Immune responses are required for host protection against infections and neoplasms. However, in order to prevent immune-related disorders, such as autoimmune and chronic inflammatory diseases, the balance between the activation and suppression of immune responses is regulated very tightly by complex immune cell types in higher organisms. Among those immune cell types, CD4+ helper T-cells play a pivotal role in regulating chronic inflammation in various disease models, including, but not limited to, chronic colitis( Reference Westendorf, Templin and Geffers 1 , Reference Coccia, Harrison and Schiering 2 ), atherosclerosis( Reference Koltsova, Garcia and Chodaczek 3 ), rheumatoid arthritis( Reference van der Lubbe, Reiter and Riethmuller 4 , Reference Zhang, Nakajima and Goronzy 5 ) and chronic eosinophilic lung inflammation( Reference Doherty, Soroosh and Broide 6 ).
Immune functions are affected by the diet. Dietary fish oil (FO) enriched with n-3 PUFA exhibits anti-inflammatory properties in both in vitro and in vivo experimental models( Reference Zhang, Kim and Zhou 7 , Reference Orr, Trepanier and Bazinet 8 ). DHA (22 : 6Δ4,7,10,13,16,19) and EPA (20 : 5Δ5,8,11,14,17) are the major n-3 PUFA found in dietary fish oil( Reference Kris-Etherton, Harris and Appel 9 ), which have been studied for their immunoregulatory properties. Furthermore, clinical and epidemiological studies have supported the favourable role of n-3 PUFA on T-cell-mediated chronic inflammatory diseases( Reference Adams, Phung and Wu 10 , Reference Rangel-Huerta, Aguilera and Mesa 11 ). These immunomodulatory effects of n-3 PUFA appear to be mediated by a variety of mechanisms, including the inhibition of pro-inflammatory eicosanoid production, the antagonism of NF-κB and the modulation of lipid rafts (reviewed previously in Kim et al. ( Reference Kim, McMurray and Chapkin 12 , Reference Kim, Khan and McMurray 13 )). Our laboratory has also demonstrated that n-3 PUFA decrease the accumulation of CD4+ T-cells in the colonic lamina propria during dextran sodium sulphate-induced colitis, thereby suppressing colitis-associated colon cancer( Reference Jia, Lupton and Smith 14 , Reference Monk, Kim and Callaway 15 ).
Previous studies have focused on CD4+ T-cells activated polyclonally by either concanavalin A, monoclonal antibodies specific to CD3 and CD28, or phorbol ester (phorbol-12-myristate-13-acetate) and ionophore (ionomycin). However, since these treatments potently activate T-cells by multidirectional signalling on the cell surface, their interpretation is complicated. In order to mimic antigen-induced clustering of proteins on the T-cell surface, researchers have used superantigens and cholera toxin B subunit, which also have limitations since they do not accurately reflect the response of T-cells to nominal antigens. In contrast, antigen-specific T-cell activation, in which polar immunological synapses (IS) to antigen-presenting cells are formed, is more physiological. Antigen-specific T-cell receptor transgenic mice of the DO11.10 strain harbour CD4+ T-cells, which respond to the ovalbumin 323–339 (OVA) epitope. Due to the genetic deletion of recombination activating gene 2 (RAG2), the antigen specificity of those transgenic T-cells are maintained during positive and negative selection in the thymus( Reference Robertson, Jensen and Evavold 16 ).
Heterogeneous liquid-ordered mesodomains of the plasma membrane, i.e. lipid rafts, effectively sort signalling proteins during CD4+ T-cell antigen receptor activation according to their affinity for raft domains, leading to the assembly of stable, larger rafts at the contact site of T-cells/antigen-presenting cells, i.e. IS( Reference Meiri 17 , Reference Jury, Flores-Borja and Kabouridis 18 ). Interestingly, lipid raft size appears to be tightly regulated, since oversized lipid raft domains negatively affect T-cell signalling( Reference Nicolau, Burrage and Parton 19 ). Using fat-1 transgenic mice in which endogenous n-3 PUFA are generated and targeted to the T-cell membrane( Reference Kang, Wang and Wu 20 , Reference Fan, Kim and Callaway 21 ), we have demonstrated that n-3 PUFA modulate lipid raft composition and down-regulate CD4+ T-cell signalling( Reference Kim, Fan and Barhoumi 22 ). The fat-1 transgenic mouse model has an advantage in that fatty acid loss to the culture medium during the functional ex vivo assessment of CD4+ T-cells is prevented( Reference Fan, Kim and Callaway 21 ). However, it remains to be determined whether dietary supplementation with n-3 PUFA can recapitulate these findings. Therefore, in the present study, we fed DO11.10-Rag2− / − T-cell receptor transgenic mice a semi-purified diet containing either fish oil (enriched with DHA and EPA), purified DHA alone, or control maize oil (devoid of n-3 PUFA) at physiologically relevant levels( Reference Sugano 23 ). Here we report the effects of dietary FO and DHA on antigen-induced lipid raft formation and subsequent T-cell signalling.
