Introduction
The European record of Pliocene canids is relatively scanty and consists mainly of a few specimens in isolated occurrences. The most common taxon recovered from early Pliocene sites is the genus Nyctereutes Temminck, Reference Temminck1838, whereas the genus Eucyon Tedford and Qiu, Reference Tedford and Qiu1996 is more elusive during this period. The genus Vulpes Frisch, Reference Frisch1775, which has been present in North America and Africa since the late Miocene, is documented in Eurasia only from the early Pliocene (Wang and Tedford, Reference Wang and Tedford2008).
Vekua (Reference Vekua1972) described the fossil assemblage of Kvabebi, a late Pliocene site (MN16a in the European Land Mammal biochronology). Mein (Reference Mein1975a, Reference Meinb) and Agustí et al. (Reference Agustí, Vekua, Oms, Lordkipanidze, Bukhsianidze, Kiladze and Rook2009) reported the co-occurrence of Nyctereutes megamastoides (Pomel, Reference Pomel1842) and Canis sp. in Eastern Georgia. Agustí et al. (Reference Agustí, Vekua, Oms, Lordkipanidze, Bukhsianidze, Kiladze and Rook2009) provided a general revision of the Kvabebi fauna and its chronological position. The authors, by providing a magnetostratigraphic calibration of the Kvabebi succession to the late Pliocene (chron 2An.1r), reviewed the faunal list ascribing the canid record to the genera Nyctereutes and Eucyon. In the framework of a joint collaborative project between the University of Florence and the Georgian National Museum (GNM), the Kvabebi Canidae sample has been revised to examine its role in determining the evolutionary history of the Plio-Pleistocene canid guild of the Old World. This revision of all the available material from Kvabebi housed in the GNM paleontological collections allowed us to identify the co-occurrence of three different canids in the same fossil assemblage: Nyctereutes, Eucyon, and Vulpes. This association is remarkable for its diversity and because it includes the first occurrence of a Vulpes alopecoides-like fox in the European fossil record.
Geological and chronological context of the Kvabebi site
Kvabebi is located south of Magaro village, in the area of the Sighnaghi region of eastern Georgia that surrounds Kvabebi Mountain, on the southern edge of the Iori Plateau. The Kvabebi section sits in the sedimentary infill of the Kura Basin. The latter, since the late Neogene, has been bordered by the Greater Caucasus to northeast and by the Lesser Caucasus and the Talysh ranges to the southwest (Agustí et al., Reference Agustí, Vekua, Oms, Lordkipanidze, Bukhsianidze, Kiladze and Rook2009, fig. 1), and it formed as a northward inland ingression of the Caspian (Popov et al., Reference Popov, Shcherba, Ilyina, Nevesskaya, Paramonova, Khondkarian and Magyar2006). The Kvabebi section, which is in the middle part of the Akchagylian stage of the Caspian Paratethys (Vekua, Reference Vekua1972), is ~170 m thick and displays a general regressive trend. The lower 110 m of the section is built up of a succession of brownish and bluish laminated mudstones. The brownish sediments are of fluvio-lacustrine origin, whereas the bluish color testifies to transgressive (marine) events characterized by a Pliocene (Akchagylian) mollusk fauna (Djikia, Reference Djikia1968). The upper part of the section (from 110 m to the top) is formed by a succession of alluvial reddish-brown sediments with no marine fauna. The section is characterised by the occurrence of several sandstone layers, including the one that yielded the Kvabebi fossil vertebrate assemblage (at meter 40), which is the result of accumulation due to tractive processes in a fluvial-influenced environment (Agustí et al., Reference Agustí, Vekua, Oms, Lordkipanidze, Bukhsianidze, Kiladze and Rook2009).
Recent re-prospecting and re-excavating conducted at Kvabebi allowed a re-evaluation of the geochronological setting of the section and a revision of the vertebrate fauna assemblage (Agustí et al., Reference Agustí, Vekua, Oms, Lordkipanidze, Bukhsianidze, Kiladze and Rook2009). A magnetostratigraphic study allowed placement of the fossiliferous level within the polarity reversals chron 2An.1r, with an age of ca. 3.07 Ma for the Kvabebi site (Agustí et al., Reference Agustí, Vekua, Oms, Lordkipanidze, Bukhsianidze, Kiladze and Rook2009). The updated revised list of the Kvabebi vertebrate fauna (Vekua, Reference Vekua1972; Hemmer et al., Reference Hemmer, Kahlke and Vekua2004; Agustí et al., Reference Agustí, Vekua, Oms, Lordkipanidze, Bukhsianidze, Kiladze and Rook2009; this paper) is as follows:
Reptilia
Testudo cernovi transcaucasica Chkhikvadze, Reference Chkhikvadze1979
Aves
Ioriotis gabuniae Burchak-Abramovich and Vekua, Reference Burchak-Abramovich and Vekua1971; Struthio transcaucasicus Burchak-Abramovich and Vekua, Reference Burchak-Abramovich and Vekua1971
Mammalia
Rodentia: Hystrix cf. H. primigenia Wagner, Reference Wagner1848
Carnivora: Nyctereutes megamastoides (Pomel, Reference Pomel1842); Eucyon sp., Vulpes cf. V. alopecoides (Del Campana, Reference Del Campana1913); Ursus minimus Devèze and Bouillet, Reference Devèze and Bouillet1827; Lynx issiodorensis (Croizet and Jobert, Reference Croizet and Jobert1828); Homotherium davitashvili Vekua, Reference Vekua1972; Puma pardoides Owen, Reference Owen1846; Dinofelis cristata (Falconer and Cautley, Reference Falconer and Cautley1836); Chasmaportetes lunensis (Del Campana, 1914); Perinium kvabebicus (Bendukize and Vekua, Reference Bendukize and Vekua2012)
Arctiodactyla: Propotamochoerus provincialis (Gervais, Reference Gervais1859); Eucladoceros sp.; ?Pseudalces sp.; Procapreolus sp.; Ioribos aceros Vekua, Reference Vekua1972; Protoryx heinrichi Major, Reference Major1891; Oryx (Aegoryx) sp.; Parastrepsiceros sokolovi Vekua, Reference Vekua1968; Eosyncerus ivericus Vekua, Reference Vekua1972; Gazella postmitilinii Vekua, Reference Vekua1972
Perissodactyla: Hipparion rocinantis Hernández Pacheco, Reference Hernández Pacheco1921; Stephanorhinus megarhinus (de Christol, Reference de Christol1834)
Hyracoidea: Kvabebihyrax kachethicus Gabunia and Vekua, Reference Gabunia and Vekua1966
Probiscidea: Anancus arvernensis (Croizet and Jobert, Reference Croizet and Jobert1828)
Materials and methods
Studied specimens and comparative sample
The present study is based on comparative morphological analyses of the Canidae from Kvabebi. The examined and described fossils are housed at the GNM (see abbreviations below). We studied the collections of the IGF as comparative fossil material, and reviewed the relevant literature on Plio-Pleistocene canids (Del Campana, Reference Del Campana1913; Thenius, Reference Thenius1954; Qiu and Tedford, Reference Qiu and Tedford1990; Teford and Qiu, 1991, 1996; Koufos, Reference Koufos1997; Rook, Reference Rook2009; Petrucci et al., Reference Petrucci, Cipullo, Martínez-Navarro, Rook and Sardella2013; Koufos, Reference Koufos2014). Extant specimens from the MZUF, MNHN, and GNM were also used for morphological and morphometric comparisons.
