Case report
A 2-week-old newborn was referred for cardiac evaluation because she had a systolic murmur since the first day of life. The first echocardiography suspected an abnormal pulmonary venous return in coronary sinus. Her parents reported no family history of CHD. On physical examination, she was eupneic with normal oxygen saturation. The electrocardiogram showed a sinus rhythm, with normal P waves’ morphology. Echocardiogram revealed a bulky outpouching of the left ventricle (LV) located behind the mural leaflet of the mitral valve. Colour Doppler showed multiple small orifices between the LV and the outpouching with a maximal Doppler velocity of the jet of 3 m/seconds. This aneurysmal structure went along the posterior wall of the left ventricle and communicated through a tiny orifice with the right atrium via the coronary sinus (see Fig 1a and b). Doppler interrogation of this unusual flow showed a continuous flow at a velocity of 2 m/seconds. The systolic pulmonary arterial pressure was moderately elevated at 40 mmHg. Coronary sinus was mildly dilated and pulmonary veins were normally connected to the left atrium. A large ostium secundum atrial septal defect was also observed. This combination of the shunt through the submitral aneurysm and the right heart via the coronary sinus and the interatrial shunt resulted in a moderate diastolic overload of the right ventricle. At that time, the patient was not eligible for invasive procedure or surgery considering her low weight of 2.5 kg. Follow-up until the left-to-right shunt would be significant was decided, but patient who lived oversea was lost to follow-up.
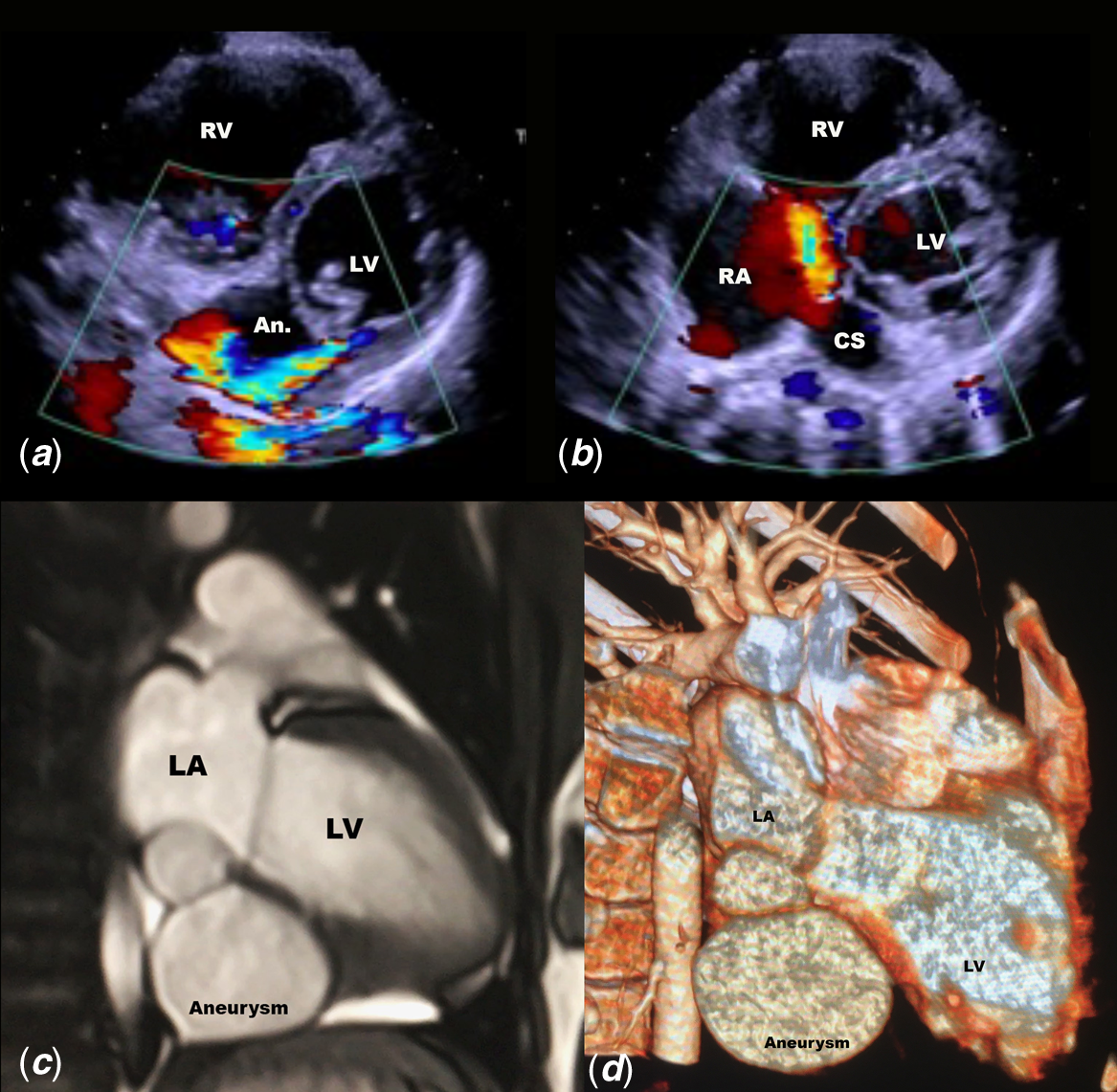
Figure 1. (a ) High velocity shunt between the LV and the submitral aneurysm behind the posterior leaflet of the mitral valve. ( b ) Dilated coronary sinus with large orifice in the right atrium and colour Doppler flow from the submitral aneurysm. ( c ) 2D-CT showing the biloculated submitral aneurysm below the left ventricle and the left atrium (see also supplementary files). ( d ) 3D-CT showing the location of the submitral aneurysm.
