Introduction
There is growing awareness that the physiology and behaviour of animals can be altered by seemingly innocuous human activities such as wildlife tourism (Bateman and Fleming Reference Bateman and Fleming2017). Increasing popularity and economic importance of wildlife tourism has led to a growth in research on the effects of human disturbance on wildlife, and ways to manage disturbance (Tin et al. Reference Tin, Fleming, Hughes, Ainley, Convey, Moreno, Pfeiffer, Scott and Snape2008). In particular, previously isolated areas such as Antarctica and the subantarctic are becoming more popular for wildlife tourism (Pertierra et al. Reference Pertierra, Hughes, Vega and Olalla-Tárraga2017). High endemism and pressures from threats such as climate change and fluctuating prey availability mean these locations are particularly vulnerable to negative effects of human disturbance (Trathan et al. Reference Trathan, Forcada, Atkinson, Downie and Shears2008, Pertierra et al. Reference Pertierra, Hughes, Vega and Olalla-Tárraga2017). An absence of habituation opportunities due to limited contact with humans may also make subantarctic and Antarctic wildlife more sensitive to human disturbance.
While many guidelines and rules in these locations are already in place to reduce disturbance, the reasoning behind, and scientific basis for, these guidelines is often limited or lacking. Studies have shown sensitivity to human disturbance is species-specific, yet many guidelines do not cater for the most sensitive species in the area (Blumstein et al. Reference Blumstein, Fernandez-Juricic, Zollner and Garity2005, Holmes Reference Holmes2007, Tin et al. Reference Tin, Fleming, Hughes, Ainley, Convey, Moreno, Pfeiffer, Scott and Snape2008, Pertierra et al. Reference Pertierra, Hughes, Vega and Olalla-Tárraga2017). Providing a sound basis for these guidelines requires a quantification of the effects of human disturbance and documenting how changes in the nature of that disturbance may impact on the animal (Møller et al. Reference Møller, Samia, Weston, Guay and Blumstein2014, Bateman and Fleming Reference Bateman and Fleming2017). It is recognised that the best approaches to study these effects are evidence-based assessments and controlled approach studies to scientifically evaluate guidelines and assess the appropriateness of these guidelines for reducing disturbance (Holmes et al. Reference Holmes, Giese and Kriwoken2005, Weston et al. Reference Weston, McLeod, Blumstein and Guay2012).
Human disturbance has been shown to have a negative impact on a number of penguin species, including the Royal Penguin Eudyptes schlegeli, Magellanic Penguin Spheniscus magellanicus, Humboldt Penguin S. humboldti, African Penguin S. demersus, Gentoo Penguin Pygoscelis papua, Chinstrap Penguin P. antarctica and Adélie Penguin P. adeliae (e.g. van Heezik and Seddon Reference van Heezik and Seddon1990, Martín et al. Reference Martín, de Neve, Fargallo, Polo and Soler2004, Trathan et al. Reference Trathan, Forcada, Atkinson, Downie and Shears2008, Lynch et al. Reference Lynch, Fagan and Naveen2010, Barbosa et al. Reference Barbosa, De Mas, Benzal, Diaz, Motas, Jerez, Pertierra, Benayas, Justel, Lauzurica, Garcia-Pena and Serrano2013, Reyes-Arriagada et al. Reference Reyes-Arriagada, Hiriart-Bertrand, Riquelme, Simeone, Pütz, Lüthi and Raya Rey2013, Villanueva et al. Reference Villanueva, Walker and Bertellotti2014). These studies looked at a variety of disturbance responses, including behaviour, heart rate, levels of stress hormones, colony distribution, juvenile survival, and population trends. From this research, it is clear the magnitude and consequences of human disturbance depend on a multitude of factors including species, the level and type of human disturbance and the amount of exposure the colony has previously had to humans, demonstrating a need for species-specific guidelines to reduce human disturbance.
The Yellow-eyed Penguin (Megadyptes antipodes, hōiho), is one of the rarest penguins in the world (Seddon et al. Reference Seddon, Ellenberg, van Heezik, Borboroglu and Boersma2013). It is endemic to New Zealand, occurring only on the south-east coast of the South Island, Stewart Island, Codfish Island and in the New Zealand subantarctic on the Auckland Islands and Campbell Island (Figure 1) (McKinlay Reference McKinlay2001). Classified as ‘Endangered’ by IUCN and the New Zealand Department of Conservation (BirdLife International 2017, Robertson et al. Reference Robertson, Baird, Dowding, Elliott, Hitchmough, Miskelly, McArthur, O’Donnell, Sagar and Scofield2017), the population is estimated at less than 2,000 breeding pairs, with 60% of the population thought to occur in the subantarctic (McKinlay Reference McKinlay2001). Studies on mainland Yellow-eyed Penguins have shown it is one of the most sensitive penguin species to human disturbance (McClung et al. Reference McClung, Seddon, Massaro and Setiawan2004, Ellenberg et al. Reference Ellenberg, Setiawan, Cree, Houston and Seddon2007, Reference Ellenberg, Mattern and Seddon2009). The presence of tourists decreases the likelihood an adult will come ashore to their nest, increases transit times to and from the nest and increases the likelihood of nest abandonment (Wright Reference Wright1998, Ellenberg et al. Reference Ellenberg, Setiawan, Cree, Houston and Seddon2007). This delay increases the amount of food digested before regurgitation, resulting in less food available for the chicks (Wright Reference Wright1998). Unregulated tourism has been shown to decrease juvenile survival in their first year as a result of lower fledging weights, affecting population recruitment (McClung et al. Reference McClung, Seddon, Massaro and Setiawan2004). Tourist presence also has a direct effect on adult penguins, causing an increase in stress-induced corticosterone concentrations, which, with prolonged or frequent disturbance, is likely to result in decreased adult fitness and survival (Ellenberg et al. Reference Ellenberg, Setiawan, Cree, Houston and Seddon2007).
