INTRODUCTION
Hepatitis E (HEV), a single-stranded RNA virus, is as an emerging disease of global importance, as it is one of the major causes of acute hepatitis worldwide [Reference Purcell and Emerson1]. It is responsible for about 50% of cases of acute viral hepatitis in endemic countries [Reference Myint and Gibbons2]. HEV is not transmitted readily from one person to another so, unlike HAV, familial clusters of disease are unusual [Reference Purcell3]. In developing countries, HEV spreads mainly by drinking faecally contaminated water and less commonly from eating uncooked meat. Accumulating evidence indicates that there are animal reservoirs of HEV (zoonotic disease) [Reference Hirano4, Reference Okamoto5]. HEV-related viruses have been found in pigs [Reference Sonoda6], deer [Reference Sonoda6], chickens [Reference Haqshenas7, Reference Huang8], fish [Reference Koizumi9], shellfish [Reference Koizumi9], rodents [Reference Hirano4] and wild rats [Reference Hirano4]. Direct HEV transmission has been reported from pigs, deer [Reference Tei10], fish [Reference Koizumi9] and shellfish to humans as a result of eating undercooked or raw meat from these animals [Reference Dalton11]. Consuming raw or undercooked meat is the most common mode of transmission in developed countries [Reference Dalton11]. Very rarely, parenteral (blood borne) [Reference Taremi12] and vertical transmission from mother to child have been also implicated in this virus transmission [Reference Kumar13].
The HEV infection incubation period varies from 15 to 45 days [Reference Malcolm14]. The clinical features of HEV infection range from asymptomatic infection to mild hepatitis to subacute and acute liver failure [Reference Dalton11]. The outcome can be serious in individuals with an underlying chronic liver disease, with a mortality approaching 70%. Hepatitis E in developed countries is far more common than previously recognized [Reference Dalton11]. The seroprevalence data from industrialized countries suggests that subclinical or unrecognized infection is common [Reference Schwartz15]. However, the incidence of HEV infection in such countries is not known. The prevalence of HEV infection in the USA has been reported in a few studies [Reference Kuniholm16]. To our knowledge, the literature does not contain reports on the incidence of HEV in the USA. Therefore, for better assessing the burden of primary HEV infection, as well as understanding the epidemiology of HEV in the US population, this analysis was conducted using a nationally representative sample of the American population to estimate HEV force of infection (incidence rate). Force of infection is defined as the instantaneous per capita rate at which susceptibles acquire infection and reflects the degree of contact with potential for transmission between susceptibles and infected [Reference Sutton17].
METHOD
The NHANES III is a national survey conducted during 1988–1994 by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). The sampling plan of the survey followed a stratified multistage probability cluster design that selected a sample representative of the US non-institutionalized population. As NHANES III was based on a complex multistage probability sample design, appropriate probability sampling weights were assigned to produce unbiased population estimates. The sampling weights incorporated the differential probabilities of selection and adjusted for non-coverage and non-response. In the present analysis participants aged ⩾6 years, who had available data from their physical and laboratory examinations, participated in a home-conducted interview. Self-reported data collected at the home interview relevant to the current analysis included demographics (age, gender, ethnicity, poverty index, residence). Participants were asked to sign a Consent Form agreeing to participate in the survey. For participants aged <16 years, parents' or guardians' consents were provided.
Race/ethnicity was self-reported and grouped as White, Black, Hispanic and other. The poverty index was calculated by dividing the total family income by the poverty threshold adjusted for family size during the year of the interview. Values <1 were considered below the poverty line. Region was defined by standard US Census Bureau categories: Northeast, Midwest, South, and West. Residence in central or fringe counties of metropolitan areas with a population of ⩾1 million was defined as metropolitan residence. Non-metropolitan residence was defined as residence in all other areas not defined as a metropolitan area. This definition, developed by the US Department of Agriculture, was used by the NCHS to assign a metropolitan or non-metropolitan residence category to each NHANES III participant. Owning pets, dogs and cats, was based on the following interview questions: ‘Do any pets live in this home?’, ‘Do any dogs live in this home?’, ‘Do any cats live in this home?’. Monthly meat consumption of fish, chicken or turkey, beef and eggs was determined by asking the participant how often did they eat the different types of meat per month.
