Copper in its raw geological form played a central role in North America's social and economic networks from the Archaic period (Hill et al. Reference Hill, Greenlee and Neff2016, Reference Hill, Seeman, Nolan and Dussubieux2018; Martin Reference Martin1999; Sanger et al. Reference Sanger, Hill, Lattanzi, Padgett, Larsen, Culleton and Kennett2018, Reference Sanger, Padgett, Larsen, Hill, Lattanzi, Colaninno and Brendon J2019; Stevenson Reference Stevenson1976) through the Early, Middle, and Late Woodland periods (Ehrhardt Reference Ehrhardt2005, Reference Ehrhardt2009; Lattanzi Reference Lattanzi2007, Reference Lattanzi2022; Levine Reference Levine, Levine, Sassaman and Nassaney1999; Penney Reference Penney, Brose, Brown and Penney1985; Railey Reference Railey and Lewis1996; Seeman et al. Reference Seeman, Nolan and Hill2019). During the Late Woodland period (ca. AD 900–ca. 1600), copper emerged as both a valuable trade commodity and a material expression of cosmology for Native societies of eastern North America (Bradley and Childs Reference Bradley, Childs and Ehrenreich1991; Erhardt Reference Ehrhardt2009; Miller and Hammell Reference Miller and Hammell1986). During the terminal Late Woodland period, copper was prized by the Algonquian polities of the Virginia Coastal Plain (e.g., the Powhatan) and Siouan polities of the interior (e.g., the Monacan; Blanton and King Reference Blanton and King2004; Gallivan Reference Gallivan2003, Reference Gallivan2007, Reference Gallivan2011, Reference Gallivan2016; Hantman Reference Hantman1990, Reference Hantman2018; Mouer Reference Mouer, Wittkofski and Browning1983, Reference Mouer1991; Potter Reference Potter, Wood, Waselkov and Thomas Hatley1989, Reference Potter1994; Rountree Reference Rountree1989, Reference Rountree and Turner2002; Turner Reference Turner and Fitzhugh1985, Reference Turner2003). There are no known surface deposits of geological or “native” copper in the Virginia Coastal Plain, so the Powhatan had to acquire it through trade with their western neighbors, the Monacans (see Hantman Reference Hantman1990, Reference Hantman2018).
Scholars have long debated whether the Monacans mined copper from local deposits in the Blue Ridge Mountains or acquired it through trade. Our study of native copper artifacts from five Late Woodland sites in Virginia determined that the majority of the copper came from remote locations (e.g., Michigan, Pennsylvania, and New Jersey; Gunter-Bassett et al. Reference Gunter-Bassett, Stevenson and Dussubieux2019)—although, as we noted in the original study (Gunter-Bassett et al. Reference Gunter-Bassett, Stevenson and Dussubieux2019), it is possible that some of the copper circulating through Monacan–Powhatan networks came from sources closer to home, perhaps from deposits in western Virginia or North Carolina (e.g., Quinn et al. Reference Quinn, Walker and Wright2022). Whatever the source(s) of native copper, documentary records make clear that Algonquian elites (e.g., Chief Powhatan [Wahunsenacawh] and his weroances [chiefs]) valued it in part for its rarity, and they tightly restricted its distribution (Gallivan Reference Gallivan2016; Mallios Reference Mallios1998; Potter Reference Potter, Wood, Waselkov and Thomas Hatley1989). Indeed, Chief Powhatan may have allowed the English to build James Fort in his territory (Tsenacommacah) because he saw their plentiful copper supply as an opportunity to break his “political dependence” on the Monacan (Hantman Reference Hantman1990:685; see also Mallios and Emmett Reference Mallios and Emmett2004:iii–2).
Although the English were oblivious to Monacan–Powhatan political dynamics when they arrived on the north shore of the James River in 1607, they were aware of copper's value to Native peoples, and to coastal Algonquians specifically. Much of their prior knowledge of Algonquian peoples came from the short-lived Roanoke Colony (1585–1590), an English settlement established 133.5 km (83 mi.) south of the mouth of the James River along the North Carolina coast. Roanoke colonists produced sketches, watercolors, and written accounts, many of which featured copper (see Hariot Reference Hariot1588, Reference Hariot1972 [1590]). Ralph Lane of the first Roanoke expedition wrote in 1585 that for Carolina Algonquians, “copper carieth ye price of all” (Quinn Reference Quinn1977:209). John White of the second Roanoke expedition in 1587 produced sketches and watercolors of Native men, women, and children, some of them wearing pierced sheet copper. Details of the expedition were published in 1588 (Hariot Reference Hariot1588), followed by an expanded publication that included replicate prints of White's watercolors (Hariot Reference Hariot1972 [1590]). Planners of the Jamestown venture likely had these publications in mind when they stipulated that ships bound for Virginia bring copper sheeting in the form of “10 seven-inch squares and five seven-inch circles, 40 four-inch squares and 20 four-inch circles, [and] 100 three-inch squares” (Quinn Reference Quinn1977:432–434). The planners also tasked the colonists with finding new zinc-rich sources of calamite stone for the production of brass. A large amount of metallurgical equipment has been recovered from Jamestown, and its analysis shows that these early experiments were unsuccessful in locating new deposits (Martinón-Torres and Rehren Reference Martinón-Torres and Rehren2007; Veronesi et al. Reference Veronesi, Rehren, Straube and Martinón-Torres2019).
John Smith's 1624 account of the Jamestown expedition (Barbour Reference Barbour1986; Smith Reference Smith1624) offers insights into the role of copper in Powhatan–English interactions (see also Mallios Reference Mallios1998, Reference Mallios2006; Mallios and Emmett Reference Mallios and Emmett2004). During the early years of the Fort (between 1607 and 1609), the English were able to acquire key food resources through trade with the Powhatan (“Their manner of trading is for copper, beads, and such like, [for] which they give such commodities as . . . skins, foule, fish, flesh, and their Country Corne” [Smith Reference Smith1624:34]). This was in large part due to copper's purchasing power in Powhatan territory (“for a copper kettle [the Powhatans] will sell you a whole Countrey” [Barbour Reference Barbour1986:3:276]). But lavish gifts of copper to Chief Powhatan and his chiefs (Mallios Reference Mallios1998:57–58, 238), combined with unrestricted trade to nonelites (Mallios Reference Mallios1998:263), quickly flooded the Powhatan market with copper. By 1609, as the quantity of copper in circulation spiked and relations between Chief Powhatan and the English soured, Native demand for copper had plummeted (“But in a short time it followed, that [corn] could not be had for a pound of Copper, which before was sold [to] us for an ounce” [Smith Reference Smith1624:51]).