Figure 1. (A) New Zealand and New Zealand subantarctic islands. (B) Enderby Island. (C) Penguin Alley on Sandy Bay, Enderby Island.
The subantarctic populations are considered separate management units to the mainland due to low levels of migration (Boessenkool et al. Reference Boessenkool, Austin, Worthy, Scofield, Cooper, Seddon and Waters2009). Because of the isolation and practical difficulties of researching in the subantarctic, comparatively little is known about the population trends, breeding success and impact of threats on these colonies (Holmes Reference Holmes2007). Enderby Island (part of the Auckland Island archipelago) is thought to have the highest density of Yellow-eyed Penguins in the world (Moore Reference Moore1990). It is also the only place in the subantarctic where tourists regularly come into contact with Yellow-eyed Penguins (Department of Conservation 2016). The beach where tourists come ashore is in the area where a large proportion of the penguin population transit to and from the sea on foraging trips daily during summer. Restrictions and guidelines are already in place, and some of these are based on an observational study of Yellow-eyed Penguins and tourists on Enderby Island (Young Reference Young2009). The current minimum approach guideline for all wildlife there is a distance of 5 m, yet the appropriateness of this has never been scientifically validated. The behavioural impact of tourism on subantarctic Yellow-eyed Penguins has also never been quantified using an experimental approach. Despite this lack of knowledge on the current impact of tourism, in 2016 the number of tourists allowed per year on the island was increased from 600 to 1,100 and the number of people allowed on the island per day increased from 150 to 200 (Department of Conservation 1998, 2016).
In this study we sought to evaluate the effectiveness of current approach guidelines in reducing disturbance to Yellow-eyed Penguins on Enderby Island. Disturbance responses can be challenging to measure without the research itself becoming a source of disturbance, particularly for a sensitive species such as the Yellow-Eyed Penguin. This study was conducted using behavioural measures only due to practical and ethical restrictions preventing the gathering of physiological data. This has some limitations; studies have shown physiological changes can occur without any measurable changes in behaviour, such as in Magellanic Penguin chicks (Walker et al. Reference Walker, Boersma and Wingfield2005). In our study it is therefore possible that physiological responses may occur even when no behavioural change is observed. Our results should thus be viewed as a minimum measure of disturbance effects.
We quantified behavioural responses to human presence through controlled approaches and monitoring penguin behaviour in the presence and absence of tourists. Based on penguin behaviour and movement data, we evaluate the appropriateness of the current minimum approach guidelines and determine more effective distances for tourists interacting with Yellow-eyed Penguins.
Methods
Fieldwork was conducted during the 2016–2017 breeding season (November–February) on Enderby Island, Auckland Island archipelago (50°29′45″S 166°17′44″E; Figure 1). The Yellow-eyed Penguin is non-colonial and nests under cover away from conspecifics (Seddon and Davis Reference Seddon and Davis1989). They begin to nest in August/September, in forest or dense scrub up to 1 km inland from shore (Darby and Seddon Reference Darby, Seddon, Davis and Darby1990). Normally two eggs are laid, which are incubated for between 39 and 51 days prior to hatching (Darby and Seddon Reference Darby, Seddon, Davis and Darby1990, Seddon et al. Reference Seddon, Ellenberg, van Heezik, Borboroglu and Boersma2013). During the incubation and guard phase (September to January), both partners take it in turns to incubate the eggs and care for the young chicks, undertaking frequent foraging trips while their partner is guarding the nest (Seddon Reference Seddon1989). Hatching occurs in November/December. In the post-guard phase (January to March), both parents undertake foraging trips at the same time, and return for brief periods to the nest to feed the chicks (Darby and Seddon Reference Darby, Seddon, Davis and Darby1990). On Enderby Island, foraging trips begin and end with a transit through the forest and across the beach to get to the sea. This transit may involve contact with people and may also involve transiting through a New Zealand sea lion Phocarctos hookeri breeding area.
