Dementia is a global public health issue with substantial personal, societal and fiscal consequences. The burden of dementia worldwide is projected to rise, mainly driven by population growth in low/middle-income countries and increased disease awareness(Reference Banerjee1). The search for a cure in the past decades has taught us that the most viable option is to target neurodegenerative processes, like Alzheimer’s disease, at as early a stage as possible. However, identifying asymptomatic or oligosymptomatic individuals has proven quite a challenge. Apart from people with a clear-cut family history of dementia, others tend to consider the earliest signs of cognitive decline as normal ageing or attribute them to anxiety. If – at some point – a dementia specialist is consulted, confirming subtle cognitive decline or an early stage of neurodegeneration(Reference Sperling, Aisen and Beckett2) requires time-consuming neuropsychological testing or expensive/mildly invasive investigations (positron emission tomography/spinal tap). In this context, subjective cognitive decline (SCD), a self-reported persistent decline in cognitive capacity, has emerged as a promising surrogate for preclinical dementia(Reference Jessen, Amariglio and Buckley3). SCD and its potential significance as a marker of future cognitive deterioration has been a subject of debate for long; recent data have confirmed this notion(Reference Slot, Sikkes and Berkhof4), and the development of research diagnostic criteria for SCD(Reference Jessen, Amariglio and van Boxtel5,Reference Rabin, Smart and Amariglio6) has rekindled interest in the topic. Even though the risk of SCD progression to overt cognitive decline has been explored(Reference Slot, Sikkes and Berkhof4,Reference Mitchell, Beaumont and Ferguson7) , very little is known about the actual course of SCD severity over time and the parameters influencing it.
Among modifiable risk factors for cognitive decline, diet stands out due to its potential for application of population-wide, cost-effective preventive strategies. Indeed, the association of dietary habits and dementia has been thoroughly researched. The role of diet in the prevention of cognitive decline has been investigated in observational(Reference Anastasiou, Yannakoulia and Kosmidis8,Reference Scarmeas, Stern and Tang9) and interventional(Reference Moll van Charante, Richard and Eurelings10–Reference Ngandu, Lehtisalo and Solomon12) studies, and the results are generally in favour of the Mediterranean Diet (MeDi) or other similar plant-based diets(Reference Singh, Parsaik and Mielke13–Reference Morris, Tangney and Wang17). There is even limited evidence for the role of dietary factors in the treatment of cognitive decline(Reference Vlachos and Scarmeas18). As it is known that patients with objective cognitive decline are well downstream the neuropathological cascade of dementia and preventive measures can have a larger effect the earlier they are introduced, the question of the effect of the MeDi on SCD (as a marker of preclinical dementia) is both important and exciting. Published research on this topic is surprisingly scarce; we have identified two randomised, placebo-controlled trials studying the effect of nutrient supplementation on the cognitive function of elderly adults with SCD: one failed to show any beneficial effect after 3 years of n-3 PUFA supplementation(Reference Andrieu, Guyonnet and Coley19) and the other showed that supplementation with fish oil and/or blueberry improved cognition after 24 weeks(Reference McNamara, Kalt and Shidler20). This discrepancy is not unexpected; the effect of each micronutrient is of small magnitude and easily gets diluted in the course of neurodegeneration. Only a combination of beneficial elements in the form of a complete diet(Reference Morris21) for a sufficient amount of time, probably accompanied by an overall ‘healthier’ lifestyle, might be envisaged to have a measurable and clinically significant outcome. That is why prospective cohort studies on SCD and nutrition, shedding light on real-world habits, might be more useful, especially when they focus on diet as a whole.
Nevertheless, observational data on the effect of dietary patterns on SCD are also very limited. A French study has revealed an inverse association between adherence to the MIND dietFootnote 1 and subjective memory complaints in individuals ≥70 years old(Reference Adjibade, Assmann and Julia22), while another study on a large American cohort showed that adherence to the MeDi(Reference Bhushan, Fondell and Ascherio23) was associated with lower odds for SCD years later. It is noteworthy that in the latter publication, which is the only one investigating the MeDi and SCD, the authors have used an extensive and detailed data set to model the association between the average historical MeDi adherence score and the average subjective cognitive function score years later and not the effect of diet on SCD evolution. We therefore decided to use our data from the Hellenic Epidemiological Longitudinal Investigation of Aging and Diet study to explore the association of MeDi adherence with the course of SCD over time in a quantitative manner. We are also proposing a novel, five-level SCD score based on eighteen items. Cross-sectional data on SCD prevalence and its determinants in our population have been discussed elsewhere(Reference Vlachos, Cosentino and Kosmidis24).
Experimental methods
Study population, research protocol and covariate assessment
The Hellenic Epidemiological Longitudinal Investigation of Aging and Diet (HELIAD study) is an investigation of the epidemiology of cognitive disorders in the elderly population in Greece, aiming to unravel their interrelation with various demographic, genetic, medical, lifestyle and dietary parameters. The study is conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human participants have been approved by the Institutional Ethics Review Board of the National and Kapodistrian University of Athens and the University of Thessaly. Written informed consent was obtained from all participants. The full cohort consists of 1984 individuals ≥65 years old from urban and suburban sites who have been randomly sampled and invited to participate in the study, so that they can be considered representative of the whole elderly population in Greece. All participants were examined at baseline and then asked to attend a follow-up visit about 3 years after the initial evaluation. The baseline visits occurred between December 2009 and June 2016, while follow-up was conducted between January 2013 and July 2019. Details about study design and methodology have been presented elsewhere(Reference Vlachos, Cosentino and Kosmidis24,Reference Dardiotis, Kosmidis and Yannakoulia25) ; only the pillars of our extensive protocol that are related to the present research will be outlined here.