Copper excavated from feature contexts at James Fort provides another line of evidence for the crash in Native demand for copper. Through an analysis of copper scrap found in features at James Fort, Mallios and Emmett (Reference Mallios and Emmett2004) determined that the highest proportions of copper (relative to other diagnostic artifacts) were found in features that were used and filled in the first 17 years of the fort's occupation (Mallios and Emmett Reference Mallios and Emmett2004:iii–3). They used these copper proportions to construct a timeline of copper supply and demand, noting four key phases: during phase 1 (pre-1607), before the English arrive, there was low supply and high demand; during phase 2 (1607–ca. 1610), there was high supply and high demand; during phase 3 (1610–1620), there was high supply and diminishing demand (and, from ca. 1615 to 1620, diminishing supply and diminished demand); and finally, during phase 4 (ca. 1620–1650), there was low supply and low demand. Because of the large volume of English copper alloy that entered Native trade networks in phase 2 (1607–ca.1610), archaeologists have hypothesized that James Fort was the main supplier of copper alloy artifacts found at contact-period Native sites across Virginia.
In this article, we use laser ablation–inductively coupled plasma–mass spectrometry (LA-ICP-MS) to test the hypothesis that objects made from European smelted copper (impure copper and copper alloy) were traded to Native peoples in the Virginia Coastal Plain between 1607 and approximately 1610 and then circulated through Native networks that extended to sites in central and southwest Virginia. Specifically, we analyze the elemental composition of a sample of smelted copper artifacts from Native sites in central and southwest Virginia, as well as a sample of smelted copper artifacts from two well-known European Fort sites—James Fort in Virginia (1607–1625) and Fort San Juan in North Carolina (1567–1568). We find that a portion of the impure copper from Native sites is chemically similar to that of the James Fort sample; however, some of the impure copper is elementally distinct from that supply. We raise the possibility that these elementally distinct metals (impure copper and copper alloy) came from an alternate European supplier located to the north of Virginia.
Elemental Characterization of Copper Alloy
Other than iron-based items, metal artifacts imported to the Americas from Europe consisted principally of smelted copper (impure copper) and copper alloy, with lesser occurrences of bronze, latten, and gunmetal (Hudgins Reference Hudgins2004, Reference Hudgins2006a). Simple visual assessments cannot reliably distinguish artifacts made from native copper (hereafter, also called American native copper [ANC]) from those made from smelted copper because of corrosion. This ambiguity has prompted chemical studies to determine compositional differences. Trace element studies have been successful at distinguishing between ANC and European smelted copper. Various regional studies have noted that smelted European impure copper contains a suite of trace elements such as nickel (Ni), indium (In), cadmium (Cd), and cobalt (Co) that cannot be detected in American native copper (Fleming and Swann Reference Fleming and Swann2000; Hancock et al. Reference Hancock, Pavlish, Farquhar, Salloum, Fox and Wilson1991).
Significant advances have been made in matching copper artifacts to geological sources of ANC in the American/Canadian Midwest (Anselmi et al. Reference Anselmi, Latta and Hancock1997; Mulholland and Pulford Reference Mulholland and Pulford2007; Rapp et al. Reference Rapp, Allert and Vitali2000), the Southeast (Goad Reference Goad, Greber and Brose1979, Reference Goad1980; Goad and Noakes Reference Goad, Noakes and Carter1978), the Northeast (Anselmi Reference Anselmi2008; Levine Reference Levine, Levine, Sassaman and Nassaney1999, Reference Levine2007a, Reference Levine2007b), and Pennsylvania and New Jersey (Lattanzi Reference Lattanzi2007, Reference Lattanzi2022). The source attributions of ANC within the assemblages studied here were completed by Gunter-Bassett et alia (Reference Gunter-Bassett, Stevenson and Dussubieux2019), who established that sources of ANC in Virginia included the upper Midwest as well as geological deposits in New Jersey and Pennsylvania. In this follow-up analysis of European trade copper, two artifacts from Fort San Juan were identified as ANC, but a provenance assignment was not attempted.
The process of determining the source of European smelted copper (impure copper and copper alloy) is also a complex undertaking. The use of additives during smelting, as well as recycling of metal, can prevent a determination of geological provenance in European smelted copper, given that elements such as silver (Ag), arsenic (As), gold (Au), and antimony (Sb) become enriched (Hancock et al. Reference Hancock, Pavlish, Farquhar, Salloum, Fox and Wilson1991; Hancock et al. Reference Hancock, Pavlish, Fox and Latta1995). When tin (Sn) is introduced, additional trace element enrichment can also occur (Hancock et al. Reference Hancock, Pavlish, Fox and Latta1995). On the other hand, copper alloy can be easily identified by the concentration of introduced zinc (Zn) because zinc ranges between 5% and 20% in “red” brass and between 20% and 36% in “yellow” brass (Anselmi et al. Reference Anselmi, Latta and Hancock1997).
Elemental characterization studies in the Middle Atlantic region have largely concentrated on distinguishing ANC from European impure and alloy copper. An early scanning electron microscope / energy dispersive spectrometer (SEM/EDS) study of trade copper from the Graham-White (44RN21) and Hurt Power Plant (44PY144) sites was successful in recognizing two compositional groupings (high and low Zn concentration) within the analyzed sample of copper alloy (Barber et al. Reference Barber, Solberg and Barfield1996, Reference Barber, Barber and Bowen1998). The study also found that copper alloy in the sample possessed inclusions that were highly enriched in tin and lead. Based on these results, the authors hypothesized that smelted copper at interior sites such as Graham-White and Hurt Power Plant originally came from James Fort (Barber et al. Reference Barber, Barber and Bowen1998). Fleming and Swann (Reference Fleming and Swann2000) also proposed a James Fort connection in their analysis of copper trade beads from Governor's Land or Paspahegh Village (44JC308).