We collected all behavioural data at Penguin Alley, a section of Sandy Bay, Enderby Island, that is frequently used by penguins transiting to and from their nests and tourists arriving/departing and setting off/returning from walks on the island (Figure 1). Penguin Alley is currently the only area that has a penguin-specific restriction (the ‘no stopping’ rule), in addition to the 5 m minimum approach guideline that is applied across the subantarctic and across all species (Department of Conservation 2013).
Unlike on the New Zealand mainland, tourism in the subantarctic is highly regulated by the Department of Conservation. The main source of tourists is from cruise ships which visit Enderby at a rate of approximately one per week over part of the breeding season of the Yellow-eyed Penguin. These vessels can hold between 50 and 200 tourists, who visit Enderby Island for one day per trip. During the 2016–2017 breeding season 12 tourist vessels visited Enderby Island at an average of one ship every nine days; at the peak period (early January) three ships visited in eight days.
Behavioural observations of penguin-tourist interactions
We used behavioural observations in the presence and absence of tourists to study the impact of tourist activity on the behaviour of penguins. We recorded behaviour of penguins, New Zealand sea lions and tourists using an ethogram adapted from a set of behaviours identified and previously used for similar projects on the mainland and on Enderby Island (Young Reference Young2009). Specifically, we recorded the following behaviours: walking (walking at a regular pace), preening (preening feathers, shaking head and/or stretching flippers out), alert (scanning or frequent head turning) and fleeing (also referred to as ’running highly alert’, running stumbling, hopping or tobogganing at a fast pace). We recorded the start and stop times of each behaviour, so that a total time spent on each behaviour could be calculated. For example, ’time spent preening’ is the total time a penguin (or penguins) spent preening during a transit. Adult and juvenile penguins were differentiated by the yellow crown present on adults but absent in juveniles (Darby and Seddon Reference Darby, Seddon, Davis and Darby1990). In addition, we recorded the distance between the penguin(s) and the tourist(s) where possible, using a digital rangefinder (Nikon Forestry 550), a compass to measure distance and angles to each target (human or penguin), and used trigonometry to calculate the unknown distance between the targets.
Controlled approaches
We carried out controlled approaches using a single observer, when tourists were not present. Approaches were limited to a 10-minute period every hour to minimise disturbance. Two different approaches were made:
(i) A stationary approach where a single observer stood in the middle of Penguin Alley, so that the penguin(s) must approach the observer in order to transit across the sward.
(ii) A moving approach, where a single observer moved slowly (approximately 0.5 m/s) towards the penguin(s) parallel to the shoreline and perpendicular to the penguin’s line of travel.
When approaching, the observer used a laser range finder to measure the distance from them to the penguin(s) every 30 seconds or 5 m, including recording the distance when the penguin(s) was first disturbed. Another observer out of sight of the penguin(s) (not on Penguin Alley) recorded penguin behaviour using the ethogram mentioned above.
Disturbance was defined as avoidance behaviour: either a change in direction of travel by more than 45 degrees away from the observer, or a change in the speed of travel from walking/stationary to fleeing. The time the penguin(s) was first sighted leaving a refuge (surf or forest) and the time the penguin(s) entered a refuge was recorded to calculate total transit time in the open.
To investigate the impact of sea lion disturbance, we also recorded penguin-sea lion interactions on days when tourist-penguin interactions were being observed, or when controlled approaches were being undertaken. We also conducted a count of adult sea lions each observation day, where the whole of Sandy Bay was divided into five sections and the number of sea lions was counted using binoculars. In addition, we recorded the number of active sea lions (classed as active when doing any behaviours except resting) for every observation. These were used as covariates in the models described below.
Analysis
We used general linear models to analyse the continuous response variables (time spent alert, time preening, time walking and transit time). To meet the assumption of normality, all these response variables were log-transformed. We analysed disturbance type as a fixed factor with four levels: control (no disturbance), stationary (stationary controlled approach), moving (moving controlled approach) and tourist (observations of actual tourist-penguin interactions). The predictor variables were treatment (a fixed factor with four levels), direction (whether the penguin(s) were leaving or returning from the nest), penguin group size, time (expressed as days since mean hatch date: 27 November), sea lion count and sea lion behaviour. Direction, group size, time and sea lion count were all consistently not significant and did not improve the fit of the models, so they were removed from the models. All continuous predictor variables were centred by subtracting the mean from each observation to avoid multicollinearity and to obtain meaningful odds ratios. We used a generalised linear model with binomial family and logit link for the binomial response variable ‘outcome’. The transit was classed as successful if the penguin(s) completed the journey in the direction it was originally travelling. The moving approach was excluded from this analysis because the penguin(s) had to be half-way up the shore before the approach could begin. Fleeing behaviour was also expressed binomially and analysed in the same way.