Neurologists, neuropsychologists and dietitians administered all structured questionnaires in face-to-face interviews. The examination had a total duration of approximately 2–2·5 h. Participants provided full medical, neurological and family history and answered questions regarding medication, tobacco and alcohol use, mental, physical, occupational and social activities, sleep and dietary habits, subjective memory or other cognitive complaints, as well as various aspects of everyday function. Changes in the performance of daily activities and self-care habits were measured using the Blessed Dementia Scale(Reference Blessed, Tomlinson and Roth26). The participants’ ability to use the telephone and transportation, to manage their medication and handle their finances independently was assessed using the Lawton Instrumental Activities of Daily Living scale(Reference Lawton and Brody27). Several items from the CARE Subjective Memory subscale(Reference Cosentino, Devanand and Gurland28) have also been included to measure functional restrictions in everyday life. Quantification of the staging of dementia was performed using the Clinical Dementia Rating scale(Reference Morris29), which globally assesses six domains of cognitive and functional performance.
Particular focus was placed on identifying potential comorbidities that could affect cognitive performance through screening participants for depression, anxiety and behavioural symptoms, as well as Parkinson’s disease, essential tremor and dementia with Lewy Bodies. Participants were screened for depressive symptoms over the past week using the fifteen-item version of the Geriatric Depression Scale (GDS)(Reference Yesavage30,Reference Fountoulakis, Tsolaki and Iacovides31) . Anxiety over the past week was quantified using the seven-item Hospital Anxiety and Depression Scale-Anxiety Subscale(Reference Zigmond and Snaith32) with a cutoff score of 8(Reference Bjelland, Dahl and Haug33,Reference Michopoulos, Douzenis and Kalkavoura34) . We also calculated the Hachinski ischaemia scale score(Reference Hachinski, Iliff and Zilhka35), a useful screening tool for vascular dementia. We typically evaluated comorbid conditions using a combined approach, gathering information from self- and/or informant-reported history, current medications and neuroimaging results, where available. Finally, a peripheral blood sample was drawn for basic biochemistry and APOE (apolipoprotein E) genotyping. The APOE genotype remained unknown for about a third of our sample because of delayed inclusion of this procedure to our protocol and some cases of refusal of venipuncture, so we used a simple approach to estimate genetic predisposition to cognitive decline for the whole sample: subjects were considered genetically predisposed to cognitive decline if they were APOE-ϵ4 carriers or reported family history of dementia.
Neurological and neuropsychological evaluation and diagnosis
All participants received standard physical and neurological examination, as well as a comprehensive neuropsychological battery, evaluating five cognitive domains in detail (memory (verbal/non-verbal), executive, language, visuospatial and attention-speed), leading to the calculation of a Z-score for each test, based on age- and education-standardised normative values of a normal Greek reference population. The average Z-score within the tests for each individual cognitive domain determined the subject’s domain performance. Subsequently, an overall cognitive Z-score was calculated by averaging the above five Z-scores. All Z-scores were calculated using mean values and sds from the baseline assessment. A subject was considered to be impaired in a certain cognitive domain when they scored more than 1·5 sd below the age- and education-standardised normative values.
Integration of all the collected data on each participant led to the formation of neurological and psychiatric diagnoses through consensus interdisciplinary meetings including all researchers and main investigators involved in the project, according to the established international criteria. Diagnosis of dementia and its subtypes was based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision(36). If a participant’s score fell below 1·5 sd in at least two cognitive domains, coupled with significant impairment in social/occupational functioning, they received a diagnosis of dementia. Mild cognitive impairment and its subtypes were diagnosed according to the Petersen criteria(Reference Petersen37).
Subjective cognitive decline and subjective cognitive decline score
Each research team usually uses their own operationalisation of the SCD definition or an original definition altogether(Reference Rabin, Smart and Crane38); we chose to adapt the research diagnostic criteria proposed in 2014(Reference Jessen, Amariglio and van Boxtel5) for use in our data set (Table 1). Complaints relevant to SCD were elicited with the help of a structured list of questions/topics for discussion with the participant (Table 2). Based on our data, subjective decline could be further classified according to the cognitive domain the participant felt they were having problems with, i.e. memory, language, visuoperceptual and executive function. Corresponding SCD types were memory decline, naming decline, orientation decline and calculation decline, with the latter namely referring to money-handling issues. Participants were considered positive for an SCD type in case of at least one positive answer in the questions of the respective domain. Our overall SCD score was the number of reported SCD domains and thus ranged from 0 to 4.
Table 1. Criteria for the diagnosis of subjective cognitive decline (SCD) in our research [adapted from Jessen et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. 2014 Nov;10(6):844–52]

SCD, subjective cognitive decline; GDS, geriatric depression scale; HADS-A, hospital anxiety and depression scale-anxiety subscale.
Table 2. Questions/topics used to elicit complaints associated with subjective cognitive decline (SCD) and SCD types in our research*

SCD, subjective cognitive decline.
* Participants were considered positive for an SCD type and SCD overall in case of at least one positive answer.
