Introduction
Open habitats such as semi-deserts and grasslands have been extensively modified throughout the world and currently much of their former natural cover has been lost to crop and cattle fields, or urban and industrial developments, with low and relatively flat areas being most affected due to their greater accessibility (Onrubia and Andrés Reference Onrubia, Andrés, Bota, Morales, Mañosa and Camprodon2005). Tropical and temperate islands are currently at a particularly high risk of this form of land transformation due to the high economic pressure to devote land to recreational facilities (hotels, golf courses) that compete for local and frequently scarce resources, most notably water in semi-desert areas. Consequently, island birds from open-country habitats frequently appear in the lists of endangered species (Groombridge Reference Groombridge1992; BirdLife International 2000).
The Black-bellied Sandgrouse Pterocles orientalis is a Palaearctic species that reaches the western border of its distribution range in the Canary Islands archipelago. Currently, the only breeding populations occur in Fuerteventura, although there are a few records of the species in the nearby and similar island of Lanzarote (Emmerson Reference Emmerson, Herranz and Suárez1999; Martín and Lorenzo Reference Martín and Lorenzo2001; Emmerson and Lorenzo Reference Emmerson, Lorenzo and Lorenzo2007). It occupies open, arid semi-desert and steppe habitats where it forages for seeds on the ground in variable-sized flocks, which can be most easily detected when flying to pools and creeks where they gather for watering (De Juana Reference De Juana, Del Hoyo, Elliot and Sargatal1997). Western Palaearctic populations (P. o. orientalis) have been estimated at 62,000 pairs, mainly distributed in the strongholds of Turkey (25,000–50,000 pairs; BirdLife International 2004) and Spain (7,824–13,273 pairs; Suárez et al. Reference Suárez, Hervás, Herranz and Del Moral2006). It is classified as ‘Vulnerable’ in the Red Book of Spanish Birds because of a decline in extent of occurrence and negative population trends (Suárez and Herranz Reference Suárez, Herranz, Madroño, González and Atienza2004). In the Canary Islands, however, it has never been adequately researched, and there is an urgent need for an accurate census of its population and knowledge of its habitat preferences to support planning and assessment, given the increasing pressure for land-use transformation in the archipelago (Fernández-Palacios and Martín Esquivel Reference Fernández-Palacios and Martín Esquivel2001).
In this study, we estimate the population size and identify the more important areas for Black-bellied Sandgrouse in the Canary Islands. We also model the habitat preferences of the species in Fuerteventura and apply the results to the nearby island of Lanzarote in order to identify potential breeding areas and discuss how many birds they could harbour and why they are currently unoccupied.
Methods
Study area
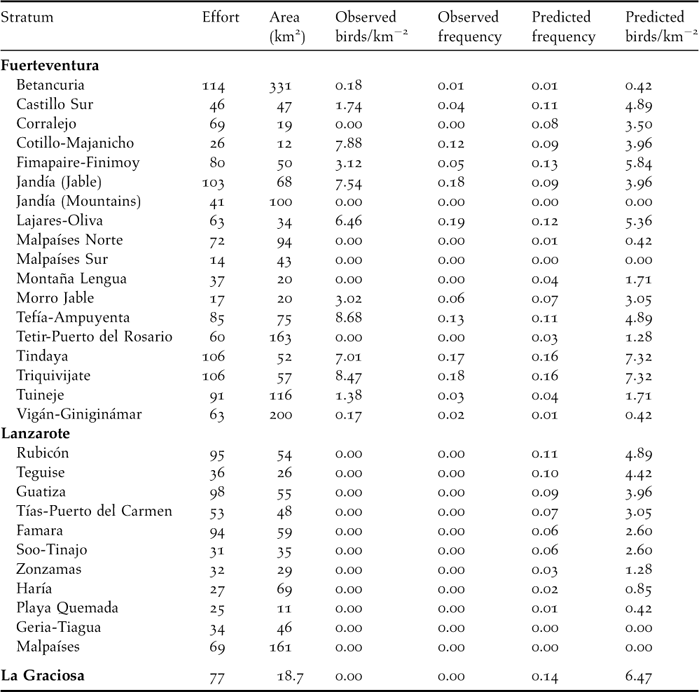
Fuerteventura is an eastern island of the Canary archipelago (area 1,730 km2; 28°27'N, 14°00'W), lying only 100 km from the North African coast. It shows a smooth relief (highest altitude 807 m) in accordance with its ancient geological origins (20–22 million years) and subsequent erosion, since the volcanic activity of the island is almost extinct. The combined effects of direct Saharan influence on climate and a prevailing flat topography result in a dominance of scarcely vegetated arid landscapes, which have been extensively grazed (mainly by goats) and cultivated. The impoverished plant communities mostly consist of a few species of xerophytic shrubs (Launaea arborescens, Lycium intricatum, Salsola vermiculata, Suaeda spp. and Euphorbia spp.), therophytic forbs and several perennial grass species. The only natural woodlands are small and patchily located tamarisk Tamarix canariensis and palm Phoenix canariensis groves. The degree of development of vegetated areas is relatively diverse due to local conditions, such as humidity, slope of terrain, soil characteristics, goat grazing, and human uses. With regard to soil lithology and compactness, the study areas also comprise a broad range of conditions, from stony lava fields to loose sand dunes (Rodríguez et al. Reference Rodríguez, García and Reyes2000; Fernández-Palacios and Martín Esquivel Reference Fernández-Palacios and Martín Esquivel2001). Just 12 km to the north, the island of Lanzarote shares the volcanic origin, climatic characteristics and many of its general habitat attributes with Fuerteventura, but is smaller (846 km2), lower (maximum altitude 846 m), younger (16–19 million years) and has a higher proportion of its surface covered by lava fields. The volcanic island of La Graciosa, 2 km north of Lanzarote, completes our study area with a smaller area (18.7 km2) mainly occupied by sandy areas and small hills (maximum altitude 266 m) covered by bushes (see Fernández-Palacios and Martín-Esquivel 2001 for more details).