The fossil comparative sample includes specimens of Nyctereutes donnezani (Depéret, Reference Depéret1890) from La Gloria and Layna; Nyctereutes megamastoides (Pomel, Reference Pomel1842) from the Lower Valdarno Basin, Villaroya, Dafnero 1; Nyctereutes vulpinus Monguillon et al., Reference Monguillon, Spassov, Argant, Kauhala and Viranta2004 from St. Vallier; Nyctereutes sinensis (Schlosser, Reference Schlosser1903) and Nyctereutes tingi Tedford and Qiu, Reference Tedford and Qiu1991 from the Yushe Basin; Eucyon monticenensis (Rook, Reference Rook1992) from the Cava Monticino and Venta del Moro; Eucyon adoxus (Martin, Reference Martin1973) from Perpignan; Eucyon marinae Spassov and Rook, Reference Spassov and Rook2006 from Muhor-Erig; Eucyon davisi (Merriam, Reference Merriam1911) from North America and the Yushe Basin; Eucyon zhoui from the Yushe Basin; Eucyon odessanus (Odintzov, Reference Odintzov1967) from Megalo Emvolon, Alatini and the Odessa Catacombs; Eucyon minor (Teilhard de Chardin and Piveteau, Reference Teilhard de Chardin and Piveteau1930) from Nihewan and Shamar; Vulpes alopecoides (Major, Reference Major1875) from the Upper Valdarno Basin, Pirro Nord and Dafnero-1; Vulpes praeglacialis (Kormos, Reference Kormos1932) and Vulpes praecorsac Kormos, Reference Kormos1932 from Spain, Hungary, and Ukraine.
The extant comparative sample includes specimens of Nyctereutes procyonoides (Gray, Reference Gray1834), Vulpes vulpes (Linnaeus, Reference Linnaeus1758), and Vulpes lagopus (Linnaeus, Reference Linnaeus1758), which are housed in the MZUF, MNHN, and GNM.
Measurements and statistical analyses
Cranio-dental and postcranial measurements were taken with a digital calliper to the nearest 0.1 mm following von den Driesch (Reference von den Driesch1976), with a few modifications. We used linear measurements and the ratio of the width/length of m1 to create scatter plots to test the affinity of the specimens of Vulpes and Eucyon from Kvabebi within the variability of Plio-Pleistocene species of these two genera. For Vulpes, we decided to include the extant species V. vulpes and V. lagopus for the widespread distribution of the former and size affinity of the latter to fossil species. Fossil species taken into consideration are European Plio-Pleistocene ones (e.g., V. praecorsac, V. praeglacialis, and V. alopecoides). We decided to keep convex hulls only for fossil species to identify their variability range compared to extant ones, which are considerably more variable. In the Eucyon scatter plot, we included six species of the Eurasian Pliocene Eucyon (E. marinae, E. adoxus, E. minor, E. davisi, E. zhoui, and E. odessanus) and preferred to leave only the convex hull of E. odessanus for its great variability.
In these analyses, we used PAST software ver. 3.08 (Hammer et al., Reference Hammer, Harper and Ryan2001; Hammer, Reference Hammer2016). We further clarified the specific attribution of the material of Nyctereutes by comparing the material from Kvabebi with that of other Eurasian Villafranchian and extant species of the genus by means of two log-ratio diagrams (Simpson, Reference Simpson1941; Simpson et al., Reference Simpson, Roe and Lewontin1960). These graphs calculate the difference between the log-transformed mean value of each single variable for each species and a selected species that is chosen as the comparative baseline. The dental variables selected are P2-P3 L, P4-M2 L, and W for the upper teeth, and p2-p3 L, p4-m2 L, and W. We chose the extant N. procyonoides as a reference baseline and plotted the following fossil species: N. megamastoides from European sites; N. tingi and N. sinensis from China; and Nyctereutes from Kvabebi.
Repositories and institutional abbreviations
IGF, Museum of Natural History, Geological and Paleontological section, University of Florence (Italy); K, Kvabebi site; MG, S. Janashia Museum of Georgia, part of the Georgian National Museum (Tbilisi, Georgia); MNHN, Museum National d’Histoire Naturelle, Paris; MZUF, Museum of Natural History, “La Specola” Zoology section, University of Florence (Italy).
Systematic paleontology
Anatomical abbreviations
Postcranial: GL, general length; Bd, breadth of the distal epiphysis; Bp, breadth of the proximal epiphysis; BPC, greatest breadth across the coronoid process; DC, depth of the caput femoris; Dd, depth of the distal epiphysis; Dp, depth of the proximal epiphysis; DPA, depth across the processus anconaeus
Order Carnivora Bowdich, Reference Bowdich1821
Suborder Caniformia Kretzoi, Reference Kretzoi1943
Family Canidae Fischer von Waldheim, Reference Fischer von Waldheim1817
Subfamily Caninae Fischer von Waldheim, Reference Fischer von Waldheim1817
Tribe Vulpini Hemprich and Ehrenberg, Reference Hemprich and Ehrenberg1832
Genus Nyctereutes Temminck, Reference Temminck1838
Nyctereutes megamastoides (Pomel, Reference Pomel1842)

Figure 1 Nyctereutes megamastoides (Pomel, Reference Pomel1842) from Kvabebi. MG 29-2013/581 (K219), cranium, in dorsal (1), ventral (2) and right lateral (3) views; MG 29-2013/456 (K217), cranial fragment, in occlusal view (4); MG 29-2013/457 (K220), cranium, dorsal (5), ventral (6), right lateral (7) and rostral (8) views. Scale bar=2 cm.