All cognitively normal individuals not meeting SCD exclusion criteria at baseline who completed their second evaluation comprised our SCD cohort.
Assessment of dietary intake, including adherence to the Mediterranean diet
Dietary intake was evaluated by trained researchers (mainly registered dieticians) through a structured interview using a semi-quantitative food frequency questionnaire (FFQ) that has been validated for the Greek population(Reference Bountziouka, Bathrellou and Giotopoulou39). Responses were corroborated by another household member, when necessary, and then converted to daily consumption of specific food items and extrapolated into macronutrient intakes. Energy intake was calculated by adding energy intake from macronutrients and alcohol, assuming 4 kcal/g for carbohydrates and proteins, 9 kcal/g for lipids and 7 kcal/g for alcohol. Dietary intake was also clustered into food groups featuring the core foods of the Greek diet(40). Adherence to the MeDi pattern was evaluated through the Mediterranean diet score (MDS)(Reference Panagiotakos, Pitsavos and Arvaniti41). This score is based on the weekly consumption of eleven food groups; an individual score for each component is calculated, ranging from 0 to 5. For the consumption of items that are presumed to closely reflect the Mediterranean pattern (i.e. non-refined cereals, fruits, vegetables, legumes, potatoes, fish and olive oil), individuals who reported no consumption were assigned a score of 0, and scores of 1–5 were assigned for rare to daily consumption (for example, weekly consumption in excess of 32 servings of non-refined cereals, >18 servings of potatoes, >22 of fruits, >33 of vegetables, >6 of legumes or fish and daily consumption of olive oil were assigned the maximum score of 5). For the consumption of foods that are presumed to diverge from this diet pattern (i.e. meat and meat products, poultry and full-fat dairy products), participants were assigned scores on a reverse scale (i.e. from 5 when they reported no consumption to 0 when they reported almost daily consumption; for example, over 10 servings of red meat and its products weekly, >10 servings of poultry and >30 servings of full-fat dairy were assigned a score of 0, while consumption of ≤1 serving of red meat, ≤3 of poultry and ≤10 of full-fat dairy were assigned 5). For alcohol intake, it was assumed that small amounts of consumption are beneficial, while either high or no consumption may be harmful. Therefore, a score of 5 was assigned for consumption of less than 300 ml of alcohol/d, a score of 0 was assigned for no consumption or for consumption of more than 700 ml/d and scores of 1–4 were assigned for consumption of 600–700, 500–600, 400–500 and 300–400 ml/d, respectively (assumed ethanol concentration: 12 g/100 ml of alcohol). The total MDS ranged from 0 to 55, with higher values indicating greater adherence to the Mediterranean dietary pattern. MDS aims to address one of the limitations of other iterations of MeDi scores, as the frequency of consumption of each food group is compared with levels assumed to be consistent or incompatible with the prototypical Mediterranean dietary pattern and not to sample-specific median values.
Statistical analyses
We used descriptive statistics to present demographic, genetic and dietary characteristics, clinical diagnoses, and duration of follow-up of our sample. Continuous variables were reported as mean values, standard deviations and ranges; categorical variables were reported as absolute and relative frequencies. We used Pearson’s χ 2 test and t-test to compare participants who returned for the second visit with those who were lost to follow-up due to refusal/relocation or being deceased.
We employed models of generalised estimating equations with SCD score as a dependent variable and baseline MDS as the predictor, including the interaction term MDS × time. We adjusted for sex, age, years of education, APOE-ϵ4 carriage and follow-up duration (model 1), sex, age, education, genetic predisposition to cognitive decline and follow-up duration (model 2), model 2 + energy intake (kcal/d) (model 3), model 2 + GDS score (model 4) and model 2 + energy intake + GDS score + Hachinski score (model 5). Baseline values were used for age, energy intake, GDS score and Hachinski score. All analyses were performed on the SCD cohort and on the cognitively normal participants at baseline with no exclusion criteria for SCD. The SCD score was treated as a continuous and as an ordinal variable. Both autoregressive and exchangeable correlation structures were tested with no difference in the results.
We then repeated our generalised estimating equation analyses in a model using the baseline daily consumption of each food group as predictors, including the (daily consumption)*time interactions. Studied food groups include milk and yogurt; cheese; cereals and legumes; fruit and juice; vegetables; red meat, poultry and cold cuts; fish; pastries, cakes; sweeteners, regular soft drinks; coffee and tea; alcoholic beverages. Adjustment was performed for sex, age, education, genetic predisposition to cognitive decline and follow-up duration, as well as the aforementioned covariates plus energy intake. Again, baseline values were used for age and energy intake; all analyses were performed on the SCD cohort and on the cognitively normal participants at baseline with no exclusion criteria for SCD. The SCD score was treated as a continuous and as an ordinal variable. Multicollinearity was excluded using a correlation matrix.
The two-sided significance level was set to 0·05. Statistical analyses were performed using the SPSS version 26 package.
Results
Sample characteristics
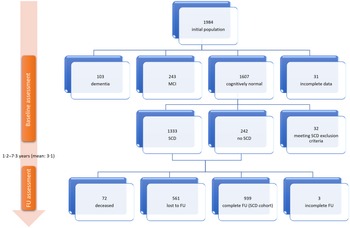
The initial visit of the Hellenic Epidemiological Longitudinal Investigation of Aging and Diet study was attended by 1984 individuals. Subject flow until the follow-up visit is illustrated in Fig. 1. There were 1607 cognitively normal participants after the baseline visit, of which 1333 (67·2 % of the total population) fulfilled criteria for SCD (Table 1), while 242 participants reported no SCD and 32 were excluded from further analysis according to our criteria. Among the 1575 cognitively normal individuals not meeting SCD exclusion criteria at baseline, 72 were deceased and 561 were lost to follow-up (35·6 %), leaving 942 to attend and finally 939 to complete the second evaluation and constitute our SCD cohort. Among the SCD cohort, 628 participants (66·9 %) were diagnosed with SCD on follow-up.