Measurement of hepatitis E antibodies
In the present study, ‘in-house’ enzyme immunoassay (EIA) developed at the US National Institutes of Health to test NHANES III serum samples for anti-HE antibodies was used. This assay has been used extensively for measuring anti-HEV in studies of HEV infection in humans, swine and for evaluation of the efficacy of hepatitis E vaccine in non-human primates [Reference Engle18, Reference Purcell19]. The antibodies measured by this assay included the principal, if not only, neutralizing antibody to the virus [Reference Zhou, Purcell and Emerson20]. In brief, wells of polystyrene microwell plates (Nunc 468667) were coated with recombinant HEV antigen diluted in a carbonate-bicarbonate (pH 9·6) buffer and allowed to bind to the solid phase at room temperature for ~18 h. The antigen concentration was ~0·025 μg/well. Next, unbound antigen was removed, and the wells were washed twice with a wash solution (KPL 50-63-00) that contained 0·02% Tween 20 in 0·002 mol/l imidazole-buffered saline by use of an automated plate washer. Negative control serum samples were obtained from HEV-naive chimpanzees. The anti-HEV standard series consisted of serial dilutions of a secondary anti-HEV standard, which is modelled to match the World Health Organization (WHO) 95/584 anti-HEV preparation (available from the National Institute for Biological Standards and Control, Hertfordshire, UK). NHANES III serum sample values were expressed, after subtraction of background optical density values, as the ratio of the NHANES III sample optical density value to the 0·01 WHO unit sample optical density value. Samples with ratios >1·0 were classified as seropositive.
Statistical analysis
The force of HEV infection or annual incidence rate (λ) [Reference Schenzle, Dietz and Frosner21] was estimated by assuming a steady state (low migration and mortality), incidence was constant over time and independent of age, seroconversion is permanent. The intercept (log μ) used to calculate force of infection (incidence rates) from the catalytic model was fitted from a generalized linear model with seronegative proportion as the outcome variable, a binomial distribution family, complementary log–log link function, and the natural logarithm of age as an offset. The following equation was used to perform the previous analysis:
where log[μ] is the intercept of the regression model, pi is the proportion of seropositives and A is age as a continuous variable. The obtained log[μ] was included in the following catalytic model to calculate the annual infection rate of HEV infection: λ=1−e−e(log[μ]). Regression and catalytic models were conducted to calculate the overall incidence rate as well as the incidence rates across the different categories of each variable. The incidence rates were considered statistically significant when their corresponding confidence intervals did not overlap.
RESULTS
The overall force of HEV infection in the USA was 66 infections per 10 000 susceptible persons per year (approximately seven infections per 1000 persons per year) (Table 1). The force of infection of HEV was significantly higher in those who served in the army, those who were born outside the USA and those who had a low poverty index. The non-Hispanic White Americans had the highest HEV force of infection (P<0·05). Finally, higher incidence rate of HEV infection was observed in those who consumed fish more frequently (P<0·05) (Table 2). Although the incidence rates of HEV were higher in females, residents of metropolitan areas and the Midwest region, these rates were not statistically significant.
Table 1. Force of infection of hepatitis E virus infection by demographic characteristics and pet-ownership status

CI, Confidence interval.
† Number of individuals infected by HEV per 10 000 susceptible individuals per year. If the relevant confidence intervals did not overlap, the parameters based on the different groups were considered to be statistically different.
‡ Data were available for participants aged >17 years.
* Significant at 0·05.
Table 2. Hepatitis E virus force of infection across the monthly consumption of different types of meat and eggs
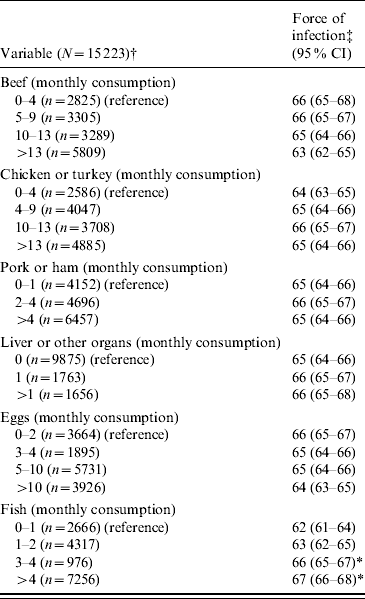
CI, Confidence interval.
† Data were available for participants aged >17 years.
‡ Number of individuals infected by HEV per 10 000 susceptible individuals per year. If the relevant confidence intervals did not overlap, the parameters based on the different groups were considered to be statistically different.
* Significant at 0·05.
DISCUSSION
Estimates of the frequency of new HEV infections are essential for understanding and preventing this virus transmission. The present analysis shows that in HEV seronegative individuals in the USA, nearly 7/1000 persons seroconvert every year. This relatively low force of infection indicates that HEV is not easily transmitted. The force of infection for HEV was higher in non-Hispanic Whites than the other ethnic and racial groups. This higher force of infection (i.e. incidence in seronegative individuals) suggests that HEV is circulating more frequently in this racial/ethnic group.