The next significant advancement in copper provenance studies in Virginia was the use of major and minor elements to identify the manufacturing location and date range of impure copper and copper alloy artifacts. Investigations into the patents, business records, and chemistry of European mining and smelting have identified when particular metal “recipes” were invented and have established the characteristic variations in minor element concentrations between country manufacturers (Hudgins Reference Hudgins2005, Reference Hudgins2006a). Minor element concentrations of less than 4% are thought to represent accidental or minor additions to create an impure copper. Major element concentrations of more than 4% in the metal are thought to reflect purposeful additions. More substantial additives to the bulk copper, such as Zn (along with Sn and Pb), can result in a “weak brass” (defined as having a concentration of 4%–20% Zn) or strong brass (20%–30% Zn). The amount of brass in copper alloy can provide a terminus post quem (TPQ) for brass. According to the patent records, brass that contains less than 30% Zn must date to the seventeenth century or earlier, given that it was only technologically possible to produce brass with higher zinc content after AD 1723 (TPQ of 1723).
In all cases, minor elements (0.10%–3.99%) and trace elements (of less than 0.1%) in copper ore are transferred to the final metal to make an impure copper. These minor elements and trace elements can reflect geographical differences due to distinctions in regional geology. Elemental variation at this level has been used to discriminate between the three major manufacturers of impure copper and copper alloy (brass) in the seventeenth century: the Falun Mining Complex in Sweden, the central European mines (e.g., Harz Mountains, Saxony; Mansfeld, Thuringia; Schwaz region, Austria; Zips Mountains and Neusohl [Banská Bystrica], Slovakia), and the English mines (e.g., Cornish region, Cumbria; Hudgins Reference Hudgins2005). Nickel (Ni), As, and Ag are the discriminating elements. Nickel is present at greater than a 0.1% concentration in continental European metals and As is correspondingly low at concentrations of less than 0.1%. In contrast, English and Swedish metals are low in Ni (<0.1%), but they are distinguished from each other by their relative As concentrations (Swedish copper has less than 0.7% As). Low As concentrations in Swedish copper result from Swedish metallurgical techniques, in which metallurgists roasted the copper sulfide prior to smelting, allowing the volatile As to vaporize and be released from the ore. An additional source of minor element variation is attributed to the desilvering of copper melt in order to extract the more precious metal. Desilvered bulk copper has an Ag concentration of 0.10%–0.15%, whereas metal that retains its original integrity may have Ag well in excess of 0.15%. Although compositional variation is informative about the smelting process, concentration levels cannot be currently linked to specific manufacturers (Hudgins Reference Hudgins2005).
Using these criteria, Hudgins (Reference Hudgins2005) compared a sample of copper from James Fort to copper found at Native sites in the immediate region. Hudgins first analyzed a sample of 258 pieces of James Fort copper by ICP-AES (inductively coupled plasma–atomic emission spectroscopy) and found that 80% of the unalloyed, impure copper came from England. He then used the same methods and protocols to analyze a sample of copper from two nearby sites: Werowocomoco (44GL32) and Kiskiak (44YO687, 44YO693). Werowocomoco and Kiskiak are both seventeenth-century Powhatan sites located on the York River (Figure 1), and Werowocomoco is believed to be the seat of the Powhatan Chiefdom (Gallivan Reference Gallivan2016). Hudgins's analysis of a sample of the 22 pieces of copper recovered from Werowocomoco determined that the assemblage consists of both impure copper and copper alloy—the impure copper had not been desilvered, and the low Ni concentration (0.002%–0.099%) reflected an English origin (Hudgins Reference Hudgins2006b; see also Gallivan Reference Gallivan2016:159). The results of Hudgins's analysis of a sample of copper-based artifacts from Kiskiak remain unpublished, but summaries of the results suggest that copper scraps excavated by Dennis Blanton from a midden context at Kiskiak likely came from James Fort (see Erickson Reference Erickson2004).
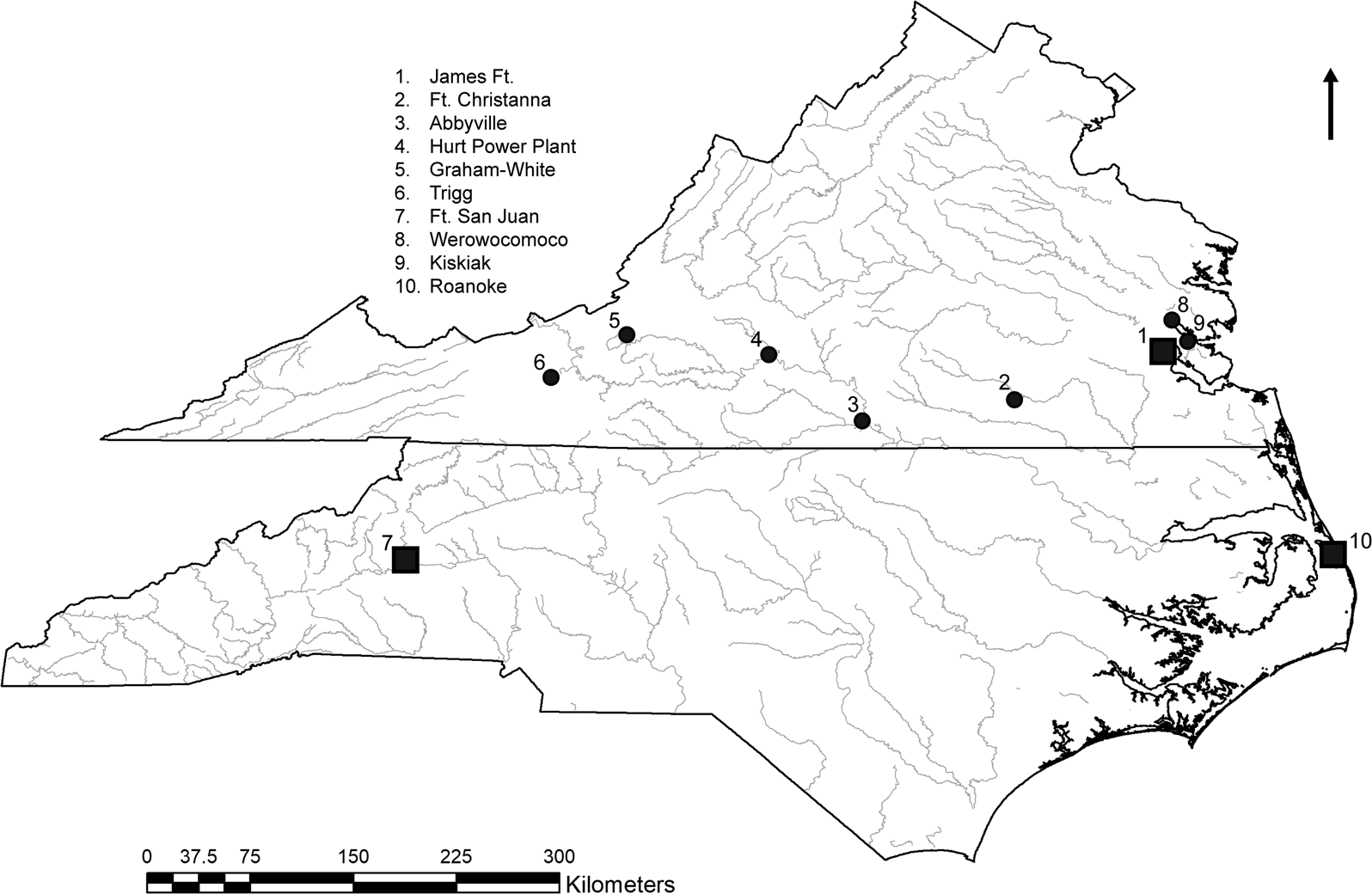
Figure 1. Late Woodland and contact period sites in Virginia and North Carolina.
Methodology of Provenance Studies