Bird and habitat data
Breeding bird surveys in the islands were carried out during the periods 12–26/02/2005 (Lanzarote), 22–23/02/2005 (La Graciosa) and 05/03/2005–09/04/2005 and 05/03/2006–14/03/2006 (Fuerteventura). The phenology of the species is little known, but recent accounts report March to July as the breeding season, so we feel confident the survey covered the early breeding period (Martín and Lorenzo Reference Martín and Lorenzo2001; Emmerson and Lorenzo Reference Emmerson, Lorenzo and Lorenzo2007). Moreover, the average flock size of the species was 2.62 sandgrouse (SD = 1.57), the interquartile range was 2–3 birds, and the percentage of flocks with four or fewer sandgrouse was 90.8% in Fuerteventura during the census period (n = 152 near contacts at less than 100 m from the observer, for which it is highly probable that all birds were detected). These figures are nearly identical to those reported for the species during the breeding season in the Iberian Peninsula (De Borbón et al. Reference De Borbón, Barros, De Juana, Herranz and Suárez1999: 78–85% of flocks with 1–4 birds in June and July, average of three birds per flock during the breeding season).
The survey method used was the line transect, frequently used in extensive assessments of abundance, general distribution patterns and habitat preferences of birds, (Bibby et al. Reference Bibby, Burgess, Hill and Mustoe2000), which we have previously used to assess land bird populations in the Canary Islands (e.g., Carrascal et al. Reference Carrascal, Seoane, Palomino and Alonso2006; Palomino et al. Reference Palomino, Seoane, Carrascal and Alonso2008). Line transects of 0.5 km (geolocated and measured by means of GPS) were performed across the whole island (Figure 1), including all of the main non-urban habitats: barren lava fields, shrubby steppe-like plains, stony/sandy desert areas, traditional cultivations, hilly/mountain slopes, and gullies. Line transects were carried out on windless and rainless days, walking cross-country or on dirt tracks at a low speed (1–3 km h−1 approximately), during the first four hours after dawn and the two and a half hours before dusk.
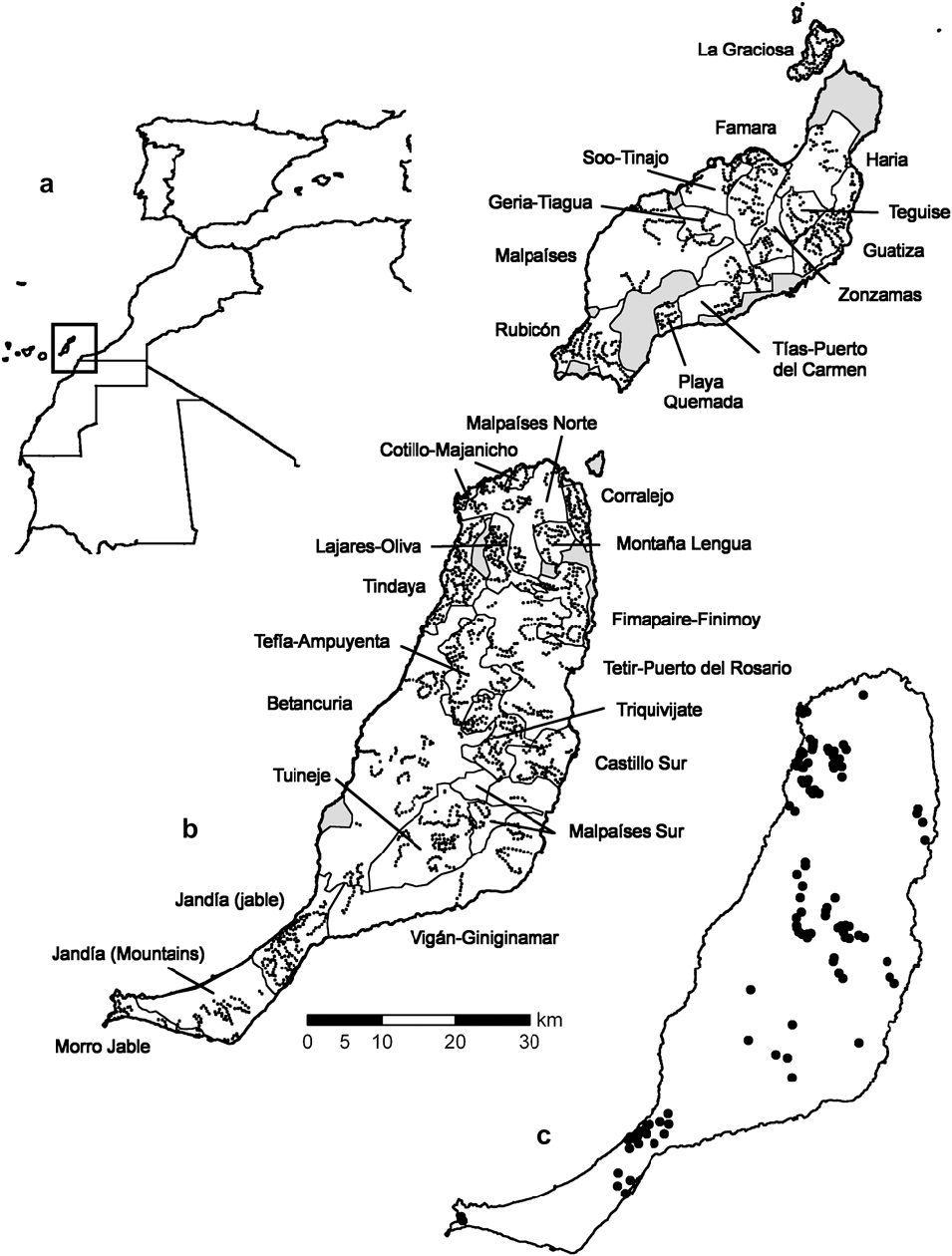
Figure 1. Location of a) the study area, b) the geographical strata (light lines) and the centres of line transects (dots) in Lanzarote, La Graciosa (above) and Fuerteventura (below) c) samples where Black-bellied Sandgrouse were detected. Areas that could not be surveyed are in grey.
Additionally, to assess seasonal changes in the distribution/abundance of the Black-bellied Sandgrouse, a habitat-stratified selection of 602 transects was repeated in the post-breeding period of 2006 (7–15/08/2006). The sampling locations and the approximate number of transects to gather on them, were roughly determined in proportion to the surface in the islands of each type of main landscape types. Apart from the mere availability of a safe place to park, the starting point of each sampling line was randomly determined. Next, the observers walked through the target area trying: a) to perform 0.5 km transects as homogeneous as possible; b) to attain an extensive cover of the surveyed area. The transect lines were not biased by an a priori potential of the habitat to harbour Black-bellied Sandgrouse, because this field work was not exclusively focused in sampling this species, and because most locations were so intensively sampled that there is little room for any geographical bias.