Figure 2 Nyctereutes megamastoides (Pomel, Reference Pomel1842) from Kvabebi. MG 29-2013/820, left hemimandible, in buccal (1), lingual (2), and occlusal (3) views; MG 29-2013/592 (K4177), right hemimandible fragment, in buccal (4), lingual (5), and occlusal (6) views; MG 29-2013/458, left humerus (K251) and ulna (K250), in caudal (7) and right lateral (8) views; MG 29-2013/602 (K503), right pelvis, in lateral view (9); MG 29-2013/600 (K226), right femur, in dorsal view (10); MG 29-2013/597 (K253), left metacarpals with phalanges, in dorso-lateral view (11); MG 29-2013/460 (K254), left phalanges, in lateral view (12). Scale bar=1 cm.
1972 Nyctereutes megamastoides Reference VekuaVekua, p. 41, pl. 2, figs. 1, 2, pl. 3, figs.1, 6.
2009 Nyctereutes megamastoides Reference Agustí, Vekua, Oms, Lordkipanidze, Bukhsianidze, Kiladze and RookAgustí et al., p. 3277.
Holotype
The holotype of Nyctereutes megamastoides comes from Perrier-Etouaires (early Pleistocene), France.
Materials
MG 29-2013/455 (K234) juvenile cranium with left dP4 and M1 and right P4–M2; MG 29-2013/456 (K217) cranium; MG 29-2013/457 (K220) cranium with left C1–M2 and right P1–M2; MG 29-2013/567 (K4173) neurocranium; MG 29-2013/568 (K215) cranium; GNM 29-2013/573 (K233) skull; MG 29-2013/581 cranium; GM 29-2013/610 (K227) left C1; MG 29-2013/609 (K237) left M1; MG 29-2013/588 (K221) left hemimandible with i2, c1–p1, m1–m3; MG 29-2013/589 (K224) right hemimandible with p4-m2; MG 29-2013/590 (K235) right hemimandible with dm1 and germinal m1; MG 29-2013/591 (K214) right hemimandible with m1; MG 29-2013/592 (K4177) right hemimandible with p4-m1; MG 29-2013/606 right hemimandible with c1; MG 29-2013/820 left hemimandible with p2–m3; MG 29-2013/612 (K236) c/C; MG 29-2013/613 incisors, c1 and p1; MG 29-2013/605 (K256) left p4; MG 29-2013/611 right m2; MG 29-2013/593 vertebral fragments (K502 and K504); MG 29-2013/458 left humerus (K251), ulna, and radius (K250); MG 29-2013/594 left humerus (K241), ulna (K239), radius (K245), metacarpals II–V, and I phalanx; MG 29-2013/596 (K232) left radius; MG 29-2013/602 (K503) right pelvis; MG 29-2013/598 (K501) left femur; MG 29-2013/600 (K226) right femur; MG 29-2013/599 (K820) right tibia; MG 29-2013/601 left calcaneum; MG 29-2013/597 (K253) metapodial fragments and phalanges; MG 29-2013/460 (K254) left phalanges.
Description
The cranium is elongated rostro-caudally, with a long muzzle and long nasals, which end beyond the maxillo-frontal suture. In lateral view, the cranial profile is rather straight on the nasals and frontals aspects and becomes arched only in the caudal portion of the braincase (e.g., Fig. 1). The frontals show a depression on the midline of the cranium at the level of the postorbital processes. These are expanded laterally and are well developed. The postorbital constriction is short and marked, so that the frontal sinuses are slightly developed and have a flat external surface. The braincase is rather inflated and globular in shape. The sagittal crest is poorly developed, with some specimens (e.g., K219) showing long parasagittal crests that fuse distally from the fronto-parietal suture and others (e.g., K215) having parasagittal crests that fuse at the level of the fronto-parietal suture. In caudal view, the supraoccipital shield is “bell”-shaped, with prominent borders and a marked knob-like expansion at level of the mastoid process. In lateral view, the inion does not overhang the condyles. The tympanic bullae are fairly inflated. The paraoccipital processes are not fused with the bulla and are posteriorly directed.
Unfortunately, the only juvenile cranium (MG 29-2013/455 [K234]) is too poorly preserved to assess correctly its morphological features. It appears to be low in dorso-ventral height, in lateral view. In dorsal view, the braincase is inflated and the postorbital constriction seems rather marked.
The incisors possess a main cusp with two small accessory cuspids on both sides of the main cusp. I3 is slightly larger compared to I1-I2, but still maintains an incisor morphology (not canine form) and does not possess the basal cingulum. C1 is very thin and its crown is not very high. Short diastemata are visible between upper premolars. The premolars do not show distal accessory cuspids. The P3 possesses a tiny cuspid on the distal cingulum. The P4 is rather slender and elongated mesio-distally. The protocone is small, pointy, and anteriorly positioned compared to the mesial margin of the tooth. Some specimens (e.g., K217) seem to show a parastyle in the mesio-lingual portion of the P4. The paracone is high and the metastylar blade is sharp and short. There is a strong lingual cingulum. The M1 is subquadrate in shape. It possesses an equally developed paracone and metacone, bounded buccally by a strong cingulum. There is a prominent parastyle. The M1 shows a large trigon basin, a prominent protocone, a large protoconule and a low, but well-developed, metaconule. Lingually, there is a cingular hypocone. On the mesial side, there is a strong cingulum that forms a bulging of enamel in the shape of a small cuspid. The M2 is large mesio-distally and possesses an equal-sized paracone and metacone, a protocone, a protoconule, and a small metaconule. There is a lingual cingulum. The mandible is long and rather shallow, with a straight ventral margin. In the angular region, the subangular lobe is well developed and high-angled, in lateral view. The angular process is large and stout. The masseteric fossa is deep.