To model the optimum minimum approach distance, we used mixed generalized linear models for the binomial response variable ‘disturbed’ with the predictor variable being distance from penguin to human. The distance was recorded multiple times for each trial, so a trial was classed as a random effect and distance a fixed effect. We generated graphs in R studio, using ‘ggplot2’ (Wickham Reference Wickham2016, R Core Team 2017). Models were created using the R packages MASS and ‘lme4’ (Venables and Ripley 2013, Bates et al. Reference Bates, Maechler, Bolker and Walker2015).
Results
Behaviour
We conducted a total of 95 controlled approaches (to groups of one or more penguins), 32 observations of penguin-tourist interactions and 81 observations where no humans were present (control). Human presence caused a significant drop in the probability of a successful transit compared to the control group for the stationary and tourist groups, from 0.99 (SE 0.01) for the control to 0.77 (0.06) and 0.76 (0.15) for the stationary and tourist groups respectively; Z = -2.9 and -2.8, P < 0.005. The presence of an active sea lion caused a decrease in the probability of a successful transit from 0.99 (SE 0.01) with no active sea lion to 0.75 (0.09); Z = -2.5, P = 0.01. The probability of a bird fleeing did not increase in the presence of a human, from 0.02 (SE 0.01) for the control to 0.05 (0.03) and 0.07 (0.04) for the stationary and tourist groups respectively; Z = 1.1 and 1.3, P > 0.05, but did increase in the presence of an active sea lion, from 0.02 (SE 0.01) for the control to 0.53 (SE 0.15) in the presence of an active sea lion; Z = 5.9, P < 0.001. Sea lion presence was not significant in any other models. The time spent alert increased with the stationary approach and in the presence of tourists (t = 2.5 and 5.3, P < 0.05), as did time spent walking (t = 3.2 and 2.7, P < 0.01). The time spent preening decreased with the moving approach and in the presence of tourists (t = -4.0 and -3.6, P < 0.001). Moving human presence significantly decreased transit time (t = -2.0 P = 0.04), and tourist presence significantly increased transit time (t = 2.1 P = 0.03) compared to the control (Figure 2).
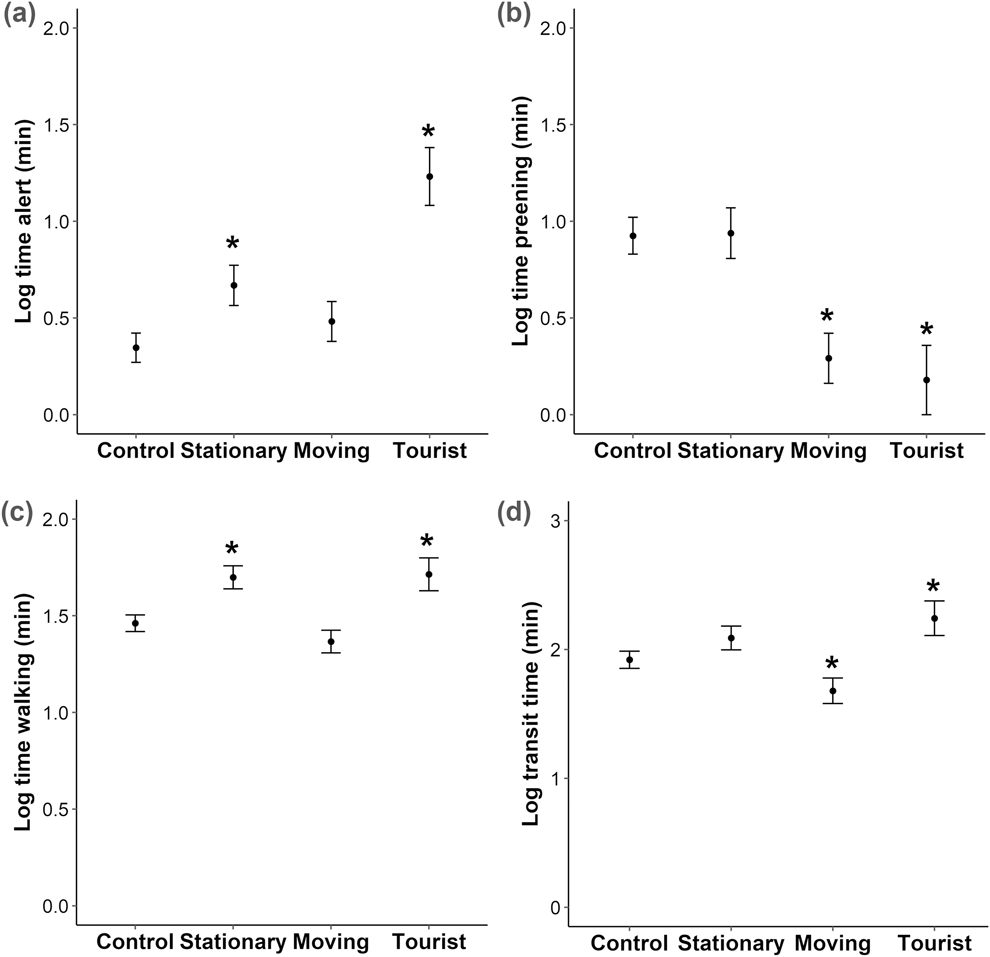
Figure 2. Effect of controlled approaches and tourist presence on behaviour of Yellow-eyed Penguins. Plots show (A) time spent alert, (B) time spent preening, (C) time spent walking and (D) transit time. Categories across the x-axes are when there is no human presence (Control), stationary human presence (Stationary), moving human presence (Moving) and in the presence of tourists (Tourist). Asterisk denotes significance from control. n = 208 for all comparisons.