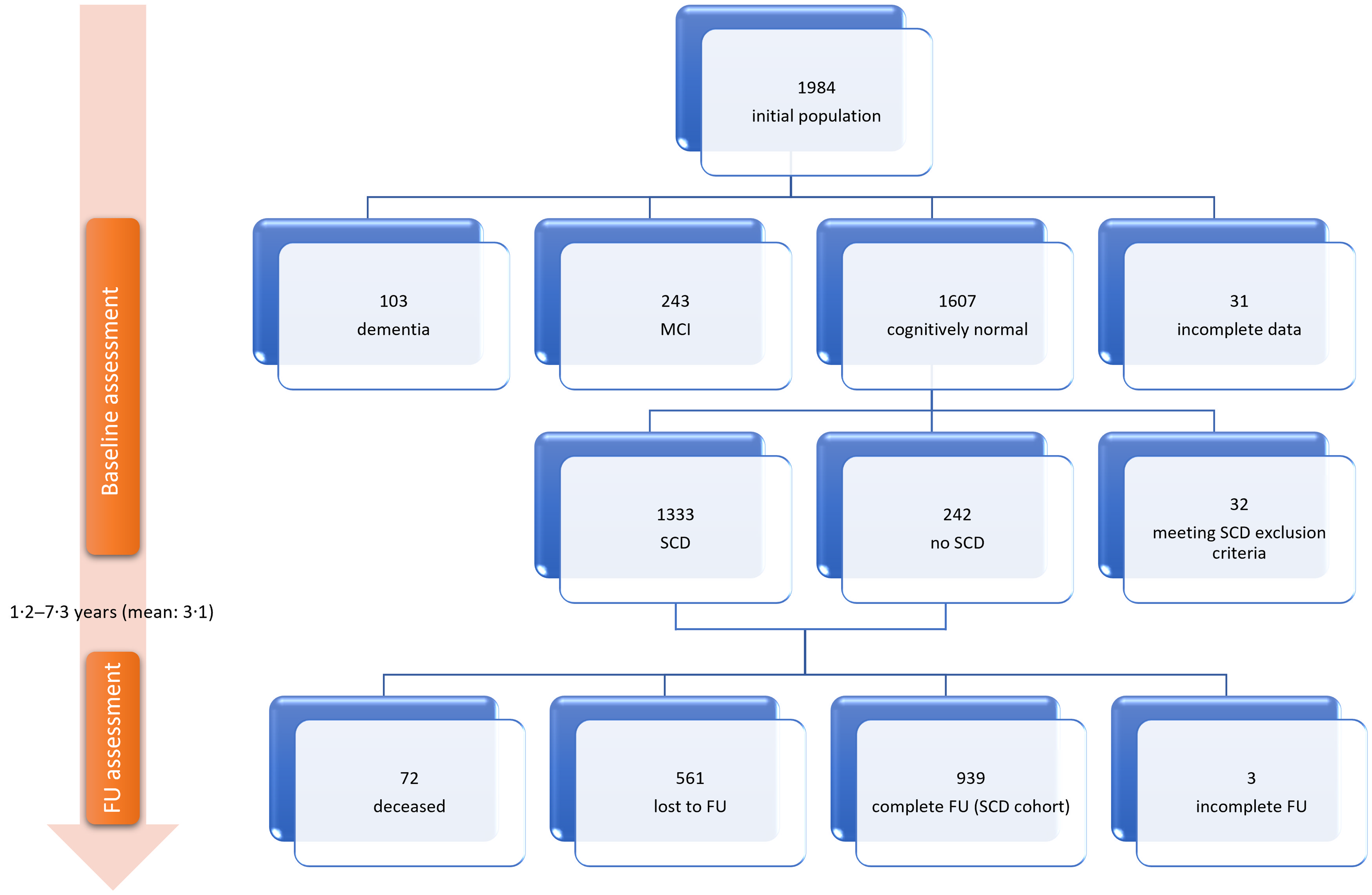
Fig. 1. Study flow chart. SCD, subjective cognitive decline; MCI, mild cognitive impairment; FU, follow-up.
A comparison within the cognitively normal population not fulfilling exclusion criteria for SCD at baseline between those who attended follow-up and those who refused to continue or relocated did not reveal any statistically significant differences in relation to sex (P = 0·485), level of educational attainment (P = 0·390), APOE-ϵ4 carriage (P = 0·486) or MDS (P = 0·364), while age was marginally different between the two groups (P = 0·049), with younger people more likely to attend follow-up; those with higher initial overall cognitive Z-score were also more likely to return (P < 0·001). A comparison within the same population between those who completed the second visit and those who were deceased at follow-up showed that male sex (P < 0·001), older age (P < 0·001), lower education (P = 0·012) and lower initial overall cognitive Z-score (P < 0·001) were associated with increased probability of death, while APOE-ϵ4 carriage (P = 0·096) and MDS (P = 0·069) remained statistically not significant.