MG 29-2013/590 (K235) possesses dm1 with a broken paraconid and a pointy and high protoconid; its talonid basin is wide and bounded by an entoconid, hypoconid, and a prominent distal accessory cuspulid. Of the lower premolars, only p4 possesses a distal accessory cuspulid, although there is a cuspulid-like distal cingulid on p3 and p4. The p4 also possesses a slightly visible mesial cingulid. The m1 paraconid is short, lower than the p4 protoconid. The protoconid is high, whereas the large metaconid is individualized from the protoconid, slight lingually, and distally positioned compared to the distal margin of the protoconid. In the talonid, the hypoconid is larger based compared to the entoconid, but not sensibly higher. In K4177, there are accessory cuspulids on the talonid (e.g., mesially to the entoconid or distally to the hypoconid). The m2 is enlarged in the mesial portion, especially with a great expansion of the mesio-buccal cingulid. The protoconid and the metaconid are equal in size. Distal to the protoconid is a hypoconid with accessory cuspulids on both mesial and lingual sides. The m3 possesses two large cuspulids and a distal accessory one.
The postcranial remains from Kvabebi are generally in a bad state of preservation, so the features of the various bones are difficult to discern. MG 29-2013/458 (K251) and MG 29-2013/594 (K241) are the distal epiphysis of the left humeri (Bd: 21.0 mm and 22.2 mm, respectively), showing a prominent medial epicondyle compared to the lateral one; the trochlea has a sharp ridge. MG 29-2013/594 (K239) is fragmentary ulna (BPC: 8.6 mm) that possesses a faint lateral coronoid process, whereas the medial one is round and expanded. The ulna of MG 29-2013/458 (K251) has DPA=18.5 mm and BPC=9.6 mm. Radius fragments MG 29-2013/458 (K251) and MG 29-2013/596 (K232) have Bp=11.8 mm and 13.5 mm, and Bd=9.0 mm and 9.0 mm. Two femur fragments are recorded, MG 29-2013/598 (K501) and MG 29-2013/600(K226); the former (Bp=23.3 mm and DC=12.3 mm) shows a large head departing from a short neck. The greater trochanter is high and slender, with a deep trochanteric fossa. The lesser trochanter of MG 29-2013/598 (K501) is prominent. In the distal epiphysis, the lateral condyle is considerably larger than the medial, which is also thinner. There is no proximal epiphysis of the tibiae preserved, but only a diaphysis with the distal portion MG 29-2013/599 (K820), with Bd=17.4 mm and Dd=11.9 mm. The calcaneum MG 29-2013/601 is rather compressed mediolaterally, so that the sustentalum tali is very reduced (GL=31.9 mm). MG 29-2013/594 and MG 29-2013/597 are metapodial fragments with associated phalanges. MG 29-2013/594 measures are: Mc II—GL=39.6 mm; Bp=4.7 mm; Dp=7.6 mm; Mc III—GL=47.4 mm; Bp=6.3 mm; Dp=72 mm; Mc IV—GL=46.5 mm; Bp=4.1 mm; Dp=6.5 mm; Mc V—GL=38.6 mm; Bp=7.5 mm; Dp=6.3 mm.
Remarks
Most of the Canidae specimens of Kvabebi are ascribable to the genus Nyctereutes based on the size and on the morphological features described above. Compared to N. procyonoides and to the Eurasian Pliocene species of raccoon dog-like canids, Nyctereutes from Kvabebi is similar in size to N. megamastoides, N. sinensis, and N. donnezani, but smaller than N. tingi. Some morphological features (e.g., the high angle of the corpus of the mandible in the region of the subangular lobe, subquadrate M1, mesiodistally enlarged M2, etc.) are suggestive of a derived form of Nyctereutes, thus differing from primitive morphologies like those characterizing N. tingi and N. donnezani (e.g., poorly developed subangular lobe, reduced width of upper molars). Therefore, Nyctereutes from Kvabebi is more similar to species like the extant N. procyonoides, N. sinensis from the Yushe Basin, and N. megamastoides from Western Europe. Among these latter species, N. procyonoides possesses the greatest number of dental differences compared to Nyctereutes from Kvabebi (e.g., the P4 protocone more mesially placed, a stronger parastyle on P4, proportionally bucco-lingually shorter M1 and M2, the M1 metacone and metaconule reduced in development, the pointy and higher M1 hypocone, the individualized metaconid and reduced bucco-lingual width of the talonid on the m1). Nyctereutes sinensis and N. megamastoides are similar in size and in morphological features, so they could represent a single taxon with a wide zoogeographic range (Tedford and Qiu, Reference Tedford and Qiu1991). Nevertheless, as pointed out by Tedford and Qiu (Reference Tedford and Qiu1991), N. megamastoides has a better-developed subangular lobe and more quadrangular upper molars compared to N. sinensis. Furthermore, the P4 protocone of N. sinensis lies more mesially when compared to the mesial margin of the tooth, more than it does in N. megamastoides; this species has an equal-sized M1 paracone and metacone, in contrast to those of N. sinensis, which appear well developed in F:AM 96759 (Tedford and Qiu, Reference Tedford and Qiu1991); the latter species does not possess any or strongly reduced protoconule on M1, which is present in the Villaroya and Dafnero N. megamastoides; the mesiolingual cingulum on M1 of N. sinensis is not continuous and does not form the cuspid-like enlargement on the mesial side of the M1 of N. megamastoides; the M2 of the Chinese species is reduced compared to that of N. megamastoides, which has a peculiar “D”-like shape; N. megamastoides from Villaroya and Dafnero-1 has a large protoconule and protocone and a low metaconule on the M2, whereas N. sinensis seems to possess only a large and prominent protocone; the m1 metaconid of N. megamastoides is larger than that of N. sinensis; in N. megamastoides, the m1 talonid is considerably enlarged compared to the trigonid, whereas in N. sinensis the talonid is not oversized compared to the trigonid; the m2 is generally slender in N. sinensis compared to N. megamastoides in which it is oval in shape. The m2 protoconid and metaconid are subequal in size in N. megamastoides, whereas N. sinensis has a larger protoconid; this latter species does not possess any accessory cuspulids on the disto-lingual side of m1, whereas N. megamastoides generally shows the entoconid and may possess accessory cuspulids. Considering these differences, the Kvabebi raccoon dog can be assigned to Nyctereutes megamastoides.