To assess whether the current absence (or scarcity) of a breeding population from the rest of the eastern islands can be explained by differences with regard to particular habitat variables on Fuerteventura, we also performed 594 line transects in Lanzarote and 77 in La Graciosa during 2005 in semidesert areas that we deemed more suitable for the species. For each bird heard or seen, the perpendicular distance to the observer's trajectory was estimated (overflying birds were disregarded) to later obtain estimates of detectability and density (Buckland et al. Reference Buckland, Marsden and Green2008). Previous training with a laser rangefinder helped to reduce inter-observer variability in distance estimates.
We measured habitat characteristics by averaging three visual estimations on 25-m radius circular plots located at 125, 250 and 375 m along the line transect. These variables were: coverage of grass, annual forbs, shrubs, and trees (%); mean height of the shrub layer (cm); rocky cover (%); soil typology (according to the following classes: 0-lava fields, 1-stone/gravel soils, 2-compact soils, 3-sandy soils, and 4-loose sand dunes); and altitude above sea level (measured with GPS units). The amount of any agricultural land-use (%) was estimated in two 250-m width bands on both sides of the transects. Other variables were measured on 1:25,000 maps: the distances from the centre of each transect to the nearest paved road (m) and the nearest city (m); the length of paved roads (m) and dirt tracks (m) within circles of radius 250-m centred on each transect; and the maximum slope terrain in a circle of radius 250-m. Finally, we also used a normalized difference vegetation index (NDVI, range 0–255) as a radiometric index of photosynthetic activity (the larger the value, the more vigorous vegetation). Raw data were ten-day synthesis at 1 km2 spatial resolution obtained from the sensor VEGETATION on board the SPOT satellite (available freely at http://free.vgt.vito.be/). We built monthly maximum composite of NDVI images, averaged from 1999 to 2004 (cloudy pixels were assigned a value of zero in the ten-day images, so they were never selected as the monthly maximum used to build the composite). These values were assigned afterwards to transects.
Transects were grouped in 18 strata in Fuerteventura, 11 in Lanzarote and just 1 in La Graciosa according to habitat characteristics and geographical proximity (Figure 1). Table 1 summarizes the range and mean values for these variables in both islands and Table 2 shows the sampling effort and area covered by each stratum.