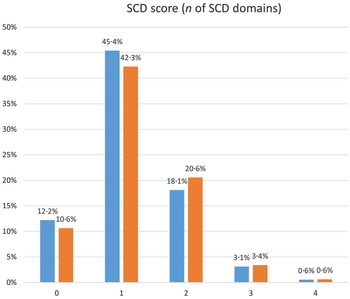
Demographic, genetic and dietary characteristics, as well as neuropsychological results, clinical diagnoses and duration of follow-up of our sample are presented in Table 3. The SCD score for the majority of our sample was 1 for both visits; a mean increase in the score by 0·20 cognitive domains during follow-up was documented. The frequency distribution of the SCD score in the two study visits is shown in Fig. 2 in detail.
Table 3. Demographic, genetic, clinical, neuropsychological and dietary characteristics and duration of follow-up
(Mean values and standard deviation, SCD cohort, n 939)
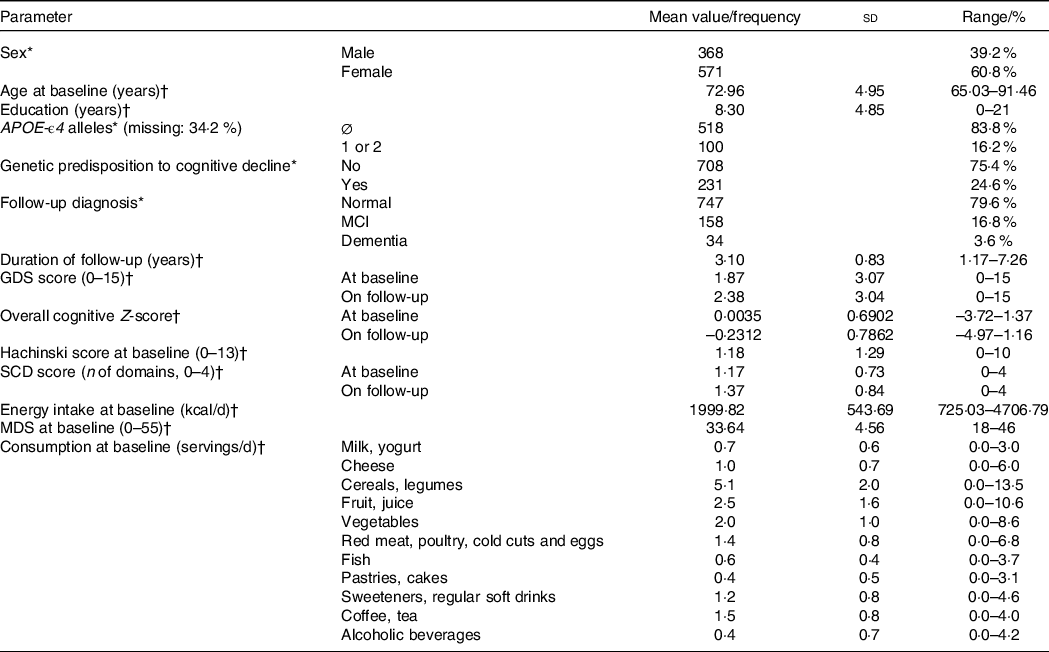
SCD, subjective cognitive decline; APOE, apolipoprotein E gene; GDS, geriatric depression scale; MDS, Mediterranean diet score.
* n, %.
† Mean (sd), range.
Continuous variables are reported as mean values, standard deviations and ranges. Categorical variables are presented as frequencies and relative frequencies. Relative frequencies of APOE-ϵ4 carriage reflect only participants with known APOE status. Subjects were considered genetically predisposed to cognitive decline if they were APOE-ϵ4 carriers and/or reported family history of dementia.
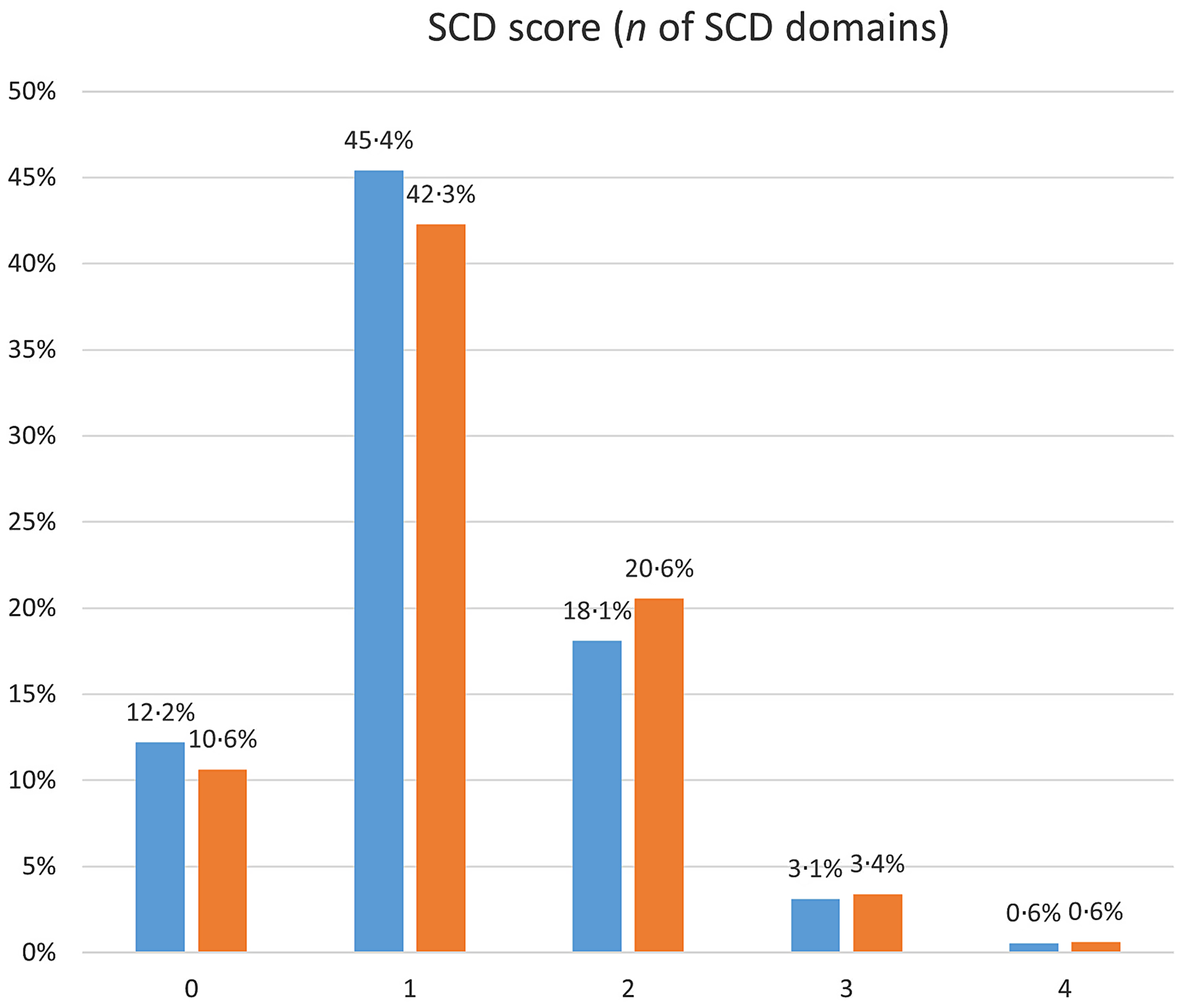
Fig. 2. Frequency distribution of the SCD score in the two study visits. SCD, subjective cognitive decline.  , Baseline;
, Baseline;  , follow-up.
, follow-up.
Association of nutritional factors and the time course of subjective cognitive decline
Table 4 shows the results of our generalised estimating equation models on the association between the interaction of MDS with time and the SCD score in the SCD cohort. We have found that an MDS higher by ten points was associated with a 7 % reduction in the progression of SCD within 1 year; this result remained statistically significant after adjustment for multiple potential confounders.
Table 4. Association between Mediterranean diet score (MDS) and the time course of subjective cognitive decline (SCD) score in the SCD cohort*

GEE, generalised estimating equations; APOE, apolipoprotein E gene; GDS, geriatric depression scale.
* The table shows the association between the interaction of MDS with time and the SCD score, after adjustment for potential confounders, using GEE. Participants were considered genetically predisposed to cognitive decline if they were APOE-ϵ4 carriers or reported family history of dementia. Baseline values were used for age, energy intake, GDS score and Hachinski score. The SCD score was calculated as the number of SCD cognitive domains reported.