Cranial, dentognathic, and postcranial measurements of the Nyctereutes sample from Kvabebi are shown in Tables 1–4. The log-ratio diagrams (Fig. 3.1, 3.2) were used to compare the mean dental measures of Nyctereutes from Kvabebi to those of the extant N. procyonoides and other Eurasian Pliocene species, using N. sinensis from the Yushe Basin (Tedford and Qiu, Reference Tedford and Qiu1991) as a standard reference. On the one hand, these diagrams show that Nyctereutes from Kvabebi differs considerably both in size and proportions from N. procyonoides and from N. tingi, the former being much smaller (as expected), and the latter substantially larger, than the Kvabebi raccoon-dog. On the other hand, the graphs allow an appreciation of how the Kvabebi sample is similar in size and proportion to both N. sinensis and N. megamastoides, although with a much greater affinity to the latter. This provides additional support for the taxonomic interpretation of the Kvabebi material as Nyctereutes megamastoides.

Figure 3 Log-ratio diagrams based on selected upper (1) and lower teeth (2) variables in the described Kvabebi material, as well as in other Pliocene and extant species of Nyctereutes (N. sinensis, N. tingi, and N. megamastoides; data taken from the literature: Tedford and Qiu, Reference Tedford and Qiu1991; Monguillon et al., Reference Monguillon, Spassov, Argant, Kauhala and Viranta2004) as compared to the extant N. procyonoides (used as the reference baseline).
Table 1 Cranial measurements of Nyctereutes megamastoides (Pomel, Reference Pomel1842) from Kvabebi. A-B=akrokranion-basion (height of the cranium without the sagittal crest); BL=basion-prosthion (basal length of the cranium); CBL=prosthion-occipital condyles (condylobasal length of the cranium); Ect=ectorbitale-ectorbitale (frontal breadth); ECW= external C1 alveoli width; Eu=greatest neurocranium breadth; FL=prosthion-frontal midpoint (facial length); FMW=width of the foramen magnum; GPW=greatest palatal width; GWOC=greatest width of occipital condyles; NcL=akrokranion-frontal midpoint (neurocranium length); PL=prosthion-staphylion (palatal length); PoCW=least breadth of postorbital constriction; SH=skull height (with sagittal crest); TL=akrokranion-prosthion (total length of the cranium); Zyg=zygion-zygion (zygomatic breadth).
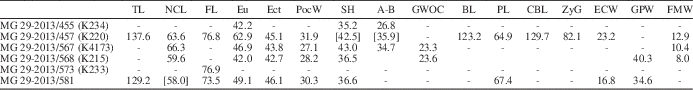
Table 2 Associated upper teeth measurements of Nyctereutes megamastoides (Pomel, Reference Pomel1842) from Kvabebi. L=mesiodistal length; LCR=upper cheek toothrow length (P1–M2); LMR=upper molar row length (M1–M2); LPR=upper premolar row length (P1–P4); W=buccolingual width.

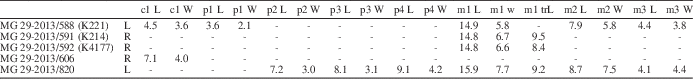
Table 3 Mandibular measurements of Nyctereutes megamastoides (Pomel, Reference Pomel1842) from Kvabebi. LLMR, length of the lower molar row (m1--m3); M m1 B, mandibular corpus breadth below midpoint of m1; M p4 H, mandibular corpus height distal to p4 alveolus; m1–m2 L, length of the first and second lower molars; p2–p4 L, length of premolar row, starting from p2.

Table 4 Associated lower teeth measurements of Nyctereutes megamastoides (Pomel, Reference Pomel1842) from Kvabebi. L=mesiodistal length; m1 trL=trigonid of m1; m1 tdL=talonid of m1; W=buccolingual width.
Genus Vulpes Frisch, Reference Frisch1775
Vulpes cf. V. alopecoides Major, Reference Major1875
1972 Canis sp. Reference VekuaVekua, p. 50, pl. 3, fig. 7.
Holotype
The holotype of Vulpes alopecoides comes from Tasso (early Pleistocene), Upper Valdarno Basin, Tuscany, Italy.
Materials
MG-29-2013/461 (K260) right hemimandible fragment with m1 and m2.
Description
The fragment MG-29-2013/461 (K260) is very small in dimensions compared to the other specimens from Kvabebi. The ventral margin of the corpus of the mandible is preserved in a small, curved portion below the m2 (e.g., Fig. 4). The m1 possesses a proportionately high paraconid, a higher and distally curved protoconid and a prominent, slightly individualized metaconid. The latter is in line with the distal margin of the protoconid, so it is not visible in buccal view. The talonid basin is deep, with a large hypoconid and a smaller entoconid. These cuspulids are of the same height and are connected by a cristid. On the buccal side, there is a cingulid. The m2 is bean-shaped, with a larger mesial part compared the distal. The protoconid has a larger base than the metaconid, but both cuspulids have the same height. Mesial to the protoconid is a prominent cristid that ends in a faint accessory cuspulid. On the mesio-buccal side, a cingulid is well developed. Distal to the protoconid is a large hypoconid, and the lingual side is bounded by a cristid with a low entoconid. MG 29-2013/461 (K260) measurements are as follows: m1 L=131 mm; m1 W=5.5 mm; trm1 L=8.5 mm; m2 L=6.2 mm; m2 W=4.4 mm; M m1 B=4.5 mm; M m1 H=11.1 mm.

Figure 4 Vulpes cf. V. alopecoides from Kvabebi. MG-29-2013/461 (K260), right hemimandible fragment, in buccal (1), lingual (2), and occlusal (3) views. Scale bar=1 cm.