Minimum approach guideline
The distance from human to penguin had a significant effect on the likelihood of disturbance behaviour being displayed (Z = -4.0, P < 0.001, Figure 3). At the current minimum approach guideline (5 m), the probability of disturbance is 0.99 (99%). At 50 m the probability of disturbance drops to less than 0.03 (3%).
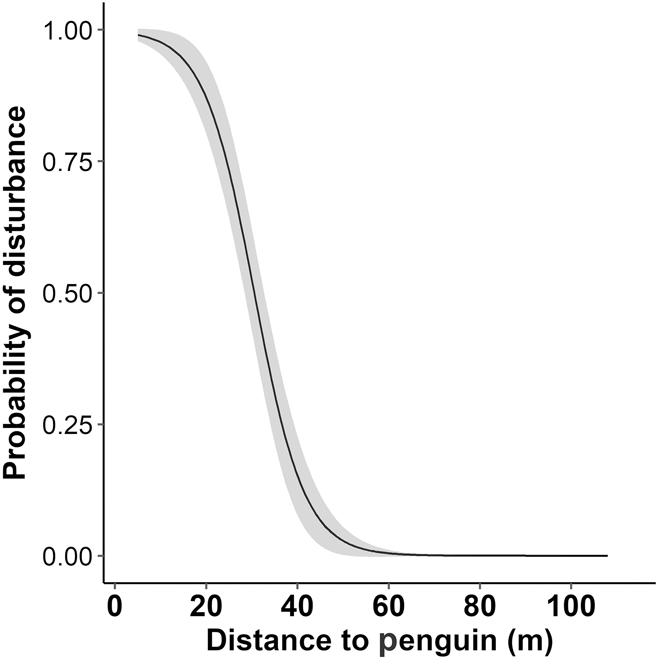
Figure 3. The probability of disturbance for Yellow-eyed Penguins as a function of approach distance by a human. The fitted line is from a generalized linear mixed model, grey shading is standard error; n = 212.
Tourist behaviour
A total of 32 penguin-tourist interactions were recorded. The distance between penguin and tourist was recorded for 18 of these interactions. The minimum distance tourists approached penguins ranged from 3 to 113 m, with a median minimum approach distance of 27 m. Thirty-nine percent of tourist groups approached Yellow-eyed Penguins to a distance of < 21 m (Figure 4). The mean group size was three tourists (SE 2.67).
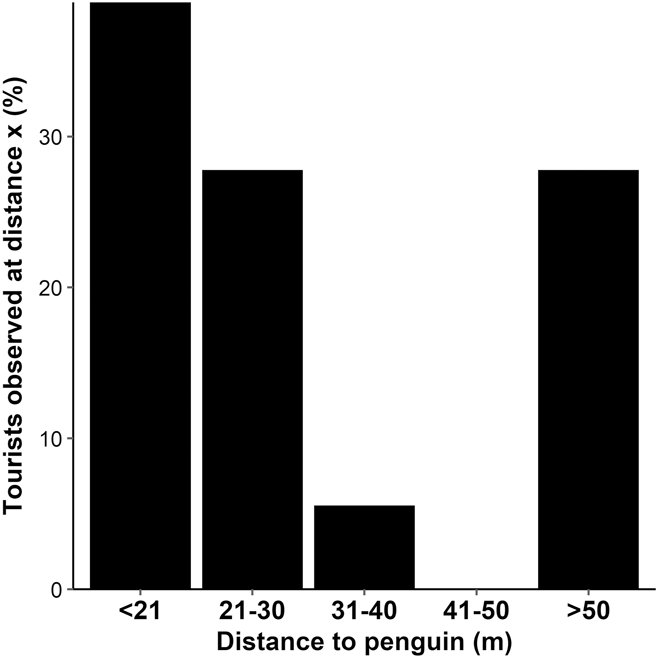
Figure 4. Percentage of tourists observed approaching to a minimum distance of < 21, 21–30, 31–40, 41–50 and > 50 m to a Yellow-eyed Penguin; n = 18.