** p values denote a statistically significant result (P < 0 05).
Table 5 shows similar models on the association between the interaction of specific food group consumption with time and the SCD score in the SCD cohort. The results reached statistical significance only for vegetables; every additional vegetable serving/d was associated with a 2·2 % reduction in SCD progression/year. This association remained statistically significant after adjustment for sex, age, education, genetic predisposition to cognitive decline, follow-up duration and energy intake.
Table 5. Association of specific food group consumption and the time course of subjective cognitive decline (SCD) score in the SCD cohort*

GEE, generalised estimating equations; APOE, apolipoprotein E gene.
* The table shows two models exploring the association between the interaction of specific food group consumption with time and the SCD score, after adjustment for potential confounders, using GEE. Participants were considered genetically predisposed to cognitive decline if they were APOE-ϵ4 carriers or reported family history of dementia. Food consumption was expressed in servings/d. Baseline values were used for age and energy intake. The SCD score was calculated as the number of SCD cognitive domains reported.
** p values denote a statistically significant result (P < 0·05).
The same analyses were performed on the cognitively normal participants at baseline with no exclusion criteria for SCD, irrespective of participation in follow-up (please see in the Supplementary Information), and also with the SCD score treated as an ordinal variable. These extra analyses yielded very similar results (not shown).
Discussion
Using our prospective data set of 939 cognitively normal adults at least 65 years old followed-up for a mean period of 3·10 years, we showed a statistically significant association of MDS with SCD score evolution; specifically, MeDi adherence higher by ten points was associated with a 7 % reduction in the progression of SCD within 1 year. This relationship remained significant after adjustment for multiple potential confounders, including sex, age, education, genetic predisposition to cognitive decline, follow-up duration, GDS score, energy intake and Hachinski score.