Remarks
The extant species V. vulpes is larger in size compared to the smallest canid from Kvabebi. Moreover, it possesses several features (such as an individualized and large metaconid, a high and pointy accessory cuspulid between entoconid and metaconid, and an oval-shaped m2 with numerous distal accessory cuspulids) that cannot be found in MG 29-2013/461 (K260). Unlike the arctic fox V. lagopus, the smallest Kvabebi canid possesses the m1 entoconid and hypoconid close to one another, a reduced talonid basin, and a slender m2. Compared to V. praecorsac from the Odessa catacombs and Püspökfürdö (Kormos, Reference Kormos1932; Odintzov, Reference Odintzov1965), MG 29-2013/461 (K260) shows some differences (e.g., a slender m1 protoconid, in lateral view; the lower carnassial less compressed buccolingually; and the m2 more elongated mesiodistally and bean-shaped in occlusal view, with a reduced talonid portion compared to the large trigonid). Following the discussion in Madurell-Malapeira et al. (Reference Madurell-Malapeira, Alba and Moyà-Solà2009), some of the distinctive features of V. praeglacialis are: (1) a large and individualized m1 metaconid; (2) a wide bicuspid talonid with a larger hypoconid; and (3) a large and wide m2, with the protoconid larger than the metaconid. All these features contrast with those of MG 29-2013/461 (K260). In fact, the morphology of the m1 of closely resembles that of V. alopecoides from Pirro Nord and Dafnero-1 in general shape, with the distally inclined protoconid, the reduced metaconid, and slightly larger hypoconid compared to the entoconid and the presence of an accessory cuspulid mesial to the entoconid. The talonid of V. alopecoides is slightly shorter mesiodistally when compared to the Kvabebi specimen.
The scatter plot of the m1 length and width of Vulpes is shown in Figure 5. Together with the two extant species, V. vulpes and V. lagopus, we also include the Eurasian Plio-Pleistocene species V. praecorsac, V. praeglacialis, and V. alopecoides for comparison.

Figure 5 Scatter-plot diagram of the m1 length and width in extant and fossil species of the genus Vulpes. Symbols are explained in the legend.
All the fossil species fall within the variability of extant V. lagopus and are generally smaller than V. vulpes. Vulpes praecorsac is characterized by extremely reduced dimensions compared to the other two fossil species, being at the lower dimensional range of the extant V. lagopus. Vulpes praeglacialis and V. alopecoides are comparable in size. An additional scatter plot of m1 L and the ratio of m1 W/L allows an appreciation of the differences in robustness of the lower carnassial of Vulpes fossil species, showing that the Kvabebi specimen, like V. alopecoides, is characterized by stouter proportions of the m1 (Fig. 6).

Figure 6 Scatter-plot diagram of the m1 width and m1 width/length ratio in fossil species of Vulpes. Symbols are the same as in Fig. 5.
MG 29-2013/461 (K260) falls within the upper dimensional range of the early Pleistocene V. alopecoides sample and shares with the same species a comparable degree of robustness. Based on its morphological features, size, and proportions, we are prone to identify this specimen as Vulpes cf. V. alopecoides.
Tribe Canini Fischer von Waldheim, Reference Fischer von Waldheim1817
Genus Eucyon Tedford and Qiu, Reference Tedford and Qiu1996
Eucyon sp.
1972 Nyctereutes megamastoides Reference VekuaVekua, p. 41.
2009 Nyctereutes megamastoides Reference Agustí, Vekua, Oms, Lordkipanidze, Bukhsianidze, Kiladze and RookAgustí et al., p. 3277.
2009 Eucyon sp. Reference Agustí, Vekua, Oms, Lordkipanidze, Bukhsianidze, Kiladze and RookAgustí et al., p. 3277.
Materials
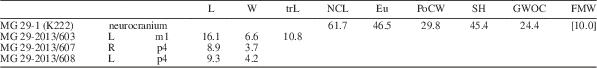
MG 29-1 (K222) neurocranium; MG 29-2013/607 right p4; MG 29-2013/608 left p4; MG 29-2013/603 right m1.
Description
MG 29-1 (K222) is a neurocranium preserving partial dorsal portions of the orbits and the frontals. The neurocranium (Fig. 7) is globular in shape, with inflated frontal sinuses that expand into the postorbital constriction. Their extension into the frontal is also testified by the dorsal outline of the frontals that bulge in lateral view, at level of the postorbital processes. The inflation of the frontal sinuses is also revealed by the smoothness of the parasagittal crest on top of them. In caudal view, the supraoccipital shield is fan-shaped, with an expansion at level of the mastoid process. The inions fail to overhang the condyles.

Figure 7 Eucyon sp. from Kvabebi. MG 29-2013/603, right m1, in buccal (1), lingual (2) and occlusal (3) views; MG 29-2013/607-608, right and left p4, in buccal (4) and occlusal (5) views. Comparison between two neurocranial fragments: Eucyon sp. MG 29-1 (K222) (6, 8, 10, 12) and N. megamastoides MG 29-2013/567 (K4173) (7, 9, 11, 13), in dorsal (6, 7), left lateral (8), right lateral (9), caudal (10, 11) and ventral (12, 13) views. Arrows highlight the morphology of the frontals at the level of the postorbital constriction and postorbital processes. Scale bar=1 cm.
The two premolars show high protoconids, slightly arched lingually; on their distal sides. There is a distal individualized and large cuspulid, which is not in line with the protoconid, but sits sits slightly buccally. Distally, there is a cuspulid-like cingulid. In the occlusal view, the m1 is elongated mesio-distally and slender. The paraconid is low, whereas the protoconid is high and slightly inclined backwards. The metaconid is reduced in height and rather individualized from the protoconid; it is positioned slightly distally compared to the distal wall of the protoconid, so that, in buccal view, it is visible behind it. The hypoconid is large and occupies almost all the talonid and has a faint accessory cuspulid on its distal side. The entoconid is reduced. There is a weak cristid connecting the talonid cuspulid. The m1 possesses a buccal cingulid.
Remarks
Cranial and dentognathic measurements of Eucyon from Kvabebi are shown in Table 5. The neurocranium MG 29-1 (K222) possesses inflated frontal sinuses, in contrast to all other cranial fragments recovered from Kvabebi. As shown in Figure 7, N. megamastoides crania (e.g., MG 29-2013/567 [K4173]) generally have marked postorbital constrictions in shape of a strong incision, in dorsal view. On the contrary, the postorbital constriction of MG 29-1 (K222) is slightly distinct. Furthermore, the dorsal portion of the frontals is bulging, compared to the depression visible in N. megamastoides. In caudal view, the supraoccipital shield in Eucyon is fan shaped (Fig. 7.10), whereas that of N. megamastoides is subrectangular (Fig. 7.11).