Table 1. Environmental characteristics (mean ± standard deviation and range) of the sampled areas in Lanzarote and Fuerteventura (eastern Canary Islands). See Figure 1 for their respective location. Number of 0.5-km transects is 1,184 for Fuerteventura and 594 for Lanzarote. ALTITUDE: mean altitude above sea level (m); SLOPE: average slope of the terrain (%); ROCK COVER: cover of rocks and stones (%); SOIL INDEX: index size of soil grain (0: volcanic soils; 1: stony soils; 2: compact sandy soils; 3: sandy soils; 4: loose dunes); DMIN-URBAN: minimum distance to the nearest city (m); DMIN-ROAD: minimum distance to the nearest paved road (m); L-TRACKS: length of unpaved tracks (m) per 20 ha; L-ROADS: length of paved roads (m) per 20 ha; FORBS COVER: cover of forbs (%); GRASS COVER: cover of grass (%); SHRUB COVER: cover of shrubs (%; mostly chamaephytes and small phanerophytes of genus Suaeda, Salsola, Launaea, Lycium and Euphorbia); H-SHRUB: mean height of shrubs (cm). TREE COVER: cover of trees (%; mainly Tamarix canariensis and Phoenix canariensis). NDVI: normalized difference vegetation index; AGRIC. COVER: cover with agricultural uses (%).
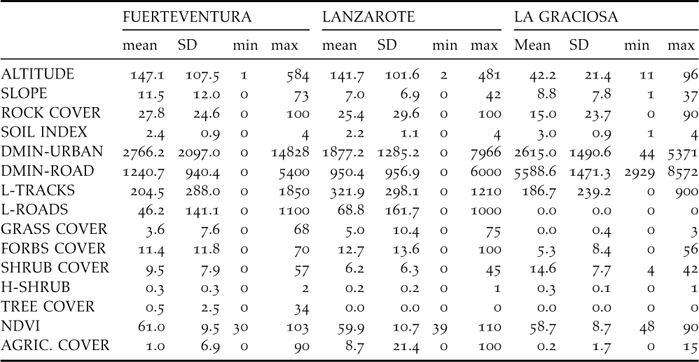
Table 2. Sampling effort per stratum (Effort: in number of 0.5-km transects) and summary of sampling results. For each stratum, it is given the area (km2, excluding urban areas from strata in Figure 1) along with the estimated density of individuals (number of birds km−2), the observed frequency (proportion of transects in which the species was detected) and the predicted frequency according to the classification tree model. Finally, the predicted density is given, according to the linear relationship between the density and the natural logarithm of one minus the frequency (that is, the observed frequency in Fuerteventura and the predicted frequency for Lanzarote and La Graciosa: r = 0.943, n = 18 strata in Fuerteventura, birds km−2 = −41.96·ln[1-frequency]).
Population size estimates
To estimate population size, we first obtained an estimate of the density in each stratum. In order to do this, we used distance sampling methods to build a model for the detectability of the species, and then consider the actual counts adjusted for this previous model (Thomas et al. Reference Thomas, Buckland, Burnham, Anderson, Laake, Borchers, Strindberg, El-Shaarawi and Piegorsch2002). For calculating the detection model, the detection distances (i.e., the perpendicular distances from the transect line at which birds were detected) were right-truncated, thus excluding outliers as recommended by Buckland et al. (Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). Then six models were fitted, all of them commonly used to explain the loss of detectability as a function of the distance from the transect line (the further the distance, the lower the probability of detecting a given individual), and the respective probabilities of detection within strips of width equal to the truncated distance were estimated. We calculated a global detection probability function, applied to every stratum. Models were evaluated according to AICc and given weights as: Wi = exp(−0.5ΔAICc)/Σ exp(−0.5ΔAICc) (Burnham and Anderson Reference Burnham and Anderson2002). Detectability models were built with Distance 5.0 software (Thomas et al. Reference Thomas, Laake, Strindberg, Marques, Buckland, Borchers, Anderson, Burnham, Hedley, Pollard and Bishop2004).
We estimated confidence intervals for the abundance by applying a randomization procedure. First, we generated 2,000 random values of probability of detection which lay within the confidence intervals given by Distance. Then we generated the same number of random bootstraps of the transects within each stratum to estimate the average number of sandgrouse recorded per 500 m transect (Davison and Hinkley Reference Davison and Hinkley1997). The density in each trial was calculated according to the probability of detection randomly assigned to that trial (and considering the truncation distance). Finally, we took the 90% confidence intervals for the abundance within each stratum using the bias-corrected and accelerated version of the bootstrap (DiCiccio and Efron Reference DiCiccio and Efron1996). To obtain the abundance estimate for the whole island, the whole sample of all transects was bootstrapped proportionally to the area of each stratum (weighting each transect in each stratum according to the balance between the proportion of area covered by that stratum and the proportion of transects made on it). This randomization procedure was carried out in Microsoft Excel using PopTools 3.0 (http://www.cse.csiro.au/poptools/).
The population size of the species in each stratum was calculated by multiplying the estimated densities (mean, lower and upper 90% confidence intervals) by its area. When the lower end of the confidence interval was lower than the actual number of individuals detected, we substituted it by this last amount.