Materials and methods
Mice and diets
All experimental procedures using laboratory animals were approved by the Texas A&M University Laboratory Animal Care and Use Committee. DO11.10 Rag2− / − T-cell receptor transgenic mice were purchased from Taconic Farms, bred and maintained at Texas A&M University. At the onset of the study, sex- and age (8–16 weeks)-matched mice were fed a semi-purified control diet containing 5 % maize oil (MO) (Dyets Company) by weight during the 7 d acclimatisation period, followed by a 2-week feeding period with either the same MO control diet, a FO diet (1 % MO+4 % FO; Omega Protein Corporation) or a DHA diet (4·05 % MO+0·95 % DHASCO® (44·9 % purity); Martek)). All the diets met the Nutrition Research Council's nutrition requirements( Reference Fan, McMurray and Ly 24 ) containing identical levels of major (fat 5 %, protein 20 %, carbohydrate 42 %, starch 22 % and fibre 6 %) and minor (vitamins 3·5 %, minerals 1 %, methionine 0·35 % and choline chloride 0·2 %) ingredients and differed only in the sources of fat as described above. The diets were stored at − 20°C and provided fresh daily in order to avoid oxidation. Food and drinking-water were provided ad libitum.
CD4+ T-cell purification and Laurdan labelling
Mice were euthanised by CO2 asphyxiation. Spleen was removed and placed in complete Roswell Park Memorial Institute (RPMI)-1640 medium with 25 mm-HEPES (Irvine Scientific), supplemented with 5 % fetal bovine serum (FBS; HyClone), 105U penicillin/l (60 mg/l) and 100 mg streptomycin/l (Irvine Scientific), 2 mm-l-glutamine (Gibco) and 10 μm-2-mercaptoethanol (Sigma). CD4+ T-cells (>90 % pure as determined by surface staining of CD3 and CD4, data not presented) were isolated from the spleen by a magnetic microbead positive selection method (Miltenyi Biotec) according to the manufacturer's recommendations( Reference Kim, Fan and Barhoumi 22 ). Purified CD4+ T-cells were labelled with Laurdan (Invitrogen) for lipid raft visualisation as described previously( Reference Kim, Fan and Barhoumi 22 ). Briefly, 5 μmol/l Laurdan was prepared in serum-free RPMI medium and incubated with 2 × 106 cells/ml for 30 min at 37°C. The cells were subsequently washed and resuspended in serum-free Leibovitz's medium (Irvine Scientific).
Analysis of fatty acid composition
Total lipids were extracted from the diets or CD4+ T-cells by the method of Folch et al. ( Reference Folch, Lees and Sloane Stanley 25 ). Briefly, lipids were extracted by the mixture of chloroform and methanol (2:1, v/v), followed by methylation with 6 % (v/v) hydrogen chloride in methanol. Fatty acid methyl esters were extracted using hexane and 0·1 m-potassium chloride and analysed by capillary GC (Fig. 1) as described previously( Reference Chapkin and Carmichael 26 ).

Fig. 1 (A) Formation of liquid-ordered mesodomains was quantified as general polarisation (GP) values at the immunological synapse (■) or the whole CD4+ T-cell (□). DO11.10 T-cell receptor transgenic CD4+ T-cells were co-cultured with ovalbumin-pulsed B-lymphoma cells (ten to twelve cells per mouse from four mice per diet were examined to obtain a total of forty to forty-eight observations). Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from that of the whole cell (P< 0·05). (B) Increase in membrane liquid order at the immunological synapse relative to the whole CD4+ T cell was assessed by calculating the fold increase of GP values. Fold increase was expressed as GPimmunological synapse/GPwhole cell. a,bMean values with unlike letters were significantly different (P< 0·05). MO, maize oil diet; FO, fish oil diet.