Once research questions have been formulated, provenance studies are generally conducted in three distinct steps (Mulholland and Pulford Reference Mulholland and Pulford2007). The first step is to establish the universe of geological sources from which the copper comprising the artifacts in question may originate. In the case of European-made copper artifacts, geological ores and smelting practices combine to form unique chemical profiles that are diagnostic of a region (e.g., Sweden, Continental Europe, England) and on occasion, temporal period [pre-1723/post-1723]; see Hudgins Reference Hudgins2005).
The second step involves the selection of the analytical technique. This is usually guided by the detection sensitivity and the degree of artifact destruction. We utilized laser ablation–inductively coupled plasma–mass spectrometry (LA-ICP-MS) for this project because the detection levels are in the low parts-per-million (ppm) for many elements (Supplemental Table 1). In addition, this technique is minimally destructive, with surface alteration of the artifact occurring at the micron scale (less than 100 μ). An advantage of the LA-ICP-MS method is that ablation of the artifacts can penetrate through surface corrosion to expose unaltered bulk metal. However, spot sampling techniques can be nonrepresentative, which are minimized by bulk analytical techniques such as neutron activation (Anselmi et al. Reference Anselmi, Latta and Hancock1997; Levine Reference Levine2007a) or ICP-AES (Hudgins Reference Hudgins2005).
Smelted metal from this period is characteristically inhomogeneous, with dispersed particles of smelting by-products that can include Pb and As (Barber et al. Reference Barber, Barber and Bowen1998; Deraisme et al. Reference Deraisme, Dussubieux, Frot, Stevenson, Creech and Bienvenu2008; Fleming and Swann Reference Fleming and Swann2000). Pre-analysis trials by the authors using LA-ICP-MS on smelted artifacts show that immiscible Pb particles can result in nonrepresentative values for the bulk metal, given that the narrow focal point of the laser may target an inclusion rather than averaging a large surface area. Pre-analysis trials also found that Zn concentrations for corroded copper alloy are significantly lower than polished bulk metal as a result of surface depletion even with pre-ablation by the laser (Dussubieux et al. Reference Dussubieux, Deraisme, Frot, Stevenson, Creech and Bienvenu2008). With the results of these trials in mind, we will omit Pb from the statistical analysis. We will, however, still use Zn concentrations as a guide for identifying copper alloy, and for distinguishing between “weak” brass and “strong” brass, given that the amount of depletion is low compared to the overall starting concentration.
The last step in provenance studies involves the treatment of the dataset to develop the statistical parameters or “elemental fingerprints” of the source groups. Here again, analytical difficulties can arise that may include the presence of undocumented sources, or sources that are chemically unrepresentative as a result of poor sampling (Mulholland and Pulford Reference Mulholland and Pulford2007). Our statistical procedure uses multivariate discriminant analysis (MDA; Koch Reference Koch2013) to guide the assignment of archaeological artifacts to the specific compositional groups represented (e.g., impure smelted copper, copper alloy).
Archaeological Context of Impure Copper and Copper Alloy Artifacts
The copper artifacts analyzed in this study come from six archaeological sites in Virginia and one site in North Carolina. The site locations are shown in Figure 1. Copper items from three contact period sites—Governor's Land or Paspahegh Village (44JC308), James Fort (VLR 047-0009), and Werowocomoco (44GL32)—were analyzed previously (Fleming and Swann Reference Fleming and Swann2000; Hudgins Reference Hudgins2005, Reference Hudgins2006a). Copper items are also found at Kiskiak (44YO687; Blanton et al. Reference Blanton, Underwood, Burkett, Lewes and Moore2005), but results of their analysis are not published (but see Erickson Reference Erickson2004). Results of those chemical analyses are discussed here, but because of differences in instrumentation and calibration, their published elemental data are not integrated into our dataset.
Copper artifacts from Native sites with contact period (AD 1607–1680) components include the following: Abbyville (44HA65), Graham-White (44RN21), Hurt Power Plant (44PY144), and Trigg (44MY3). These sites provided the majority of the copper samples included in this analysis. These sites also included other forms of evidence for direct or down-the-line exchange with Europeans in the late sixteenth and early to mid-seventeenth centuries, including glass trade beads.