Habitat-relationships models
Species occurrence (absence = 0; presence = 1) in the sample of 0.5 km line-transects in Fuertenventura was modelled with the 15 original descriptors as explanatory variables, and analysed using classification trees with Statistica 6.0 (StatSoft Reference StatSoft2001). This is a statistical tool where the response variable undergoes successive univariate splits, according to threshold values of the explanatory variables that maximize the differences between the two resulting groups of samples. Classification trees deal with nonlinear relationships between response and explanatory variables, and with interactions among the latter, and thus are suitable for modelling complex ecological scenarios (Venables and Ripley Reference Venables and Ripley1999; De'Ath and Fabricius Reference De'Ath and Fabricius2000). We applied misclassification costs according to the detectability of the species to account for false negatives (the recorded absence in 500 m transects not being always true). In order to do that, we defined misclassification costs as 100 for presence of the species and 55 for absence (i.e., proportional to the detectability). To classify samples in the absence or presence groups we used a threshold equal to the prevalence of the species in the whole sample of transects (7.9%). The predictive power of the obtained classification tree was evaluated by means of a cross-validation procedure using 20 four-fold random sampling iterations.
To test the adequacy of Lanzarote and La Graciosa to the Black-bellied Sandgrouse, the classification tree model built for Fuerteventura was applied to the line transects made in these islands. Likewise, the correlation between the predicted probabilities of occurrence (from the tree model) and the estimated abundance (from distance sampling) in Fuerteventura was applied to strata in Lanzarote and La Graciosa to predict the abundance that the sandgrouse could potentially reach.
We also tested for seasonal differences in habitat preferences. In order to do so, we first chose the non-urban transects that were sampled in both March and July and selected those in which we detected any Black-bellied Sandgrouse. Then we performed a multivariate analysis of variance on this reduced set of data, including the season (March, July) as a factor and the same environmental predictors used in the classification tree as response variables (except percent cover of agricultural land, and cover and height of trees, which were zero for all these transects).
Results
Detectability models and abundance estimates
We did a total of 1,864 line transects during the study period in the three islands in which we registered 156 contacts with 436 individuals (only on Fuerteventura). After visual inspection of the data, the perpendicular distance was truncated at 130 m, thus excluding nine contacts with a total of 29 birds.
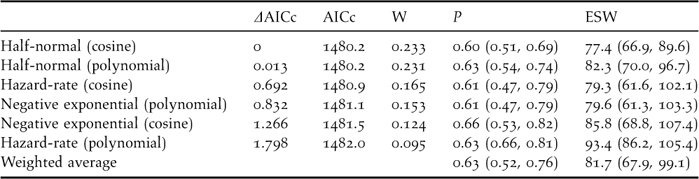
The best detectability model was the half-normal key function (Wi = 0.23), although the others were fairly sensible alternatives (ΔAICc lower than 1.80, see Table 3). In addition, all of them provided reasonable fits according to the Cramer-von Mises goodness-of-fit test and the diagnostic plots. Confidence intervals for both the probability of detection and the effective strip width were wide, ranging from 30% to 52% of the mean value. The weighted average probability of detection within 130 m was 0.63, resulting in an average effective strip width (ESW) of 81.7 m at each side of the transect line (95% CI, 67.9–99.1).
Table 3. Models fitted to the detection distances truncated at 130 m (n = 156 contacts with 436 individuals), ordered increasingly according to their Akaike's Information Criterion corrected for small sizes (AICc) values (i.e., from larger to smaller reliability). W is the weight given to each model according to the formula Wi = exp(−0.5ΔAICc)/Σ exp(−0.5ΔAICc) (Burnhman and Anderson 2002). It is also given the detection probability within 130 m and its 95% confidence interval (P), and the effective strip width ESW (note that ESW = P*130, allowing for rounding errors). The Cramer-von Mises goodness of fit test, which measures the difference between the empirical distribution function and the probability distribution function in a quantile-quantile plot, was non-significant in every model (Buckland et al. Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2004).
The areas with the highest densities recorded were Cotillo-Majanicho, Jandía jable, Tefía-Ampuyenta and Triquivijate, with >7 sandgrouse km−2 (Table 2). Sandgrouse abundance was highly and significantly correlated with the frequency of occurrence of the species in the 30 sampling sectors of Fuerteventura, Lanzarote and La Graciosa (r = 0.958, P < 0.001; birds km−2 = –41.96 x ln [1 – frequency]; data from Table 2).
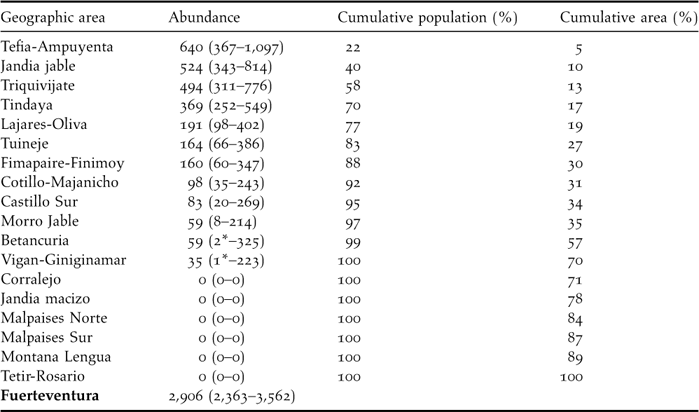
The average number of sandgrouse per sampling stratum ranged from zero individuals in Jandía mountains, Montaña Lengua, Tetir-Rosario and the volcanic bedrocks, to more than 300 individuals in Jandía jable, Tefía-Ampuyenta, Tindaya and Triquivijate (Table 4). These four areas include only 16.7% of surface of Fuerteventura (251 km2) but 70% of the whole sandgrouse population. The estimate of total population size was 2,906 (90% CI: 2,363–3,562) for the whole of Fuerteventura island. No birds were recorded in nearby Lanzarote or La Graciosa.