Antigen-specific stimulation and lipid raft assessment
For antigen-specific activation, A-20 B-lymphoma cells (ATCC) at a logarithmic growth phase were incubated with 25 μg/ml of OVA peptide (Quality Controlled Biochemicals, custom synthesised, peptide sequence: ISQAVHAAHAEINEAGR) for 4 h at 37°C in complete RPMI medium. After incubation, excess OVA was removed by washing with PBS, and OVA-pulsed B cells and Laurdan-labelled CD4+ T-cells were mixed at a 1:2 ratio. Cell mixtures were seeded onto poly-l-lysine-precoated chambered cover glass slides (2 × 106 CD4+ T-cells/chamber; Sigma-Aldrich) in serum-free Leibovitz's medium. After 30 min of incubation at 37°C, Laurdan-labelled plasma membranes were visualised by two-photon microscopy (Zeiss LSM 510 META NLO) with a 40 × objective 1·3 numerical aperture (NA) oil at wavelengths of 400–460 and 470–530 nm. The Coherent Chameleon femtosecond-pulsed Ti:Sapphire laser was set at an excitation wavelength of 770 nm. All images were converted to 8-bit/channel TIFF format and were processed using Adobe Photoshop CS3®. The mean intensity of each colour channel was measured in areas of interest either at the contact regions of the IS by drawing an oval at the T-cell membrane proximal to the B-lymphoma cell or on the same whole CD4+ T-cell by drawing a polygon around the cell as described previously( Reference Kim, Fan and Barhoumi 22 ). The fold increase of generalised polarisation (GP) values, which indicate the liquid-ordered state of the membrane( Reference Kim, Fan and Barhoumi 22 , Reference Gaus, Chklovskaia and Fazekas de St Groth 27 , Reference Gaus, Zech and Harder 28 ), was calculated as GPcontact region/GPwhole cell.
Assessment of the co-localisation of PKCθ with lipid rafts
Isolated CD4+ T-cells were mixed with OVA-pulsed A-20 B-lymphoma cells (2:1) and seeded onto poly-l-lysine-precoated chambered cover glass slides. After a 30 min incubation period, cells were fixed in 4 % formaldehyde for 20 min, rinsed with PBS and incubated with 10 mm-glycine in PBS for 10 min at room temperature to quench excess aldehyde groups. Cell membranes were permeabilised by exposure to 0·2 % Triton X-100 in PBS for 5 min at room temperature, followed by washing with PBS. The cells were subsequently covered with blocking solution (1 % bovine serum albumin/0·1 % NaN3 in PBS) and incubated at 4°C overnight with a primary rabbit antibody specific to PKCθ (Santa Cruz Biotechnology). After washing with PBS, cells were incubated with a secondary Alexa Fluor® 568 goat antibody specific to rabbit IgG (Molecular Probes) and monosialotetrahexosylganglioside (GM1)-specific cholera toxin B subunit conjugated with FITC (Sigma-Aldrich) for lipid raft visualisation. Following serial ethanol dehydration steps, samples were mounted onto glass slides with ProLong Antifade reagent (Molecular Probes). Fluorescence images were acquired by confocal microscopy to determine the co-localisation of PKCθ with GM1 using a Bio-Rad Radiance 2000MP multiphoton system (63 × objective 1·4 NA water). Cell images were captured in 8-bit TIFF format, and the co-localisation of PKCθ with GM1 at the IS was calculated by Pearson's correlation using Image J software (NIH).
Statistics
All data were tested for normality followed by one-way ANOVA with Tukey's post hoc test using GraphPad Prism version 5.00 for Windows (GraphPad Software, Inc.). Differences in data at P< 0·05 were considered statistically significant.