Discussion
Our results showed that human presence on the beach on Enderby Island when Yellow-eyed Penguins were transiting caused the birds to alter their behaviour, and that the type of human presence had an impact on the nature of the behavioural change. The presence of a human caused the probability of a transit being successful to decrease, with around a fifth of the attempted transits being aborted when people were present. Transit times (in successful transits) decreased with the moving controlled approach by the observer, whereas they increased when a tourist group was present. This may be because the moving controlled approach caused an increase in speed towards the refuge (the forest or sea), whereas the tourist group often blocked the penguin(s) from the refuge. Nonetheless, both events result in a change of movement behaviour which is likely to be negative – the increased transit time results in less food regurgitated to the chicks and the decreased transit time may increase stress levels and energy expenditure (Wright Reference Wright1998, Ellenberg et al. Reference Ellenberg, Setiawan, Cree, Houston and Seddon2007).
Other behaviours were affected differently by stationary and moving controlled approaches. The stationary controlled approach resulted in an increase in the time spent alert, while the moving controlled approach resulted in a decrease in the time spent preening. Both may reflect a change in vigilance by the bird and a consequent change in alternative maintenance behaviours.
Consequences of disturbance
While increases or decreases in these behaviours may not be that important in themselves, they are a reflection of the behavioural impact of human presence, which may contribute to physiological or population level changes. An increase in the time spent alert may indicate an increase in the levels of stress hormones (such as corticosterone). Increases in base-level corticosterone may, over the lifetime of the bird, decrease fitness and survival (Ellenberg et al. Reference Ellenberg, Setiawan, Cree, Houston and Seddon2007). In Humboldt Penguins, alert behaviour is correlated with an increase in stress hormones (Ellenberg et al. Reference Ellenberg, Mattern, Seddon and Jorquera2006). Alert behaviour is also a strong predictor of increased heart rate in Royal Penguins (Holmes et al. Reference Holmes, Giese and Kriwoken2005).
Physiological data are difficult to collect in the wild and are one of the biggest gaps in understanding of wildlife tourism worldwide (Trave et al. Reference Trave, Brunnschweiler, Sheaves, Diedrich and Barnett2017). There is evidence to suggest that behavioural changes do not always indicate a decrease in the health of the animal, physiological changes or population level effects (Gill et al. Reference Gill, Norris and Sutherland2001, Tarlow and Blumstein Reference Tarlow and Blumstein2007). Conversely, physiological changes can occur as a result of human presence when no behavioural changes are observed (Beale and Monaghan Reference Beale and Monaghan2004a, Bejder et al. Reference Bejder, Samuels, Whitehead, Finn and Allen2009). Magellanic Penguin chicks exposed to humans showed evidence of lower behavioural responses to humans but higher physiological stress responses, compared to chicks not exposed to humans (Walker et al. Reference Walker, Boersma and Wingfield2005). However, as methods for gathering physiological data are often invasive and stressful themselves, it can be difficult to separate the effects of capture and sampling from the effects of tourist disturbance (Trave et al. Reference Trave, Brunnschweiler, Sheaves, Diedrich and Barnett2017). It may also be more difficult to link specific physiological data (such as peaks in corticosterone) to specific behaviours or events. Other metrics including heavy metal levels, genotoxic damage and immunological responses can also be useful in determining human disturbance effects, as has been found in Gentoo Penguins when comparing disturbed and non-disturbed sites (Barbosa et al. Reference Barbosa, De Mas, Benzal, Diaz, Motas, Jerez, Pertierra, Benayas, Justel, Lauzurica, Garcia-Pena and Serrano2013).
The isolated and harsh conditions of the subantarctic make studying physiology extremely challenging, however several studies of mainland Yellow-eyed Penguins have shown higher base-level corticosterone at unregulated tourism sites, and an increase in heart rate of nesting birds when exposed to an approaching human (Ellenberg et al. Reference Ellenberg, Setiawan, Cree, Houston and Seddon2007, Reference Ellenberg, Mattern and Seddon2013).
An increase in transit time and the number of aborted transits is likely to decrease the food available to chicks, which may lead to decreased fledgling weight. Studies on mainland Yellow-eyed Penguins have found lower fledging weights in areas of high levels of unregulated tourism (McClung et al. Reference McClung, Seddon, Massaro and Setiawan2004, Ellenberg et al. Reference Ellenberg, Mattern, Seddon and Jorquera2006). Low fledging weight leads to decreased juvenile survival, meaning that lower fledging weights can have long term population consequences (McClung et al. Reference McClung, Seddon, Massaro and Setiawan2004). The increased number of aborted transits observed on Enderby Island could therefore have negative repercussions for population recruitment. This effect has also been seen in other penguins: in Gentoo Penguins some sites frequently visited by tourists had a significant decline in breeding pairs, and in Humboldt Penguins, breeding success was found to be significantly reduced at tourist sites due to the foraging partner being prevented from returning to the nest and feeding the chicks (Ellenberg et al. Reference Ellenberg, Mattern, Seddon and Jorquera2006, Trathan et al. Reference Trathan, Forcada, Atkinson, Downie and Shears2008).