Table 4. Abundance (with 90% confidence interval) of Black-bellied Sandgrouse in the geographic areas considered. Lower-level confidence intervals marked with asterisks were estimated to be zero but are substituted here for the actual number of birds detected. No birds were registered in either Lanzarote or la Graciosa and thus we estimate that there is no current breeding population in these islands.
Habitat relationships in the breeding season
The classification tree was highly significant (![]() = 122.5, P ≪ 0.001, Figure 2), with a correct prediction of presence-absence of the species in 68.6% of all transects, and 87.1% of transects where the species was present. The 20 cross-validations showed relatively high correct classifications of the whole sample (61.8%; SD = 2.2%) or the sub-sample of the 93 transects where the species was present (70.9%; SD = 3.6%), that were significantly different from the null hypothesis (50%; P < 0.001 in both t-tests).
= 122.5, P ≪ 0.001, Figure 2), with a correct prediction of presence-absence of the species in 68.6% of all transects, and 87.1% of transects where the species was present. The 20 cross-validations showed relatively high correct classifications of the whole sample (61.8%; SD = 2.2%) or the sub-sample of the 93 transects where the species was present (70.9%; SD = 3.6%), that were significantly different from the null hypothesis (50%; P < 0.001 in both t-tests).
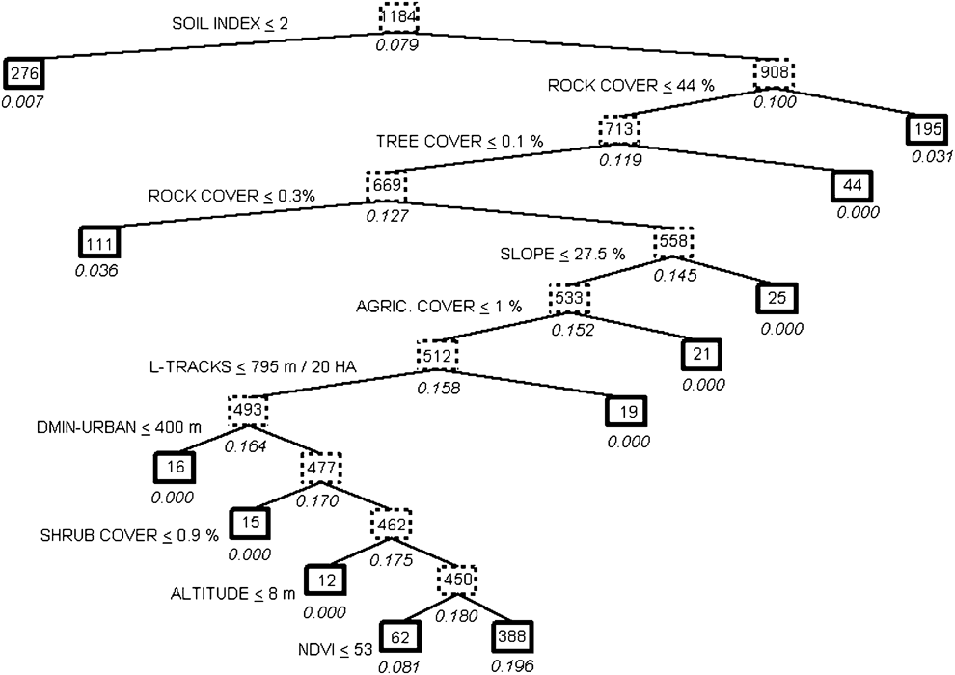
Figure 2. Classification tree describing the pattern of habitat preferences of the Black-bellied Sandgrouse in Fuerteventura (Eastern Canary Islands). The probability of presence of the species is expressed below each box as a percentage. Bold-lined boxes indicate final environmental conditions. The number of transects meeting the previous set of conditions is shown inside each box. The splitting variables and threshold values selected refer to left branches of the tree, so that right branches met opposite conditions. See Table 1 for the acronyms of the variables.
The most important variables in habitat preferences of Black-bellied Sandgrouse were those related to lithology. The probability of occurrence increased significantly (P < 0.05 in all the splits below mentioned) in locations with a soil typology higher than 2 (compact soils) and a rock cover ranging between 0.3% and 44%. Sandgrouse avoided lava fields and stone/gravel soils with a high rock cover, although they also avoided loose sand dunes or plains without rocks or stones present. Relief characteristics also played a prominent role in the habitat preferences of the species, as the probability of occurrence was higher in areas of relatively flat terrain: less than 27.5% in maximum slope, and at altitudes above 8 m asl (i.e., avoiding strictly coastal areas). The most important vegetation habitat structure variables were tree and shrub cover: the sandgrouse was only present in completely treeless areas (cover of trees < 0.1%) with shrub cover > 0.9%. Vegetation productivity, measured by the NDVI index, determined a probability of occurrence of the sandgrouse many times higher when the NDVI was greater than 53. Finally, human influence on sandgrouse was relatively important, as the species showed a strict avoidance of cultivated fields and places near urban areas (closer than 400 m), and preferred sectors of Fuerteventura with a low density of dirt roads (> 795 m per 20 ha).