Results
Incorporation of dietary n-3 PUFA into CD4+ T-cells
The fatty acid composition of the diets, expressed in mol% of individual fatty acids, was assessed by GC( Reference Chapkin and Carmichael 26 ) and is presented in Table 1. As expected, n-3 PUFA, i.e. α-linoleic acid (ALA, 18 : 3n-3), EPA (20 : 5n-3) and docosapentaenoic acid (22 : 5n-3), were enriched in the FO diet, at the expense of oleic acid (18 : 1n-9) and linoleic acid (18 : 2n-6) when compared with the MO control diet. DHA (22 : 6n-3) was present in both the FO (5·55 (sem 0·50) mol%) and DHA (4·09 (sem 0·36) mol%) diets, and this difference was statistically significantly (P< 0·05). Consequently, the total n-3 PUFA content was highest in the FO diet (18·03 (sem 1·11) mol%) followed by the DHA (4·63 (sem 0·41) mol%) and MO (0·67 (sem 0·05)) diets (P< 0·05 between the groups). During the 2-week dietary intervention, body weights were recorded at days 0, 7 and 14 and did not differ significantly between the groups (data not shown). In order to determine the enrichment of dietary fatty acids in CD4+ T-cells, total fatty acid profiles in purified splenic CD4+ T-cells were analysed (Table 2). CD4+ T-cells from FO-fed mice exhibited comparable ALA (FO 5·25 (sem 1·99) v. MO 5·05 (sem 2·43) mol%) and arachidonic acid (AA, 20 : 4n-6) (FO 4·51 (sem 0·28) v. MO 5·63 (sem 1·32) mol%) when compared with the MO control group, while EPA (0·86 (sem 0·11) mol%), docosapentaenoic acid (0·47 (sem 0·03) mol%) and DHA (1·66 (sem 0·53)%) were detected in CD4+ T-cells from the FO group but not in the cells from the MO control group. In contrast, the cells from mice fed the DHA diet contained trace amounts of ALA (0·80 (sem 0·65) mol%) and AA (1·98 (sem 1·62) mol%) when compared with the MO (ALA 5·05 (sem 2·43) and AA 5·63 (sem 1·32) mol%) and FO (ALA 5·25 (sem 1·99) and AA 4·51 (sem 0·28) mol%) dietary groups, while DHA (5·54 (sem 2·01) mol%) was highly enriched (P< 0·05). Total amounts of n-3 PUFA in CD4+ T-cells were not significantly different between the dietary groups, but the cells from mice fed the DHA diet exhibited significantly reduced (P< 0·05) levels of n-6 PUFA when compared with the MO group (6·76 (sem 2·48) v. 13·59 (sem 0·76) mol%, respectively). Consequently, the membrane ratio of n-3:n-6 was enhanced most by DHA feeding (0·94 (sem 0·16)), followed by FO (0·67 (sem 0·11)) and MO control (0·37 (sem 0·20)) feeding (P< 0·05).
Table 1 Fatty acid composition of the diets (Mean values with their standard errors, n 4 mice)

MO, maize oil diet; FO, fish oil diet; ND, not detectable.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Table 2 Fatty acid analysis of CD4+ T-cells following the dietary intervention (Mean values with their standard errors, n 4 mice)
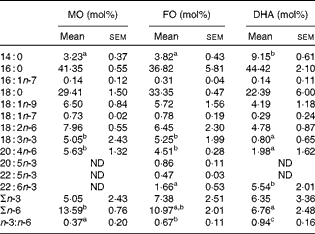
MO, maize oil diet; FO, fish oil diet; ND, not detectable.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Increase in membrane liquid order at the immunological synapse following fish oil and DHA feeding
The formation of liquid-ordered mesodomains at the IS in CD4+ T-cells was assessed by Laurdan labelling as described previously( Reference Kim, Fan and Barhoumi 22 , Reference Gaus, Zech and Harder 28 ). Following antigen-specific IS formation and T-cell activation, DO11.10 T-cell receptor transgenic CD4+ T-cells from the MO control diet-fed mice exhibited an insignificant increase in membrane liquid order at the IS as determined by GP values (whole cell 0·18 (sem 0·01) v. IS 0·20 (sem 0·01), P>0·05) (Fig. 1(A)). In contrast, GP values at the IS when compared with those of the whole T-cell for FO-fed (whole cell 0·16 (sem 0·01) v. IS 0·22 (sem 0·01), P< 0·05) and DHA-fed groups (whole cell 0·17 (sem 0·01) v. IS 0·20 (sem 0·01)) were increased. In order to investigate whether GP values at the IS relative to the whole cell were significantly different among the dietary groups, we further calculated the fold increase by normalising GPimmunological synapse to GPwhole cell. Using this relative index, the FO-fed mice exhibited a significant increase (P< 0·05) in liquid-ordered mesodomains at the IS as expressed by the fold increase of GP values at the IS relative to the whole cell (FO 1·40 (sem 0·11) v. MO 1·10 (sem 0·06); Fig. 1(B)). These data indicate that membrane enrichment with n-3 PUFA following FO or DHA feeding accelerated the formation of liquid-ordered mesodomains during antigen-specific T-cell activation.