Is habituation possible for Yellow-eyed Penguins?
The results of this study also indicate that sea lions may cause a similar level of disturbance to human presence. The endangered endemic New Zealand sea lion has a similar distribution to the Yellow-eyed Penguin, with 18% of pups born on Sandy Bay, Enderby Island (Childerhouse et al. Reference Childerhouse, Burns, French, Michael and Muller2017). These two species have co-existed on Enderby Island for thousands of years, indicating the Yellow-eyed Penguin is able to tolerate some level of disturbance (Collins et al. Reference Collins, Rawlence, Prost, Anderson, Knapp, Scofield, Robertson, Smith, Matisoo-Smith and Chilvers2014). However, as this natural disturbance already exists penguins may be more vulnerable to additional disturbance by humans. By avoiding a human, they may also increase the likelihood of an interaction with a sea lion. Trade-off scenarios may also occur, where fleeing from a human then results in the penguin moving closer to an active sea lion. Trade-offs were found to affect fleeing behaviour in juvenile Chinstrap Penguins, where a trade-off existed between fleeing from the predator (a human) and entering a colony where they may be attacked by adults (Martin et al. 2004).
Yellow-eyed Penguin behaviour in this study indicates a lack of habituation to sea lion behaviour, likely due to occasional land predation by sea lions (personal observation). New Zealand sea lions have also been shown to depredate Eastern Rockhopper Penguins (E. chrysocome) on Campbell Island (Morrison et al. Reference Morrison, Armstrong, Battley, Jamieson and Thompson2017).
Some studies have shown habituation can have positive effects on the ability of the animal to adapt to the presence of human disturbance, by reducing stress levels and preventing negative changes in behaviour (Walker et al. Reference Walker, Boersma and Wingfield2005, Baudains and Lloyd Reference Baudains and Lloyd2007). The capability and degree of habituation appears to vary greatly among penguin species, for example there is evidence of behavioural and physiological habituation in Magellanic Penguins but not in Humboldt Penguins (Ellenberg et al. Reference Ellenberg, Mattern, Seddon and Jorquera2006, Walker et al. Reference Walker, Dee Boersma and Wingfield2006, Villanueva et al. Reference Villanueva, Walker and Bertellotti2012). As tourist visitation is infrequent and at low levels on Enderby Island, these penguins may not have had the opportunity to become habituated to humans. However, the lack of habituation by Yellow-eyed Penguins on mainland New Zealand (where the level of tourism is much higher and continuous year-round) indicates that habituation to tourism may not be possible. There is some evidence of habituation by Yellow-eyed Penguins to invasive research (including frequently approaching the nest and taking blood samples) (Ellenberg et al. Reference Ellenberg, Mattern and Seddon2009). However, this effect appears to be restricted to nest visits only, as Yellow-eyed Penguins exposed to unregulated tourism were observed to have a higher hormonal response than birds from undisturbed sites (Ellenberg et al. Reference Ellenberg, Setiawan, Cree, Houston and Seddon2007).
Management implications
The results of this study indicate that human presence has an impact on behaviour of the subantarctic Yellow-eyed Penguin. This has also been found for the mainland Yellow-eyed Penguin (McClung et al. Reference McClung, Seddon, Massaro and Setiawan2004, Ellenberg et al. Reference Ellenberg, Setiawan, Cree, Houston and Seddon2007, Reference Ellenberg, Mattern and Seddon2009) and other penguin species (e.g. Ellenberg et al. Reference Ellenberg, Mattern, Seddon and Jorquera2006, Villanueva et al. Reference Villanueva, Walker and Bertellotti2014). The mere presence of humans will have some behavioural impact on most species, so a key question which is often not addressed is what level of impact is considered acceptable (Trave et al. Reference Trave, Brunnschweiler, Sheaves, Diedrich and Barnett2017). At one extreme, mortality or abandoned breeding attempts could result from disturbance, a far more serious impact than conservation managers would accept. Given the serious declines of Yellow-eyed Penguins on mainland New Zealand and the suite of stressors faced by birds there, a precautionary approach should be taken with the subantarctic population to prevent a similar decline (Boessenkool et al. Reference Boessenkool, Star, Seddon and Waters2010, Seddon et al. Reference Seddon, Ellenberg, van Heezik, Borboroglu and Boersma2013). One approach could be to prevent all visible signs of disturbance, such as alert and avoidance behaviour, as has been proposed for Royal Penguins (Holmes et al. Reference Holmes, Giese and Kriwoken2005).
On Enderby Island, tourists approached to a median minimum distance of 27 m, where the probability of disturbance is 0.65, and 39% of tourists approached to a minimum distance of < 21 m, where the probability of disturbance is > 0.87 (see Figure 3). At the current minimum approach distance (5 m), the probability of disturbance is 0.99. These disturbance probabilities imply that a new minimum approach guideline is warranted. Due to the small size of the area (the length of penguin alley is approximately 70 m), reducing the probability of disturbance to near-zero (a distance of c. 50 m) may be impractical, but our results can help managers to decide on and justify a distance that is acceptable. At 40 m for example, the probability of disturbance is still low (0.15) so this distance may be an acceptable compromise between minimising disturbance and practical limitations.