The inverse association of MeDi adherence with SCD progression further supports the preventive role of the MeDi against the whole continuum of cognitive impairment. The MeDi has already been shown in a number of studies to exert a protective effect against future mild cognitive impairment/Alzheimer’s disease(Reference Scarmeas, Stern and Tang9,Reference Martínez-Lapiscina, Clavero and Toledo11,Reference Singh, Parsaik and Mielke13) and overall dementia(Reference Scarmeas, Anastasiou and Yannakoulia15). Considering the most frequent causes of dementia (neurodegenerative and vascular), our finding can be attributed to the established effect of the MeDi on vascular risk factors: many studies have identified the beneficial role of the MeDi on all-cause and coronary heart disease mortality, stroke prevention, type 2 diabetes, lipid metabolism, blood pressure and body weight control, inflammatory and coagulation processes and brain oxidative stress(Reference Panagiotakos, Pitsavos and Arvaniti41–Reference Román, Jackson and Gadhia44). Several nutrients and non-nutritive compounds that are abundant in the foods of the MeDi, namely long-chain ω-3 fatty acids, polyphenols (including flavonoids), probiotic bacteria, fibre and prebiotic compounds, phytosterols, folic acid and other B vitamins, and antioxidants, are hypothesised to have neuroprotective properties and modulate beta amyloid aggregation and tau hyperphosphorylation(Reference Román, Jackson and Gadhia44). Given that a proportion of individuals with SCD will progress to overt cognitive impairment, incorporation of the elements of the MeDi in the daily dietary pattern could be advocated as part of the lifestyle counselling for this population(Reference Jessen, Amariglio and Buckley3).
SCD is gaining acceptance as a surrogate for preclinical dementia. Although it has been shown that individuals with SCD are at risk of objective cognitive decline(Reference Slot, Sikkes and Berkhof4,Reference Mitchell, Beaumont and Ferguson7) , very little is known about the trajectory over time of a quantitative measure of SCD severity and of the factors affecting it. This is, in part, due to the relative scarcity of quantitative SCD metrics. In our operationalisation of SCD (Tables 1 and 2), we used eighteen questions spanning four cognitive functions (memory, naming, orientation and calculations). Due to the lack of objective measures for SCD, as its definition suggests, we must rely on the individuals’ metacognitive ability, which might already be compromised in some members of our elderly SCD cohort. Reiteration of similar topics in our question set is a way to capture more data with our queries, but at the same time construction of a global score based on the sum of all positive answers may suffer from lower validity in terms of reflecting the progression of SCD severity over time. Therefore, we opted to grade our SCD score by domain: any number of positive answers in the questions for a given domain scored one point, yielding an SCD score of 0 to 4, changes to which might be more representative of substantial differences. So, if a participant reported SCD in one cognitive domain at baseline and two during follow-up, it would be relatively safe to assume that this spread of SCD into adjacent cognitive abilities was a sign of deterioration. The validity of this concept is further strengthened by the inverse association of our SCD score with the overall cognitive Z-score in our population(Reference Vlachos, Cosentino and Kosmidis24). As anticipated, the mean SCD score in our cohort worsened slightly by 0·20 cognitive domains during the mean 3·10 years of follow-up; such slow evolution is to be expected, given the long-term trajectory of neurodegenerative processes(Reference Sperling, Aisen and Beckett2).
Because of the subjective nature of SCD and its sole reliance on self-reporting, it is essential to mitigate potential confounding. The construct of SCD has been criticised as heavily dependent on concomitant depression, anxiety and personality traits pertaining to neuroticism, fear of dementia and excessive self-monitoring(Reference Jessen, Amariglio and Buckley3,Reference Liew45) . Comparison of SCD scores in the same person through prospective monitoring mostly negates any personality effect, while persistence of the association with MDS after adjustment for the baseline affective state further corroborates our findings.
Trying to elucidate the effect of individual food group consumption on SCD evolution, we employed similar models using food group intake as predictors. The results reached statistical significance only in the case of vegetable intake; every additional vegetable serving/d was associated with a 2·2 % reduction in SCD progression within a year. A similar result has already been published regarding subjective cognitive function(Reference Yuan, Fondell and Bhushan46), and also in many studies on dementia prevention, and can be attributed to the high vitamin, antioxidant, flavonoid and fibre content of vegetables, as well as their low energy density(Reference Scarmeas, Anastasiou and Yannakoulia15,Reference Angelino, Godos and Ghelfi47) . B vitamins are essential parts of cell energy production and reduce homocysteine levels; polyphenols can modulate the circadian rhythm and together with carotenoids and certain essential minerals found in vegetables may enhance the brain’s defense against oxidative stress. Moreover, higher intake of fibre increases the diversity of gut microbiota, potentially exerting neuroprotective and anti-inflammatory effects through the gut-brain axis(Reference Godos, Currenti and Angelino48).
Literature regarding the effects of vegetable consumption on disease prevention in general is not consistent; stronger evidence exists for a probable protective effect on cardiovascular disease and age-related cataract, with further possible evidence of decreased risk for stroke and depression(Reference Angelino, Godos and Ghelfi47). Given the established role of cerebrovascular disease in brain ageing, one could hypothesise that the potential protective effect of vegetables on cognitive decline might also be attributable to the beneficial effect of their consumption on cardiovascular health. Considering all the aforementioned data, the small effect size for vegetable consumption in our study and the lack of statistical significance for other food groups may appear counter-intuitive at first, but the impact of diet as a whole surpasses the sum of effects of its components, as has been noted previously in the literature(Reference Scarmeas, Stern and Tang9). Potential explanations include the inevitably limited role of each single nutrient in the multifaceted synthesis of the neuronal milieu, the integration within a complete dietary regimen of non-nutritional (lifestyle etc.) factors that are difficult to isolate(Reference Scarmeas, Stern and Tang9) and a possible lack of statistical power.