The results of the current study revealed that HEV infections in the USA could be acquired from developing countries. First, the observed incidence rate of HEV infection in those who were born outside the USA was higher than that of those who were born in the USA. This finding is consistent with the hypothesis that HEV infection is common in many developing countries (most foreign-born individuals in the USA emigrate from developing countries) [Reference Kuniholm16]. Second, the infection rate of HEV of the military service group was higher than that of the civilian group. Serving in the American army often necessitates travel to developing countries [Reference Kuniholm16].
The obtained data showed that the incidence rate increased as fish consumption increased. Therefore, it could be concluded that HEV infection is not only acquired from developing countries but is also locally acquired (autochthonous). The previous observation (the incidence rate increased as fish consumption increased) indicates that HEV infection is dose-dependent. The dose dependence characteristic was seen in some experimental studies [Reference Meng23–Reference Huang25]. Finally, a concern that faecal contamination of irrigation or coastal waters could contaminate produce or fish harvested for human consumption is warranted [Reference Huang25]. Thus, fish consumption becomes a potential source of HEV transmission. The current study shows that poverty is an important factor that increases HEV force of infection. Poverty is associated with an insanitary environment, such as insanitary water sources and food. An insanitary environment can increase the chance of contaminating water and food with HEV.
The prevalence of HEV in the US swine population is very high [Reference Meng23]. HEV was isolated from pork offered to the public in grocery stores [Reference Feagins26]. Nevertheless, the incidence of HEV virus did not differ by the amount of pork consumption. A plausible explanation for the previously obtained data is that the consumed pork meat could have been well cooked. HEV is inactivated by the temperature produced during cooking [Reference Feagins27]. Hence, the US population included in this analysis was exposed to inactivated HEVs when they consumed cooked pork [Reference Feagins27]. Unlike pork which is usually cooked before consumption, individuals could consume raw or uncooked fish. Therefore, the probability of acquiring HEV during fish consumption is higher than that of acquiring the same infection when pork is consumed. It is noteworthy to mention that the dataset did not have any information about how the pork or fish was cooked (rare, medium or well cooked). Hence, the assumed role of cooking, justifying why consuming fish not pork was associated with significant HEV transmission, can not be verified in this study.
The results of this study are very valuable. First, to our knowledge, this is the largest epidemiological study which used a national representative sample of the US population to report the incidence rate (force of infection) of HEV infection. Second, this study has public health importance. There are no nationally representative data on the incidence of HEV infection in the USA. Nationally representative estimates of HEV incidence are essential for assessing the burden of primary HEV infection in the US population. Third, the variables used in the analysis were collected using standardized protocols with rigorous quality control procedures.
The results of this study should be interpreted in the context of its limitations. First, the data used for the current analysis were obtained from a single, cross-sectional study so that time trends were not able to be addressed. Direct measurement of incidence is difficult because infection is most often asymptomatic and unrecognized. Furthermore, hepatitis E infection is not reportable in most states. Determining seroconversion rates in a large, representative cohort would be possible but costly. A more practical approach is to estimate the past incidence of infection from existing seroprevalence data by means of ‘catalytic modelling’ as it was described previously. Second, as with any model, the estimates and confidence intervals are conditional on the assumptions of the model. Some of the predefined assumptions especially, steady state, age independence and permanent immunity could be violated, e.g. in our model seroconversion was assumed to be permanent but some studies have documented that HEV antibody levels in infected persons can fall below the level of detection; such seroconversion would cause our model to underestimate incidence.
In summary, this study shows that in the USA HEV can be acquired locally and from developing countries. HEV is circulating more frequently in the non-Hispanic White racial/ethnic group, and in American individuals who consume fish more frequently. This observation raises the concern that faecal contamination of irrigation or coastal waters could contaminate produce or fish harvested for human consumption. Nevertheless, with the relatively low annual force of infection (annual incidence rate) of HEV infection, the health benefits of fish consumption should not be overshadowed by the risk of HEV transmission, but it is wise to take precautions and raise awareness on possible infectious diseases to help limit their transmission.
ACKNOWLEDGEMENTS
We respectfully acknowledge the assistance, support and cooperation of Jacqueline Lee Endt from the Chronic Disease and Health Promotion Department in the World Health Organization (WHO) for editing and preparing the manuscript for publication.
DECLARATION OF INTEREST
None.