Copper from the central water well at James Fort is represented by a deposit of European copper that was imported between AD 1607 and 1609. Also included is the early eighteenth-century site of Fort Christanna (44BR3), because it was purposefully established to trade with Native groups of the Virginia Piedmont. Finally, copper samples from Fort San Juan, a late sixteenth-century Spanish military outpost in western North Carolina (Beck et al. Reference Beck, Moore and Rodning2006, Reference Beck, Rodning and Moore2016), were tested. Copper from Fort San Juan was primarily included in this study to assess the possibility of a Spanish source of copper, which seemed plausible to us given the proximity of investigated Native sites to North Carolina (Figure 1). The archaeological contexts at each site (Table 1) are discussed in Supplemental Text 1.
Table 1. Archaeological Sites with Copper Artifacts Analyzed in This Work.
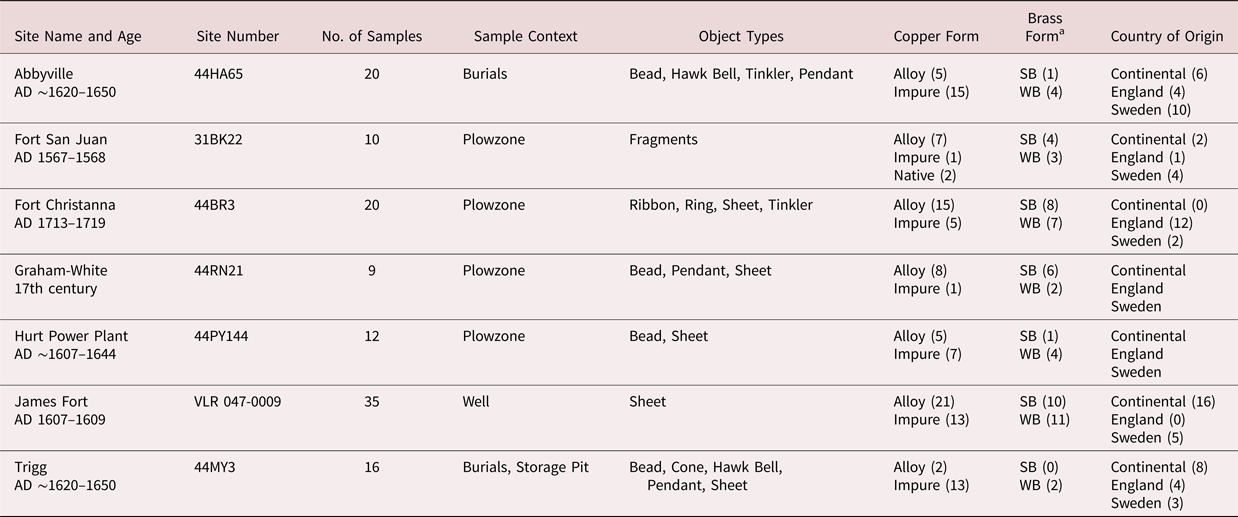
a SB = Strong brass; WB = Weak brass.
LA-ICP-MS Analytical Protocol
The elemental composition—including major, minor, and trace elements—was determined by LA-ICP-MS at the Field Museum (Chicago, Illinois) with an ICAP Q inductively coupled plasma–mass spectrometer (ICP-MS) coupled to an ESI-Elemental Scientific Lasers NW213 laser for direct introduction of vaporized solid samples. The analytical protocol used for this study is derived from the one described by Dussubieux (Reference Dussubieux, Glascock, Speakman and Popelka-Filcoff2007).
The laser operates at a wavelength of 213 nm. A CCD (charged-coupled device) camera allows for the observation of the surface of the sample prior to ablation in the sample chamber. Helium is used as a carrier gas at a flow rate of 0.20 l/min. Stability and sensitivity requirements for the signal are met when the laser operates at 70% of its maximum energy (0.4 mJ) and at a pulse frequency of 20 Hz. The single point analysis mode was selected. When a noncorroded surface is ablated, a laser beam diameter of 60 μ is selected. A 20-second pre-ablation time is set to be sure that possible surface contamination does not affect the results of the analysis and to eliminate the transient part of the signal. When the surface of the artifact is corroded, two ablations are performed at the same location. The laser beam diameter is set to 70 μm for a first ablation and 60 μm for a second one. The laser beam is focused on the bottom of the first crater before starting the second ablation. Only the signal acquired during the second ablation is recorded. The average of four measurements corrected from the blank is considered for the calculation of the concentrations.
Quantitative results were obtained by comparing the signal intensity measured for a given element in a sample to the signal intensity for the same element in a standard reference material (SRM) with certified concentrations. We selected seven different SRMs with the largest number of elements and the widest range of concentrations possible: B10 and B12 from the Centre de Développement des Industries de Mise en Forme des Matériaux, France; 71.32-4 and 51.13-4 from the Bureau of Analysed Samples Ltd, England; and 500, C1123, and 1275 from the National Institute for Standards and Technology. Fully quantitative analysis is possible for 20 elements using the following isotopes: 9Be, 24Mg, 27Al, 29Si, 31P, 53Cr, 55Mn, 57Fe, 59Co, 60Ni, 65Cu, 66Zn, 75As, 78Se, 107Ag, 118Sn, 121Sb, 125Te, 206Pb, 207Pb, 208Pb, and 209Bi. The concentrations of the elements present in the samples are calculated assuming that the sum of their concentrations, in weight percent, is 100%. The calculation method is adapted from Gratuze (Reference Gratuze1999). To improve reproducibility of measurements, 65Cu was selected as the internal standard to correct for possible instrument drift or changes in the ablation efficiency.
More details, including limits of detection and the performance of our protocol, are available in Dussubieux (Reference Dussubieux2019). A full listing of the dataset is available in Supplemental Table 1.