Suppressed co-localisation of PKCθ with GM1 by the fish oil- and DHA-containing diets
In order to determine whether enhanced formation of liquid-ordered mesodomains further alters CD4+ T-cell function, i.e. cellular signalling, the conjugates of CD4+ T-cells and antigen-presenting cells (OVA-pulsed B-lymphoma cells) were fixed on a glass slide followed by immunostaining for PKCθ (an essential T-cell signalling protein) and cholera toxin B subunit that binds to ganglioside GM1 (a lipid raft marker) (Fig. 2(A)). Subcellular immunofluorescence was captured by confocal microscopy and the co-localisation of the markers was determined at the IS by drawing an oval around the contact site between the T-cells and antigen-presenting cells. As determined by Pearson's coefficient, CD4+ T-cells from mice fed either the FO (0·37 (sem 0·04)) or DHA (0·38 (sem 0·04)) diet exhibited suppressed (P< 0·05) co-localisation of PKCθ with lipid rafts at the IS when compared with the MO control diet (0·48 (sem 0·04)) (Fig. 2(B)).

Fig. 2 (A) Representative immunofluorescence images for the analysis of the co-localisation of PKCθ with monosialotetrahexosylganglioside (GM1). Purified CD4+ T-cells and ovalbumin-pulsed B-lymphoma cells were co-incubated, fixed, permeabilised and labelled with a PKCθ-specific antibody and Alexa Fluor® 568 conjugated with a secondary antibody and GM1-specific fluorescein isothiocyanate–cholera toxin B subunit (CTX). The co-localisation of fluorescence signals was assessed at the immunological synapse. (B) The co-localisation of PKCθ at the immunological synapse, expressed as Pearson's coefficient (twelve to fifteen cells per mouse from four mice per diet were examined to obtain a total of forty-eight to fifty-seven observations). * Mean value was significantly different from that of the MO control diet (P< 0·05). MO, maize oil diet; FO, fish oil diet.
Discussion
The anti-inflammatory effects of n-3 PUFA on CD4+ T-cells have been studied extensively by us and others( Reference Zhang, Kim and Zhou 7 , Reference Fan, Kim and Callaway 21 , Reference Kim, Fan and Barhoumi 22 , Reference Fan, McMurray and Ly 24 , Reference Fowler, Chapkin and McMurray 29 – Reference Chapkin, Arrington and Apanasovich 32 , Reference Ly, Smith and Chapkin 33 , Reference Yog, Barhoumi and McMurray 34 – Reference Shaikh, Jolly and Chapkin 37 ). The manipulation of lipid rafts, liquid-ordered mesodomains of the plasma membrane, by dietary fatty acids has emerged as one of the molecular targets of n-3 PUFA( Reference Kim, Khan and McMurray 13 , Reference Chapkin, Wang and Fan 38 , Reference Shaikh 39 – Reference Williams, Batten and Harris 41 ). We have demonstrated previously that endogenous n-3 PUFA produced by fat-1 transgenic mice accumulated in the plasma membrane and up-regulated the formation of lipid rafts in CD4+ T-cells at the IS( Reference Kim, Fan and Barhoumi 22 ). Subsequently, the function of CD4+ T cells, as assessed by the localisation of signalling proteins and cell proliferation, was inhibited by n-3 PUFA( Reference Kim, Fan and Barhoumi 22 ). Interestingly, Rockett et al. ( Reference Rockett, Teague and Harris 42 ) recently demonstrated that dietary fish oil increased lipid raft size in B cells, indicating that n-3 PUFA-induced formation of lipid rafts is not a cell type-specific phenomenon.