Table 2 shows the predicted frequencies of occurrence of the sandgrouse in transects of 500 m in the Eastern Canary Islands according to the classification tree in Figure 2. The frequencies observed and predicted in the 18 strata of Fuerteventura were significantly correlated (r = 0.791, P < 0.001). Although the species was not detected during our sampling in Lanzarote and La Graciosa in 2005, there are several sectors in these islands where the predicted frequencies of occurrence are relatively high in comparison with the frequencies observed in Fuerteventura. The sectors potentially more adequate to the Black-bellied Sandgrouse are La Graciosa, Rubicón, Teguise and Guatiza, where the predicted frequency of occurrence of the species was higher than the actual occurrence of the sandgrouse in Fuerteventura. According to the equation relating density to frequency of occurrence, sandgrouse could hypothetically reach densities as high as 4–6 birds km−2 in the abovementioned sectors of Lanzarote and La Graciosa.
Seasonal differences in habitat relationships
A total of 602 line non-urban transects were sampled in both March and July. Within those, we detected Black-bellied Sandgrouse in 49 transects in March and 27 in August. Habitat use was significantly different between months (MANOVA, F11,64 = 2.05, P = 0.039), although seasonal differences in habitat preferences explained a low amount of variance (25.9%; Wilk's Lambda = 0.741). The distance to urban settlements was the only variable with significant seasonal differences (a posteriori one-way ANOVA: F1,74 = 7.07, P = 0.010). Sandgrouse were closer to human settlements in summer than in March (Figure 3).

Figure 3. Minimum distance to urban settlements of 0.5 km transects where Black-bellied Sandgrouse was present in spring (n = 49) and summer (n = 27) in Fuerteventura working with the same sample of 602 transects common to both periods. It also shows the average minimum distance to the nearest city from all transect centres (in m).
The number of birds detected did not significantly vary between seasons (Wilcoxon matched pairs test: Z = 0.533, P = 0.594) and the number of birds recorded in the 16 sampled strata were significantly related (Spearman rank order correlation: r s = 0.573, P = 0.020). The only exception to this common pattern of spatial variation in sandgrouse numbers was Lajares-Oliva and Triquivijate, which showed a notable reduction from March to summer.
Discussion
Population size and distribution
The estimated population of the Black-bellied Sandgrouse in the Canary islands is considerably larger than previously reported (Emmerson Reference Emmerson, Herranz and Suárez1999; BirdLife International 2004). Several sources of bias could explain the discrepancies between the 2,906 birds reported in this study and the 700–1,200 sandgrouse estimated previously for the Canaries. First, previous counts did not consider the likelihood of underestimation due to detectability problems. Second, we made a very large sampling effort trying to cover the entire island of Fuerteventura, while previous field work was restricted only to the presumed better areas for the species on this island (350 km2 in Emmerson Reference Emmerson, Herranz and Suárez1999). Third, our survey was carried out at the beginning of the breeding season (March), while previous ones were made in the winter season (December–January) or in the driest part of the summer (July) when the birds may be more mobile and seemingly more difficult to census because they aggregate in large flocks. Thus, we suggest our estimate as the reference guide for future comparisons on the size of the breeding population of Black-bellied Sandgrouse in Fuerteventura.
Four geographic areas include a large part of the population (70%) in a relatively small area of the island (17% of the island area). Of these, three are largely within Special Protection Areas classified under Birds Directive and could benefit from the protection regime established there (these are Jandía jable [63% within SPA], Triquivijate [59%] and Tindaya [74%], that together group half of the island population). Contrastingly, the Tefia-Ampuyenta area, first in absolute number of individuals and in population density, is mostly unprotected (11% within SPA). Therefore, this important area should be considered in future conservation programmes of the regional Canaries Government, especially considering that it is also an important area for other bird species of conservation concern (Cream-coloured Courser Cursorius cursor: Carrascal et al. Reference Carrascal, Seoane, Palomino and Alonso2007; Houbara Bustard Chlamydotis undulata: Carrascal et al. Reference Carrascal, Palomino, Seoane and Alonso2008).