Analytical Results
The 122 samples included in this analysis were derived from Late Woodland period (AD 900–1607) and contact period (1607–1680) Native sites in Virginia, as well as from European fort sites (sixteenth, seventeenth, and eighteenth century) in Virginia and North Carolina (Table 1; Supplemental Table 1). This investigation revealed that three forms of copper were represented in the present data. American native copper (ANC) was identified in two instances in the sample of copper from Fort San Juan in North Carolina (082-490, 082-526). Impure copper was the next most frequent and was identified in 42 instances. The most frequent compositional category consisted of copper alloy, with 78 instances (Supplemental Tables 2–8). The assignment of individual artifacts to one of these three categories was based upon the following: (a) the results of the prior analyses on ANC that document very high purities in Cu and low trace element abundances and (b) the presence of additions such as zinc that clearly distinguish impure copper from copper alloy (Hudgins Reference Hudgins2005, Reference Hudgins2006a).
To examine these compositional differences, the entire sample was initially grouped using a statistiXL (version 2.0) discriminant analysis (www.statistixl.com) using the elemental values of Cu, Ni, Ag, As, Sb, Sn, and Zn (Supplemental Table 1). An initial analysis revealed that the early eighteenth-century Fort Christanna samples were chemically different from the sixteenth- and seventeenth-century copper samples, so they were removed to facilitate the statistical analysis of the remaining artifacts. Next, the groups were plotted to show the dispersion for each of the major copper compositional forms (Figure 2). Impure copper from the James Fort sample and impure copper from the archaeological sample overlap substantially, but the dispersion is not identical. One artifact from Fort San Juan appears to be impure copper. An artifact from James Fort, originally determined to be an alloy, also falls within this impure copper group. The impure copper group is distinct from the copper alloy group on the left side of the plot. In the upper left quadrant, there are two outliers from Fort San Juan that represents the ANC samples.
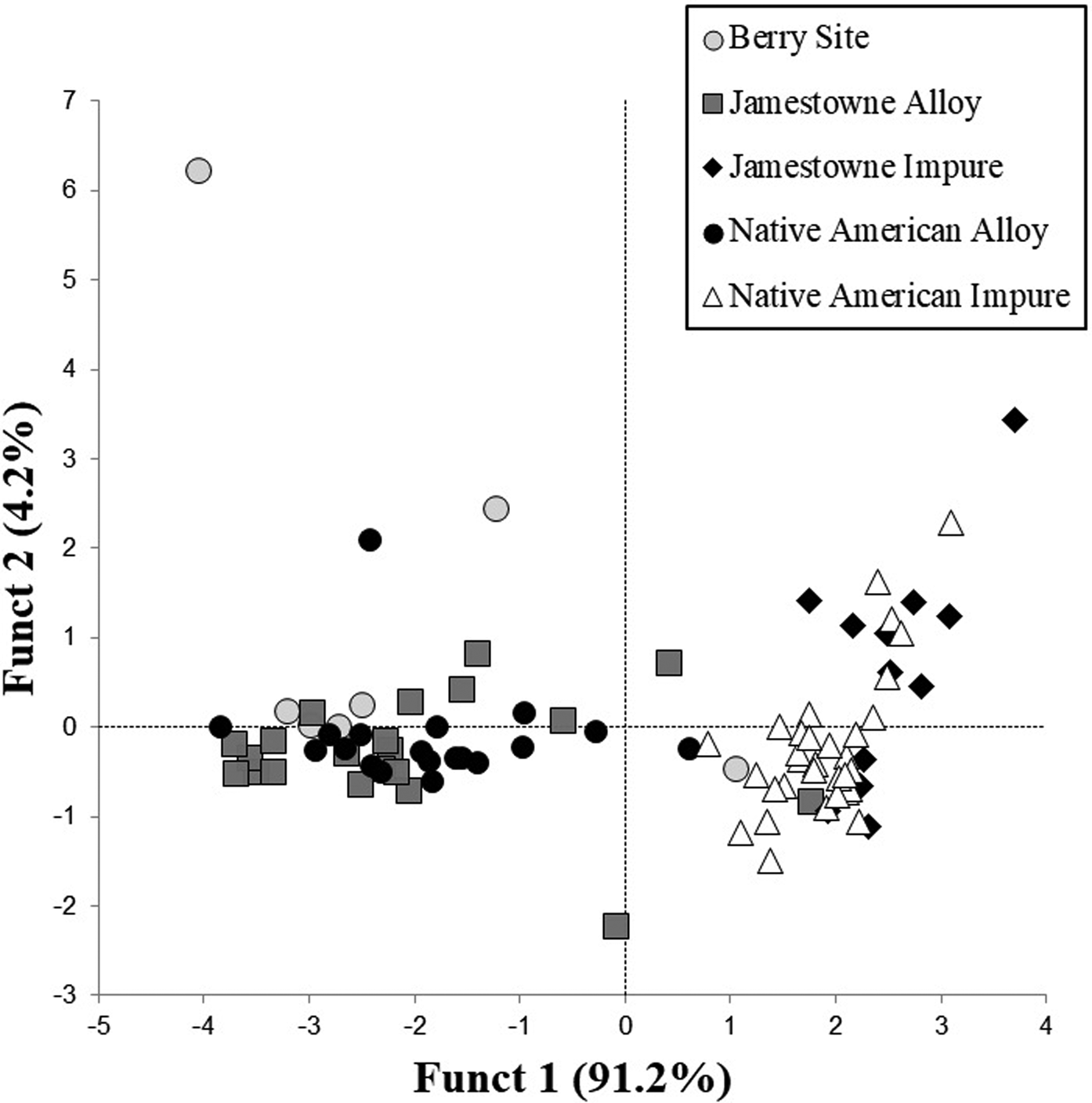
Figure 2. Discriminant analysis plot of archaeological impure copper and copper alloy, with reference samples from James Fort and the Fort San Juan.
Copper alloy from Fort San Juan, James Fort, and the central Virginia archaeological sites are all chemically similar in their major elements. Specimens determined to be copper alloy were then grouped a second time with discriminant analysis using low-concentration trace elements that are not intrinsic to geological copper (Ni through Bi in supplemental data) and not added during smelting (i.e., Pb, Sn, and Zn were excluded). This resulted in two groups—the James Fort alloy and Native archaeological alloy—that exhibited almost no overlap (Figure 3). Two of the Fort San Juan alloy artifacts have chemistries similar to the Native alloy and James Fort alloy, but the remaining five samples are different.