Ten years later, the patient had developed normally and remained asymptomatic. Echocardiogram showed a mildly enlarged LV with a preserved systolic function. The submitral aneurysm was still present with a restrictive shunt seen in the right atrium via the dilated coronary sinus. The ostium secundum atrial septal defect measured 19 mm, with adequate rims for percutaneous closure. Right atrium and right ventricle were dilated. Pulmonary artery systolic pressure was estimated at 40 mmHg.
CT scan is shown in Figure 1. The submitral aneurysm measured 5 cm, and the defect between the LV and the outpouching was a narrow 7-mm channel. The wall of the aneurysm was thin and not muscular. In order to further assess the relationship with adjacent structures, right and left heart catheterisations were performed. Angiogram of the coronary arteries was normal and showed no fistula between the submitral aneurysm and coronary arteries. The left ventriculography evidenced the shunt between the left ventricle and the biloculated cavity (Fig 2). The communication between the submitral aneurysm and the coronary sinus was narrow.

Figure 2. (a ) Angiogram in the left ventricle showing the multiple necks between the left ventricle and the submitral aneurysm. ( b ) Direct angiography into the submitral aneurysm with a microcatheter showing the biloculated shape. ( c ) Angiography in front of the neck of the submitral aneurysm. ( d ) Closure of the neck of the aneurysm with a device.
The shunt was closed under local anaesthesia and sedation by a 6 × 4-mm Amplatzer Duct Occluder (ADO) device. Ostium secundum was closed during the same procedure by an Amplatz device of 24 mm. No immediate nor late complication occurred after 3 years follow-up.
Discussion
Submitral aneurysms are rare and usually of congenital aetiology. Reference Ohlow, von Korn and Lauer1 They are most likely due to a congenital weakness of the fibrous annulus of the valve, with a disjunction between the LV musculature and the left atrium–mitral valve region due to complex disturbance of embryogenesis. Reference Baruah, Kumar, Reddy and Babu2 They can also be observed as a complication of cardiac surgery, ablation procedures, ischaemic cardiac disease, or endocarditis. Reference Baruah, Kumar, Reddy and Babu2 There is a racial predilection of the disease that is more frequently seen in the African or Indian population. Reference Kumar, Satheesh and Selvaraj3 The role of inflammation in the progression of the defect has been proposed. The most common form is the pseudoaneurysm of the mitral-aortic fibrosa. Reference Zhao, Chen and Yang4 Autopsy observations had revealed that extension of the membranous septum tissues beyond the nadir of the non-coronary cusp to the posterior medial commissure can separate the left ventricular musculature from the mitral valve annulus and fragilise this area. Reference Nayak and Victor5 The location of the aneurysm behind the mural leaflet of the mitral valve is extremely rare. The neonatal diagnosis in our patient is in favour of a congenital anomaly that may be a developmental disease or an anomaly acquired during fetal life as for other left ventricular aneurysms. Reference Malakan Rad, Awad and Hijazi6 Communication between submitral aneurysm and left atrium has been described but remains exceptional. Reference McGarry, Stark and Macartney7 We found only three cases of congenital left ventricle to coronary sinus fistula in the literature. Reference Gnanapragasam, Houston and Lilley8–Reference Shetty, Lachma, Manohar and Rao10 Two of these “fistulae” were isolated and one was associated with a transposition of the great arteries with ventricular septal defect and subvalvar pulmonary stenosis. There were not associated with large submitral aneurysms.
Surgery is the commonly performed treatment with patch closure of the multiple necks within the aneurysm. Percutaneous closure has been scarcely reported.
Conclusion
Outpouchings of the left ventricle can be very difficult to classify due to the lack of clear criteria and consensual classification in literature. This lack is due to its rare occurrence and the variety of presentations. Clinical course is often asymptomatic, but complication can be severe. Clinicians should carefully examine risks and benefices of percutaneous or surgical treatment of this rare condition.
Acknowledgements
None.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S104795112100442X
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.