SCD prevalence in our population is high (67·2 % at baseline and 66·9 % on follow-up), but that is to be expected, as reported values in the literature range from 9·0 to 58·1 %(Reference Garcia-Ptacek, Eriksdotter and Jelic49) and according to the COSMIC collaboration from 6·1 to 52·7 %(Reference Röhr, Pabst and Riedel-Heller50). There is lack of standardisation, as well as great heterogeneity in definitions, ethnicities, ages and clinical settings in studies worldwide. According to our research on SCD prevalence, 84·2 % of the cognitively normal population ≥65 years old reported subjective decline in at least one cognitive domain(Reference Vlachos, Cosentino and Kosmidis24). We believe that our structured interview seeking cognitive complaints helps elicit more positive answers than just asking open-ended questions: inquiring about specific items may prompt the subject to search their daily experiences and result in more accurate reporting and, thus, a higher prevalence rate(Reference Cosentino, Devanand and Gurland28,Reference Rabin, Smart and Crane38) . Therefore, we do not attribute high SCD prevalence to sample selection bias.
Among the limitations of the present study, it should be noted that we evaluated dietary intake only through a self-reported tool, a validated FFQ, without the added value of blood biomarkers that may have indicated adherence to at least some of the components of the MeDi more objectively. Concerns have been raised in the literature regarding retrospective assessment of dietary habits in the elderly population, but it has not been shown irrefutably that cognitively healthy older adults provide less valid self-reports(Reference Biró, Hulshof and Ovesen51,Reference de Vries, de Groot and van Staveren52) (our population may have expressed subjective cognitive complaints, but they were all considered normal in their objective evaluation). In any case, questionnaire administration by trained researchers (mainly dietitians) and cross-reference of responses by another household member – when present – served as extra levels of response validity, while it should also be noted that the validation study of the FFQ included participants up to 82 years old(Reference Bountziouka, Bathrellou and Giotopoulou39,Reference Bountziouka, Constantinidis and Polychronopoulos53) . Moreover, our clinical evaluation was not complemented by routine neuroimaging or cerebrospinal fluid biomarker collection, and APOE genotyping was not performed in a third of our sample; despite that, we believe our detailed and thorough clinical and neuropsychological protocol followed by consensus interdisciplinary team meetings integrating all collected data ensured a high level of diagnostic accuracy. Lastly, 35·6 % of the cognitively normal population not fulfilling SCD exclusion criteria at baseline were lost to follow-up, which, although unfortunate, is not uncommon in studies of this kind and in similar populations(Reference Chatfield, Brayne and Matthews54). It should be noted here that participant selection was random, and invitations to enroll and attend the follow-up visit were made by phone call, with multiple attempts when needed. Comparison of those who attended the second visit and those who were lost to follow-up did not reveal significant differences, except for an age and cognitive score discrepancy. Among those who missed follow-up because of death, there was a preponderance of men, older and less educated individuals, as well as those with lower cognitive performance, as expected.
Conversely, a strength of our research is that, to our knowledge, it is the first one trying to elucidate the complex interplay in time between MeDi adherence and SCD evolution in an elderly population. Because of our methodology, we consider our sample to be representative and our diagnoses to be fairly accurate. Our subjects have been given a comprehensive neurological and neuropsychological evaluation and have answered a meticulously designed FFQ. As SCD operationalisation and development of relevant metrics is still a matter of active research in the scientific community, another small contribution of our work is the creation of a quantitative SCD score that funnels data from eighteen questions into four potential domains and the proposition of its use as a method of longitudinal assessment of SCD severity over time.
In conclusion, based on the prospective follow-up of a cohort of people at least 65 years old in Greece, we have found that MeDi adherence higher by ten points was associated with a 7 % reduction in the progression of SCD within 1 year, and every additional vegetable serving/d was associated with a 2·2 % reduction in SCD progression/year. Our results provide support to the notion that MeDi has a protective role against the whole continuum of cognitive decline, starting at the first subjective complaints. Replication of this finding, especially for individual food groups, and further clarification of the underlying mechanisms may strengthen the role of the MeDi as a cost-effective and easily implemented preventive strategy targeting the modifiable risk factors for cognitive decline.
Acknowledgements
Many thanks are due to all HELIAD collaborators.
This work has been supported by the following grants: IIRG-09-133014 from the Alzheimer’s Association, 189 10276/8/9/2011 from the NSRF-EU program Excellence Grant (ARISTEIA) (which is co-funded by the European Social Fund and Greek National resources) and ΔY2β/oικ.51657/14·4·2009 from the Ministry for Health and Social Solidarity (Greece) (all awarded to N.S.); funding from the Research Committee, University of Thessaly, Code 2845 (awarded to G.H.) and from the Research Committee, Aristotle University of Thessaloniki, Code 89272 (awarded to M.H.K.). Our funders had no role in the design, analysis or writing of this article.
G. S. V. participated in data collection and consensus meetings, performed statistical analysis and drafted the manuscript. M. Y., C. A. A., S. C. and N. S. participated in statistical analysis. M. Y., M. H. K., P. S. and N. S. participated in study design, consensus meetings and manuscript review. E. D. and G. H. participated in study design and manuscript review. L. S. reviewed the manuscript. All authors read and approved the final manuscript.
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114521005109