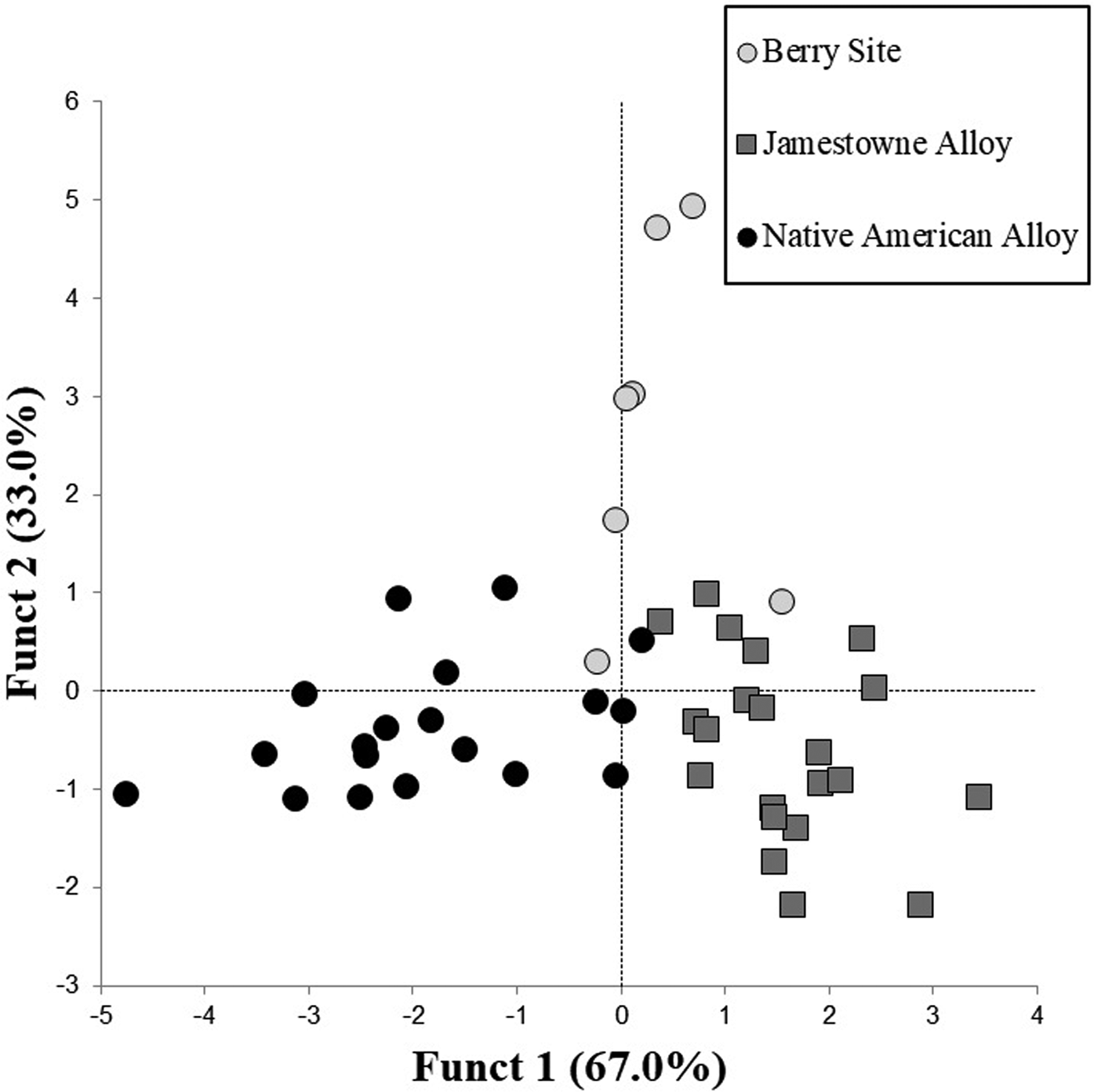
Figure 3. Discriminant plot of Native American archaeological alloy and alloy from James Fort and Fort San Juan.
A further review of the elemental data (Supplemental Tables 2–8) shows that the James Fort alloy comes exclusively from continental Europe (89%) and Swedish (11%) sources (Hudgins Reference Hudgins2005). The Native copper alloy artifacts in our sample come from continental Europe (n = 38), English (n = 32), and Swedish sources (n = 44; Supplemental Tables 2, 5, 6, 8). Samples from the Abbyville, Hurt Power Plant, and Trigg sites included artifacts made from all three regional metals. However, the nine artifacts from the Graham-White site all come from Sweden (Supplemental Table 5). A review of the elemental data shows that seven of the nine artifacts from Graham-White are highly similar, and one may infer that a single supply of copper alloy was used to fashion most of the artifacts.
Copper alloy specimens from the Native sites are a mixture of both “weak” brass and “strong” brass. Weak brass accounts for 87% (n = 52) of the alloy artifacts, whereas strong brass occurs 13% (n = 16) of the time. The low frequency of strong brass is a reversal of the proportions found at James Fort, where strong brass was three times more frequent. These ratios argue for a hinterland copper supply other than James Fort. Last, the samples from Fort San Juan consists of three weak brass, four strong brass, and a single occurrence of impure smelted copper dispersed among the three countries of origin.
Discussion
The English Virginia Company arrived on the banks of the James River with an abundance of sheet copper to engage in trade with local Native populations. Evidence for this trade in sheet copper comes from documentary records, James Fort's many archaeological features, and chemically similar artifacts found at nearby contact period sites, such as Governor's Land or Paspahegh Village (44JC308), Werowocomoco (44GL32), and Kiskiak (44YO687; Figure 1). Previous elemental analyses of sheet copper scraps from James Fort suggest that most of the English supply of sheet copper consisted of smelted and impure copper that was produced in England and supplied by the Society of Mines Royal and the Society of Mineral and Battery Works (although a small percentage of their copper originated from continental Europe and Swedish manufacturers; Hudgins Reference Hudgins2005). Although much of this copper arrived in 1607, two resupplies of copper were delivered in 1608 (Mallios Reference Mallios1998:207).