Table 5 Summarizing table of Eucyon sp. measurements from Kvabebi. Eu=greatest neurocranium breadth; FMW=width of the foramen magnum; GWOC=greatest width of occipital condyles; L=mesiodistal length; NcL=akrokranion-frontal midpoint (neurocranium length); PoCW=least breadth of postorbital constriction; SH=skull height (with sagittal crest); W=buccolingual width.
The p4 of Nyctereutes possess a short protoconid characterized by large mesial and distal sides (resulting in a low isosceles triangular shape in the buccal shape) and a distal accessory cuspulid that is almost entirely attached to the protoconid. In contrast, GM 29-2013/607 and GM 29-2013/608 show a high and slender protoconid with a prominent and individualized distal accessory cuspulid, features similar to those of Canini species. Because of its small size (Table 5) and some missing morphological features (e.g., weak cristid between hypoconid and entoconid), MG 29-2013/603 cannot be attributed to Canis. The paraconid is large mesiodistally with a subvertical mesial margin similar to E. odessanus from Alatini, unlike E. davisi and E. zhoui from Yushe and E. adoxus from Roussillon, which is shorter and distally inclined. Eucyon zhoui and E. adoxus possess very stout protoconids and larger metaconids compared to the specimen from Kvabebi. Furthermore, E. adoxus has a prominent cristid on the buccodistal side of the protoconid that is hardly visible in other Eucyon species. Eucyon marinae from Muhor-Erig, disregarding its larger size, has a considerably more individualized and larger metaconid compared to MG 29-2013/603, which is also distally pointed.
The talonid cuspids of the Kvabebi Eucyon bear resemblance to those of E. davisi and E. odessanus, with a large hypoconid and the entoconid is reduced and placed rather lingually. In fact, E. zhoui possesses a thinner talonid (reduced in buccolingual width), whereas E. marinae and E. adoxus have better-developed entoconids. Unlike the majority of Eucyon species analyzed, MG 29-2013/603 does not possesses accessory cuspulids in the talonid, apart from a small one on the distal side of the hypoconid, A comparable condition occurs in the E. odessanus mandibles of Alatini.
Species variability of the lower carnassial of the Pliocene Eucyon species from Eurasia is reported in the scatter diagram of Figure 8.1. MG 29-2013/603 is smaller than the lower carnassial of all Eurasian eucyons (E. marinae, E. zhoui, E. davisi, E. minor, and E. adoxus). Eucyon odessanus shows a wide dimensional range, and is proportionally slender when compared with the other species. Our specimen falls among the shortest individuals of E. odessanus range, although differing for the relatively wider carnassial. Given its relatively small size and robustness (e.g., Fig. 8.2), we prefer to leave open the specific attribution of the Kvabebi sample, referring to it as Eucyon sp.

Figure 8 Scatter-plot diagrams of the m1 length and width (1) and the m1 width and m1 width/length ratio (2) in species of the genus Eucyon. Symbols are explained in the legend.
Discussion
Vekua (Reference Vekua1972), who was the first to report canid material from Kvabebi, described several cranial and postcranial elements of Nyctereutes megamastoides and a single hemimandible fragment identified as Canis sp. Our revision confirms, in general terms, these original taxonomic attributions (Vekua, Reference Vekua1972; as updated in Agustí et al., Reference Agustí, Vekua, Oms, Lordkipanidze, Bukhsianidze, Kiladze and Rook2009), with additional recognition of a third taxon (Vulpes cf. V. alopecoides).
The occurrence of three sympatric canids is not surprising, since the family shows a great degree of sympatry. At present, Africa, Asia, and South America support the greatest canid diversity, with >10 living species for each continental area. The red foxes, golden jackals, and gray wolves are each sympatric with more than ten other canids (from different geographical regions) within one location; however, canid diversity is usually limited to a maximum of four or five species (Sillero-Zubiri and Macdonald, Reference Sillero-Zubiri and Macdonald2004). The fossil record of canid diversity does not contradict this rule, and the Kvabebi record documents a guild of three sympatric canids.
Nyctereutes
The first occurrence of Nyctereutes is in early Pliocene, with the species Nyctereutes tingi from the Yushe Basin and Nyctereutes donnezani from Western European localities. Subsequently, a more derived species (N. sinensis) appears in some Chinese sites, together with the more primitive N. tingi (Teilhard de Chardin and Pei, Reference Teilhard de Chardin and Pei1941). By the late early-middle Pleistocene, N. sinensis seems to have been replaced by another derived taxon (Nyctereutes sp. in Tedford and Qiu, Reference Tedford and Qiu1991). In contrast to the coexistence in Asia of primitive and derived forms, in late Pliocene localities in Western European (e.g., San Giusto, Villaroya, Perrier-Etouaires) raccoon dog-like canids are represented by the derived species N. megamastoides (Bartolini Lucenti, Reference Bartolini Lucenti2017). This species, probably related to N. donnezani, may be regarded as the European counterpart of N. sinensis for the retention of comparable derived features.
In the early Pleistocene, there is no or little record of Nyctereutes in Eurasia. The extant N. procyonoides appears in the Middle Pleistocene deposits of Zhoukoudian (localities 1 and 13). Even though the phylogenetic relationships of late Pliocene Eurasian species of Nyctereutes are still a matter of debate (see Tedford and Qiu, Reference Tedford and Qiu1991; Monguillon et al., Reference Monguillon, Spassov, Argant, Kauhala and Viranta2004), we deem a closer relation plausible between the extant N. procyonoides and N. sinensis. The occurrence in the Kvabebi sample of Nyctereutes megamastoides, as identified by Vekua (Reference Vekua1972), testifies to the wide geographic range expansion of this western European taxon.