To our knowledge this is the first research using controlled approaches on transiting Yellow-eyed Penguins, and the first research to evaluate minimum approach guidelines. There are many minimum approach guidelines for mainland breeding sites of the Yellow-eyed Penguin. However, to our knowledge none of these guidelines has been based on controlled approach experiments, and none has been tested for appropriateness. There is a clear need for a scientifically-validated minimum approach guideline that caters for sensitive species such as the Yellow-eyed Penguin.
The minimum approach modelling conducted in this study was based on experimental approaches, which was a single observer quietly and slowly approaching. In general, tourist groups are likely to be larger in numbers and noisier. Different movement behaviours can affect the magnitude of disturbance, such as walking and jogging (Lethlean et al. Reference Lethlean, Van Dongen, Kostoglou, Guay and Weston2017, Radkovic et al. Reference Radkovic, Van Dongen, Kirao, Guay and Weston2017). Group sizes can also have an effect; it has been shown in other colonial seabirds (Kittiwake Rissa tridactyla and Guillemot Uria aalge) that the effects of disturbance increase with numbers of visitors (Beale and Monaghan Reference Beale and Monaghan2004b). The behavioural results from this study concur: observations of penguin behaviour in the presence of tourists (with an average group size of three) showed a greater reaction (more time alert, less time preening) than during the experimental approaches by a single observer. Therefore, the minimum approach modelling is likely to be an underestimate of the actual reaction a penguin may have in the presence of a group of tourists.
Researchers also visit the island for weeks to months at a time and have done so annually for at least the last 25 years. Like tourists, they follow the ‘minimum impact code’ so are bound by the same guidelines and restrictions as tourists when not conducting research (Department of Conservation 2013). All research is also subject to the New Zealand Department of Conservation and animal ethics approval. While researchers are in lower numbers than tourists and may be expected to behave differently and have a lesser impact (Stein et al. Reference Stein, Young, Seddon, Darby and van Heezik2017), they have a constant presence throughout a large portion of the Yellow-eyed Penguin breeding season. In particular, New Zealand sea lion researchers must cross penguin alley multiple times each day to access the sea lion breeding area. It has been shown with mainland New Zealand Yellow-eyed Penguins that researcher disturbance at nests has no short- or long-term effects on breeding success or lifetime reproductive success (Stein et al. Reference Stein, Young, Seddon, Darby and van Heezik2017). However, researchers on Enderby Island would mostly be disturbing penguins in a similar way to tourists (i.e. on their transit rather than at their nest). Therefore, researcher impact must not be ignored by policy makers when considering the number of people visiting the island and activities conducted.
Recommendations
Properly managed tourism can be effective at reducing the negative impacts of human disturbance (Trave et al. Reference Trave, Brunnschweiler, Sheaves, Diedrich and Barnett2017). Tourism in the New Zealand subantarctic is presently at low levels, so the current effect is likely to be low. However, the negative impact of human presence shown in this study indicates the importance of minimising the number of human-penguin interactions. This can be done by keeping the total number of tourists visiting the island to low levels, regulating the timing of tour boats landing and departing to avoid peak penguin activity, and limiting the number of tourists ashore at one time. In addition, regulations such as a suitable minimum approach distance are important for reducing the disturbance during a penguin-human interaction. Modelling from this study indicates the current guideline needs to be revised from 5 m to ensure disturbance is minimised.
Modelling the appropriateness of minimum approach guidelines by predicting the probability of disturbance is a useful technique that could be applied to other species and systems. With the number of participants in wildlife tourism worldwide expected to double in the next 50 years (French et al. Reference French, González-Suárez, Young, Durham and Gerber2011), regulations and guidelines with a scientific basis will become more important than ever for reducing human disturbance in wildlife.
Acknowledgements
We conducted our research under a New Zealand Department of Conservation Wildlife Act permit 39214-FAU, Massey University animal ethics permit no. 14/67, and with the approval of local iwi (Māori tribe). Funding was provided by a Massey University MSc Scholarship (to R.K.F.) and PhD Scholarship (to C.G.M.), and Massey University Research Funding 17946 and Institute of Veterinary Animal and Biomedical Sciences Post-graduate studies grant. Thank you to the New Zealand Department of Conservation for logistical, funding and practical support including transport to the island, and providing essentials including food, fuel, and the loan of equipment. Thanks also to the following for supplying funding and/or equipment: Ecology Group Massey University, Yellow-eyed Penguin Trust, Birds NZ, Freemasons Charity and Blue Planet Marine. Thanks to Melanie J. Young for helpful advice and guidance, and thanks to the two anonymous reviewers for their useful suggestions and advice.