Results of our analysis suggest that the English at James Fort were a major source of the copper found at Native sites in central Virginia, and that the Spanish at Fort San Juan were not a source. Although much of the copper found at Native sites in central Virginia likely came from an English source, the sample of James Fort scrap that we analyzed does not account for the wide range of European compositional types found at Native sites. The wide range of European compositional types for finished artifacts, as represented by variation in elemental similarity (Figures 2 and 3), leads us to infer that copper sheeting was either from one trading partner with access to different types of impure and alloy copper over time or from multiple trading partners with distinct supplies. We suspect that some combination of these scenarios is at play, especially for hinterland sites. It is possible that the two 1608 resupplies of copper to James Fort account for some variation in the compositional types represented at hinterland sites—and that alternative trading partners with distinct supplies also account for variation. The presence of Swedish impure copper, which was not originally present at James Fort (Hudgins Reference Hudgins2005), bolsters the interpretation that European copper came from multiple trading partners with distinct supplies.
James Fort copper found at hinterland sites likely entered circulation between 1607 and 1609, when Native demand for English copper was highest (Mallios and Emmett Reference Mallios and Emmett2004), and prior to the start of the Anglo-Powhatan Wars (ca.1609–1614; 1622–1633; Potter Reference Potter, Wood, Waselkov and Thomas Hatley1989, Reference Potter1994; Rountree and Turner Reference Rountree and Turner2002). The unraveling of Powhatan social hierarchies followed. Within this context of extended violence, Potter (Reference Potter, Wood, Waselkov and Thomas Hatley1989) attributes the loss in political authority of the Powhatan chief and his weroances to multiple factors that include intentional population displacement and loss of land, the inability to combat European diseases, population decline, interruption of the traditional hereditary system (Potter Reference Potter, Wood, Waselkov and Thomas Hatley1989:225), and weakening of social authority. The latter can be inferred from Potter's (Reference Potter, Wood, Waselkov and Thomas Hatley1989) analysis of pre- and postcontact mortuary contexts, through which he is able to show that copper and other European trade goods become more frequent mortuary items in the graves of nonelite individuals. As European copper flooded the coastal market and lost its value, Powhatan elites and commoners alike may have sought to offload it to other Native groups to their west. From there, it would have been transported into the Piedmont and Blue Ridge Mountains via a network of land routes.
But from where, and by what processes, did other sources of metal make it to hinterland sites? We suggest that smelted and alloy copper arrived at sites in central and western Virginia by way of the same trade networks through which native copper and marine shell flowed during earlier periods. As we discussed in our previous phase of research (Gunter-Bassett et al. Reference Gunter-Bassett, Stevenson and Dussubieux2019), native copper found at sites in central and southwest Virginia is compositionally consistent with geological sources in the Northeast and Great Lakes regions and could have been transported via one of a series of north–south trade routes, such as the paths that later become the Great Wagon Road (Farrell Reference Farrell2010; Hofstra and Mitchell Reference Hofstra and Mitchell1993; Tietze Larson Reference Tietze Larson2014). This is not to say that all artifacts with compositions matching Great Lakes sources necessarily originated in the Great Lakes region. As Lattanzi (Reference Lattanzi2022:21–23) points out, it is possible that glaciers transported nuggets of Great Lakes copper away from their original source rock and deposited them much farther to the south. That said, the dominance of Northeast and Great Lakes copper in the Native copper artifacts found at hinterland sites (Gunter-Bassett et al. Reference Gunter-Bassett, Stevenson and Dussubieux2019) suggests strong ties between western Virginia and the northeast and northern Middle Atlantic. We suspect that these ties persisted through later periods and that European copper objects circulated through the same north–south networks through which native copper flowed during earlier periods.
It is certainly possible that further chemical analysis of copper artifacts, perhaps in conjunction with an analysis of other artifact types, will lead to the identification of European suppliers north of James Fort. Documentary and archaeological evidence hint at this possibility. When Captain John Smith first met with the Susquehannocks in 1608, he noticed that they had iron hatchets and inferred that they were in contact with a Dutch supplier to their north (Donehoo Reference Donehoo1995). Connections to Dutch suppliers north of Virginia (perhaps in Pennsylvania) are also suggested by the types of glass trade beads found at sites in central and western Virginia, such as the polychrome beads found at the Trigg site (Wells Reference Wells2002). Copper and glass were likely exchanged jointly in the same social contexts. Therefore, one fruitful line of inquiry in the reconstruction of trading relationships may be to examine diagnostic glass bead types alongside copper. We will pursue this lead in the next phase of analysis.
Acknowledgments
We are grateful to Leah Stricker (Preservation Virginia) and David Moore (Warren Wilson College) for providing copper samples for this phase of analysis.
Funding Statement
This research was funded by the Virginia Department of Historic Resources (VDHR), Richmond, Virginia, and the Virginia Museum of Natural History (VMNH) in Martinsville, Virginia.
Data Availability Statement
The analytical data generated by this research are fully available to researchers upon request.
Competing Interests
The authors declare none.
Supplemental Material
For supplemental material accompanying this article, visit https://doi.org/10.1017/aaq.2023.99.
Supplemental Table 1. Elemental data collected by LA-ICP-MS on Virginia copper artifacts.
Supplemental Table 2. Copper artifacts from the Abbyville Site (44HA65).
Supplemental Table 3. Copper artifacts found at Fort San Juan (31BK22).
Supplemental Table 4. Copper artifacts found at Fort Christanna (44BR3).
Supplemental Table 5. Copper artifacts from the Graham-White Site (44RN21).
Supplemental Table 6. Copper artifacts from the Hurt Power Plant Site (44PY144).
Supplemental Table 7. Copper artifacts found at the James Fort water well.
Supplemental Table 8. Copper artifacts from the Trigg Site (44MY3).
Supplemental Text 1. Archaeological Site Descriptions.