Recently, Asahara and Takai (Reference Asahara and Takai2016) attempted to infer dietary preferences in extant and extinct Nyctereutes species using the ratio of m2 and m1 surfaces (see also Kavanagh et al., Reference Kavanagh, Evans and Jernvall2007). Their analyses confirmed that primitive species like N. donnezani and N. tingi had omnivorous diets. In addition, the authors found that N. sinensis had a more carnivorous m2/m1 score than for N. megamastoides, suggesting a more omnivorous diet for the latter. Using the methodology of Asahara and Takai (Reference Asahara and Takai2016), Nyctereutes from Kvabebi possesses an m2/m1 of ~0.53, intermediate between the two derived species. The Kvabebi sample fits with the general framework proposed by Asahara and Takai (Reference Asahara and Takai2016), in which members of the genus Nyctereutes underwent dietary transitions or had considerable dietary plasticity in their evolution.
Vulpes
The genus Vulpes appeared in the late Miocene (ca. 9 Ma, Hemphillian) of North America (Tedford et al., Reference Tedford, Wang and Taylor2009). As with other members of the subfamily Caninae, it expanded its range early into the Old World, as testified by the African species Vulpes riffautae de Bonis et al., Reference de Bonis, Peigné, Likius, Mackaye, Vignaud and Brunet2007 from the late Miocene Toros-Menalla site and by the early Pliocene Chinese Vulpes beihaiensis Qiu and Tedford, Reference Qiu and Tedford1990 from the Yushe Basin, as well as Vulpes qiuzhudingi Wang et al., Reference Wang, Tseng, Li, Takeuchi and Xie2014 from the Tibetan Plateau (the Zanda and Kundun basins). This latter large-sized species possesses remarkable hypercarnivorous adaptations, suggesting a close relationship to the extant V. lagopus.
The earliest European record of Vulpes is that of early-late Pliocene V. praecorsac in the Odessa catacombs (MN 15, Odintzov, Reference Odintzov1965). This small-sized fox has been historically considered the ancestor of the extant Vulpes corsac (Linnaeus, Reference Linnaeus1768) (see Kormos, Reference Kormos1932). Another well-known European species is V. alopecoides described by Major, 1975 from Tasso (Upper Valdarno), and recovered from St. Vallier, Senéze, France (Viret, Reference Viret1954), and Villaroya and La Puebla de Valverde, Spain (Kurtén and Crusafont Pairó, Reference Kurtén and Crusafont Pairó1977). Kurtén (Reference Kurtén1968) suggested the synonymy between V. alopecoides and V. praeglacialis. Nevertheless, the literature reveals no unanimous opinion on the status of these two species (Bonifay, Reference Bonifay1971; Rabeder, Reference Rabeder1976; García and Arsuaga, Reference García and Arsuaga1999). The relationship between the two taxa is beyond the scope of this paper; therefore, we have included both in the comparative analyses. MG 29-2013/461 represents the first occurrence of a member the vulpine taxon V. alopecoides, the most widespread fox species in the early Pleistocene of Western Europe.
Eucyon
The genus Eucyon appears in the late Miocene (late Clarendonian) record of North America with the species Eucyon davisi (Merriam, Reference Merriam1911). Its geographic range remained limited to North America until the latest Miocene, when the genus spread into the Old World, occurring in Central Asia, Africa, and Western Europe (the “Eucyon event”, Sotnikova and Rook, Reference Sotnikova and Rook2010). Eucyon davisi disappeared in North America in the earliest Pliocene (latest Hemphillian), and survived into the early Pliocene of eastern Asia (China and Mongolia). The genus Eucyon reached a relative high diversity in the Pliocene of Eurasia (Rook, Reference Rook2009; Sotnikova and Rook, Reference Sotnikova and Rook2010), surviving until the late Pliocene in China (E. minor), Mongolia (E. marinae), Tadzhikistan (E. kuruksaensis), Kazakhstan (Eucyon cf. E. odessanus), and southeastern Mediterranean regions (E. Eucyon cf. odessanus, Sarikol Tepe, Turkey).
The occurrence of a Eucyon representative within the fauna of Kvabebi completes our knowledge of the late Pliocene history of the advanced eucyons surviving in Western Europe and central Asia. The Kvabebi finding predates the explosive radiation of the wolf- and coyote-sized Canini that took place in Central Asia (reflected in the appearance of Canis teilhardi, C. longdanensis, C. brevicephalus, and Sinicuon cf. S. dubius), which continued until the end of the Pliocene (Qiu et al., Reference Qiu, Deng and Wang2004; Sotnikova and Rook, Reference Sotnikova and Rook2010).
Conclusions
The evolutionary history of the Pliocene Canidae in Eurasia is characterized by taxonomic diversification and range expansion. Diversification of Vulpini and Canini showed a maximum in Central Asia at the beginning of the early Pliocene, as evinced by the appearance of several new taxa (Nyctereutes tingi, V. beihaiensis, V. qiuzhudingi, Nurocyon chonokhariensis, Eucyon zhoui) and by the increase in frequency of other species (like the well-known Eucyon davisi).
In Western Europe, at the beginning of the Pliocene, the canid record is limited to the primitive vulpine Nyctereutes donnezani. The peak of canid diversification in Western Europe is recorded later in the early Pliocene, during the late Ruscinian (MN 15), with the differentiation of the advanced raccoon-dog Nyctereutes megamastoides, the appearance of the small fox V. praecorsac, and the emergence of the enigmatic “Canis” michauxi, together with the advanced Eucyon adoxus and Eucyon odessanus.
The revision of the Kvabebi assemblage documents the earliest occurrence of the typical (later) early Pleistocene species Vulpes alopecoides in the European fossil record. The contemporary early occurrence at Kvabebi of Vulpes with Nyctereutes and Eucyon clearly supports the established niche partitioning among these three sympatric canids.
Acknowledgments
We dedicate this paper to the late Professor A. Vekua for his pioneering work on Kvabebi. The Italian Ministry for Foreign Affairs (DGPCC-V) is acknowledged for financially supporting Italian paleontological research in Georgia. Comparative data were partly derived from Projects FR-TAF-3311, BE-TAF-3607, HU-TAF-6520 awarded to LR and SBL by the SYNTHESYS initiative (http://www.synthesys.info) and financed by the European Community Research Infrastructure Action under the FP7 “Capacities” Program.















