferences of the prescribing GP or medical specialist. We have tried to include all possible factors that could have influenced the relationship between antibiotic treatment regimen and hospitalization in the multivariate analysis. However, we cannot exclude residual confounding or selection bias. An example of this is that, as an unexpected result, we found that a long therapeutic delay (>7 days) significantly decreased the risk for hospitalization. This is possibly because patients with a short therapeutic delay have a more severe disease at the initial presentation. We attempted to correct for ‘disease severity at the initial presentation’ by adding ‘number of self-reported symptoms in the first week of the disease’ as an indicator of this in the multivariate analysis, but apparently there is residual confounding. Furthermore, in our analysis we only focused on the initial antibiotic therapy. However, some patients switched between antibiotics during the treatment and we cannot exclude that antibiotics received after the initial treatment also had influence on the clinical course. Therefore, randomized controlled trials are still needed to provide unbiased evidence regarding the effectiveness of the various antibiotic treatments for Q fever.
Nevertheless, the study suggests that doxycycline as well as moxifloxacin and the group of other possible effective antibiotics reduces the risk of hospitalization in acute Q fever patients, with doxycycline showing the highest and most significant risk reduction. Therefore, based on the present study, there is no reason to change current guidelines that recommend doxycycline as antibiotic of first choice and moxifloxacin as second choice for the treatment of acute Q fever.
ACKNOWLEDGEMENTS
We thank all the patients and GPs who participated in this study. This work was financed from the regular annual budget that is made available by the Dutch Ministry of Health, Welfare and Sport to the Centre for Infectious Disease Control.
DECLARATION OF INTEREST
None.