In the present study, we investigated the effects of dietary n-3 PUFA on the formation of liquid-ordered mesodomains, which are thought to be the building blocks of lipid rafts, using a biologically relevant dose of fish oil. In addition, purified DHA, a major component of fish oil thought to mediate anti-inflammatory properties, was tested alone at levels approximating fish oil supplementation. It should be noted that the other highly bioactive n-3 PUFA, i.e. EPA, is also present in the FO, but not the DHA, diet. Following a 2-week dietary intervention, T-cell membrane fatty acids were significantly enriched with n-3 PUFA. By comparing fatty acid profiles of the experimental diets (Table 1) and purified CD4+ T-cells (Table 2), it was noted that the profound difference in the amount of linoleic acid in the diets did not affect the small pool of linoleic acid in CD4+ T-cells. However, the membrane content of AA, a metabolite of linoleic acid and a precursor to pro-inflammatory eicosanoids, was decreased in the DHA group, indicative of an alternative pathway by which DHA controls inflammatory responses. As expected, n-3 PUFA such as ALA, EPA, docosapentaenoic acid and DHA were enriched in CD4+ T-cells following the consumption of the FO diet. In contrast, DHA deposition following DHASCO® (DHA only) feeding was greater than that in the CD4+ T-cells of mice fed the FO diet. Consequently, the total amounts of n-3 PUFA in CD4+ T-cells from mice fed the FO and DHA diets were not significantly different, even though EPA was enriched only in the cells from the FO dietary group. Since the different dietary sources of n-3 PUFA resulted in unique cellular n-3:n-6 ratios, we further tested the effect of n-3 PUFA on CD4+ T-cell function.
We have reported previously that CD4+ T-cells from fat-1 transgenic mice, which were enriched endogenously with n-3 PUFA, exhibited enhanced lipid raft formation at the IS( Reference Kim, Fan and Barhoumi 22 ). We now report that the same effect was obtained by altering the lipid composition of the diet to include either FO or DHA (Fig. 1(A)), thus demonstrating both the robustness of the n-3 PUFA effect and its relevance for assessing the impact of dietary supplementation with n-3 PUFA. With regard to the magnitude of the diet-induced changes in GP values, Kaiser et al. (43) have reported that the difference in GP values between liquid-ordered large unilamellar vesicles and a mixed large unilamellar vesicles was approximately 0·1 (comparing liquid-ordered and liquid-disordered membrane states). In addition, incubation of Jurkat cells with a 2:1 ratio of cholesterol:7-ketocholesterol resulted in a change in the GP value of approximately 0·12, with a corresponding 40 % decrease in IL-2 secretion when compared with control cells( Reference Rentero, Zech and Quinn 44 ). These data suggest that small changes in GP values are biologically relevant, i.e. associated with perturbations in cell function.
A further investigation of the effects of the diets on GP values at the IS revealed that DHA affected the liquid order at the IS differently with respect to the combination of EPA and DHA provided in the FO diet (Fig. 1(B)). Specifically, the fold increase of the GP values at the IS relative to the whole cell was significantly different in FO-fed mice, but not in DHA-fed group, when compared with the MO control. This difference between the FO and DHA diets may be attributed to the higher levels of n-3 PUFA in the FO diet when compared with the DHA diet (Table 1). In this regard, Williams et al. ( Reference Williams, Batten and Harris 41 ) demonstrated that both DHA and EPA can be incorporated into lipid rafts, though the avidity of DHA was as twice as that of EPA.
The elevated GP ratio at the IS was previously reported to be linked to the suppression of T-cell signalling( Reference Kim, Fan and Barhoumi 22 ). In the present study, we demonstrated that the co-localisation of PKCθ with GM1 at the IS (Fig. 2(A)) was suppressed comparably by the FO and DHA diets (Fig. 2(B)), indicating an effect of dietary n-3 PUFA on the formation of liquid-ordered mesodomains and subsequent localisation of signalling proteins. Indeed, Hou et al. ( Reference Hou, Monk and Fan 35 ) recently reported that DHA inhibited actin remodelling in T-cells, resulting in a suppression of signalling cascades.
In conclusion, we demonstrated that n-3 PUFA from distinct dietary sources (FO v. DHA) can be integrated into antigen-activated CD4+ T-cells, resulting in the modulation of plasma membrane liquid order and PKCθ translocation to the IS. The previously unappreciated effect of EPA, which was present only in the FO diet, will be pursued in future studies.
Acknowledgements
The present study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2013R1A1A1006288) to W. K., and by NIH grant CA129444 and the US Department of Agriculture CSREES Special Grant, ‘Designing Foods for Health’ 2010–34402-20875 to R. S. C. The NRF, NIH and USDA had no role in the design, analysis or writing of the manuscript.
The authors' contributions are as follows: W. K., D. N. M. and R. S. C. contributed to the study design, data analysis and interpretation, and manuscript preparation and revision; W. K. and R. B. contributed to the data acquisition.
None of the authors has any conflicts of interest.