Habitat relationships: biotic vs. abiotic features
The pattern of habitat relationships found for the species in Fuerteventura largely agrees with those reported in continental populations, in particular the fact that its abundance correlates negatively with the extent of agricultural land in active use (Suárez et al. Reference Suárez, Martinez, Herranz and Yanes1997; but see Cardoso et al. Reference Cardoso, Poeiras and Carrapato2007). Abiotic features of the landscape had a much greater relevance for the Black-bellied Sandgrouse in this study, which shows that there is room for habitat selection even in apparently simple habitats in terms of human uses and vegetation structure. For example, the species mainly occurs in flat plains of compact soils with an intermediate abundance of stones, while both bare bedrock and loose sand dunes are avoided. This preference pattern may be advantageous against predation, first because the stones (and short shrubs) could help to conceal clutches (Lloyd et al. Reference Lloyd, Plaganyi, Lepage, Little and Crowe2000; Cardoso et al. Reference Cardoso, Poeiras and Carrapato2007; Znari et al. Reference Znari, Aourir, Radi and Melin2008), and second because flat ground–as opposed to undulating terrain–may help in detecting predators (Ferns and Hinsley Reference Ferns and Hinsley1995). The low dependence of the species on vegetation structure is somewhat unexpected given its seed-feeding habits and the use they make of shrubby vegetation when foraging at high temperatures (Hinsley Reference Hinsley1994; Suárez et al. Reference Suárez, Hervás, Levassor, Casado, Herranz and Suárez1999; Mian Reference Mian2003). This habitat relationship probably show the high capacity of the Black-bellied Sandgrouse to exploit food and the thermal patchiness of habitat under constraining environmental conditions (Hinsley et al. Reference Hinsley, Ferns, Thomas and Pinshow1993; Hinsley Reference Hinsley1994).
Overall, the habitat relationships of the sandgrouse do not substantially change between seasons (nor does the abundance pattern), which is in agreement with the studies in continental populations (Martínez et al. Reference Martínez, Suárez, Yanes and Herranz1998; but see Cardoso et al. Reference Cardoso, Poeiras and Carrapato2007). Nonetheless, we identified a seasonal change linked to anthropogenic disturbance: tolerance to nearby cities and villages is higher during the summer than during the breeding period. The explanation for this seasonal change in habitat use might be the intensive use of artificial water supplies, which are mostly located around villages and attract the flocks during the severe aridity of the summer months (Knight Reference Knight1989).
It is important to stress that the density of roads has a detrimental effect on the sandgrouse, as has been reported for this species in continental studies (Cardoso et al. Reference Cardoso, Poeiras and Carrapato2007) and for a number of other steppe birds in the Western Palaearctic (Little Bustard Tetrax tetrax: Silva et al. Reference Silva, Pinto and Palmeirim2004; Houbara Bustard: Carrascal et al. Reference Carrascal, Seoane, Palomino and Alonso2006; Cream-coloured Courser: Palomino et al. Reference Palomino, Seoane, Carrascal and Alonso2008). This result is particularly worrying if we take into account that infrastructure and urban development have been identified as a new threat for birds in areas that until recently maintained a large proportion of well conserved habitats (such as in Spain: Madroño et al. Reference Madroño, González and Atienza2005). The Canaries are most vulnerable to this form of land transformation due to their small size and current pressure to devote land to recreational facilities.
Why are there no Sandgrouse in Lanzarote?
Black-bellied Sandgrouse Pterocles orientalis orientalis are potent fliers that could seemingly cross the narrow stretch of sea between Fuerteventura and Lanzarote (13 km) or perhaps towards the northwest coast of Africa (100 km), where the same subspecies exists and shows some degree of nomadism (see references and maps in Birdguides Reference Birdguides2004). Lanzarote and Fuerteventura are similar regarding the environmental characteristics that successfully depict the habitat preference of the species, and there is indeed evidence of a former unquantified breeding population in Lanzarote (Martín and Lorenzo Reference Martín and Lorenzo2001). In the same vein, our models predict a medium density of Black-bellied Sandgrouse in several areas of Lanzarote, most notably for those in which some sparse observations have recently been recorded (Emmerson and Lorenzo Reference Emmerson, Lorenzo and Lorenzo2007). Thus, the current absence of a breeding population of Black-bellied Sandgrouse in Lanzarote is intriguing.
Lanzarote has fewer water resources than Fuerteventura, in particular natural or artificial ponds for cattle that sandgrouse use as watering-places (pers. obs. and Polatzek 1909 in Emmerson Reference Emmerson, Herranz and Suárez1999). Thus, the scarcity of watering-places may constrain the potential range within the island, because availability of water has been identified as a main factor responsible for the presence of the species (Cardoso et al. Reference Cardoso, Poeiras and Carrapato2007). Also, the present environmental conditions in the islands derive from the past history of human disturbance by agriculture and grazing, which could partly explain the absence of the species in Lanzarote. For example, in a study of biotic and abiotic factors that limited the range size of the endemic Canary Islands Stonechat (Saxicola dacotiae), Illera et al. (Reference Illera, Diaz and Nogales2006) found that habitat structure and food availability at fine scales differed between the otherwise similar islands of Lanzarote and Fuerteventura, being more favourable in the latter. The authors attribute these differences to the lasting effects of longer and stronger human impacts (via clearance of shrubs and erosion) in Lanzarote (see also Achord et al. Reference Achord, Levin and Zabel2003 for another example on lasting effects of previous human impacts). Similar fine-grained effects may be hindering the settlement of sandgrouse in this island.
Acknowledgements
This paper was funded by projects CGL2005-02642 BOS of the Spanish Ministry of Educación y Ciencia and by a CENTINELA for the monitoring and management of Macaronesian endangered species (Interreg III-B Açores-Canarias-Madeira 2000–2006). We also thank Claire Jasinski for improving the English.









