One of the explanations for the increased prevalence of allergic diseases lies in the well-known ‘hygiene hypothesis’, which postulates that a lack of opportunities for exposure to immunostimulating pathogens during early childhood increases the prevalence of allergic diseases(Reference Strachan1). For example, allergic children in Estonia and Sweden were found to be colonised with lactobacilli less often than non-allergic children(Reference Bjorksten, Naaber and Sepp2). Lactic acid bacteria (LAB) are widely considered to exert a number of beneficial effects including immunomodulation, interference with enteric pathogens and maintenance of a healthy intestinal microflora(Reference Mowat3, Reference Rakoff-Nahoum, Paglino and Eslami-Varzaneh4). The disturbance in the gut flora induced by the ingestion of live microbiota could favour appropriate maturation of the immune system(Reference Bjorksten, Sepp and Julge5, Reference Kirjavainen, Arvola and Salminen6). In particular, lactobacilli are considered to induce reactions involving Th1 cells and to improve allergic diseases(Reference Cross, Stevenson and Gill7). Orally administered heat-treated Lactobacillus casei (strain Shirota) was found to inhibit IgE production induced by ovalbumin in mouse serum(Reference Matsuzaki, Yamazaki and Hashimoto8). Moreover, heat-treated L. plantarum L-137 injected intraperitoneally was demonstrated to suppress IgE production in response to casein allergy in mice(Reference Murosaki, Yamamoto and Ito9). In human subjects, L. rhamnosus strain GG administered perinatally reduced the incidence of atopic eczema in children at risk during the first 2 years of life(Reference Kalliomaki, Salminen and Arvilommi10) and beyond infancy(Reference Kalliomaki, Salminen and Poussa11). L. rhamnosus and L. reuteri administration moderately improved the clinical severity of eczema in children with atopic dermatitis(Reference Rosenfeldt, Benfeldt and Nielsen12). Our double-blinded, randomised, controlled clinical trial for the effect of L. gasseri on asthmatic children with allergic rhinitis also shows significantly improved mucosal symptoms and pulmonary function in the treated subjects as compared to placebo controls(Reference Chen, Lin and Jan13).
Allergic asthma is characterised by chronic airway inflammation with intense eosinophil and lymphocyte infiltration, mucus hyper-secretion and airway hyper-responsiveness to a variety of stimuli. It is now generally thought that antigen-specific helper T lymphocyte (Th2) cells and their cytokines orchestrate these pathological features of asthma. On the other hand, Th17 cells and IL-23, a cytokine that preferentially expands Th17 cells, play a significant role in the development of chronic inflammatory diseases, including autoimmune diseases. Recently, several lines of evidence suggest that IL-17A and IL-17F are also involved in airway inflammation, especially in neutrophilic inflammation(Reference Hellings, Kasran and Liu14, Reference Oda, Canelos and Essayan15). In addition, it has been shown that IL-17A is expressed in the airways of asthmatic patients(Reference Molet, Hamid and Davoine16) and its expression is correlated with the severity of asthma(Reference Chakir, Shannon and Molet17). Moreover, our previous study results on the genetic factors associated with childhood allergic asthma indicated a possibility that the polymorphisms of IL-17A gene may confer risk for paediatric asthma in the Taiwanese population(Reference Wang, Shyur and Wang18). In a mouse model of asthma, it is also found that IL-23 and Th17 cells enhance not only neutrophilic airway inflammation but also Th2 cell-mediated eosinophilic airway inflammation in a murine asthma model(Reference Dong19, Reference Wakashin, Hirose and Maezawa20).
To date, the precise mechanism of Lactobacillus-induced immunomodulation has not been fully elucidated, although it was reported that these probiotics can directly but differentially modulate dendritic cells maturation and induce cytokine secretion through toll-like receptors including toll-like receptor 2(Reference Mohamadzadeh, Olson and Kalina21–Reference Foligne, Zoumpopoulou and Dewulf23). On the basis of experimental data, the anti-inflammatory effects of probiotics may be, in part, a consequence of the modulation of the cytokine balance: down-regulation of pro-inflammatory (e.g. IL-12 and TNF-α) cytokine production and/or stimulation of anti-inflammatory (e.g. IL-10) cytokine production(Reference Di Giacinto, Marinaro and Sanchez24). However, whether the effect of probiotic bacteria such as Lactobacillus can act on IL-17-producing cells is currently unknown.
Materials and methods
Animals and reagents
Female BALB/c mice aged 6–8 weeks were obtained from the Laboratory Animal Center of the College of Medicine, National Cheng Kung University. All experimental animal care and treatment followed the guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals, USA. Dermatophoides pteronyssinus (Der p) extract (1 g lyophilised whole-body extract in diethyl ether; Allergon, Engelholm, Sweden) was dissolved in pyrogen-free isotonic saline, filtered through a 0·22 μm filter, and stored at − 70°C before use. Lipopolysaccharide concentration of the Der p preparations was < 0·96 ng/ml (Limulus amebocyte lysate test; E-Toxate; Sigma-Aldrich, St Louis, MO, USA).
Probiotic bacterial preparations
L. gasseri A5, which exhibits probiotic characteristics, was isolated from the intestinal tract of normal, healthy humans at ProMD Biotech Company Limited (Tainan, Taiwan) by using the high-throughput detecting system. Acute oral toxicity studies (FR-AC00235), conducted by the Development Center for Biotechnology (Taipei, Taiwan), in test mice administered the highest dose of 5000 mg/kg L. gasseri A5 orally for 14 d, revealed that the treatment had no side-effects on the mice. To prepare the stock solution for ex vivo experiments, bacteria from frozen stocks were suspended in de Man–Rogosa–Sharpe liquid medium (Difco Laboratories, Detroit, MI, USA), plated in de Man–Rogosa–Sharpe agar, cultured anaerobically at 37°C for 24 h, then inoculated in fresh de Man–Rogosa–Sharpe broth and grown at 37°C under anaerobic conditions for 48 h in 50-ml tubes. A high-density culture system was used to produce freeze-dried powder of L. gasseri A5 at concentrations of more than 1011 colony-forming units (CFU)/g supplied by ProMD Biotech Company Limited, and then the powder was stored at a temperature below 4°C. Stability tests demonstrated that cell viability could be maintained at room temperature for up to 3 months. To prepare heat-killed bacterial extracts, cultured bacteria were washed twice with sterile distilled water, heat-killed at 100°C, lyophilised and suspended in PBS.
Treatment protocols
Each experiment included the following groups (n 6). Controls were sensitised and the allergen-challenged mice were subcutaneously immunised twice at 1-week intervals with a mixture containing 40 μg of Der p in incomplete Freund's adjuvant (Difco Laboratories) and subsequently challenged intra-tracheally with 50 μl of Der p (0·5 mg/ml) 7 d apart after the last immunisation. Treatment groups received oral administration of high (4 × 106 CFU), middle (2 × 106 CFU) or low dose (1 × 106 CFU) of L. gasseri in 700 μl PBS daily via a gavage needle, started from 14 d before the first immunisation with mite extract (day 0) and continued until the day of allergen challenge (day 14). Non-treated sensitised and challenged mice received PBS instead. Der p-sensitised, saline-challenged mice served as control animals. Mice were subjected to measurements of airway responsiveness followed by bronchoalveolar lavage (BAL).
Measurement of airway hyper-responsiveness and bronchoalveolar lavage examination
The airway resistance of mice was measured by a single-chamber, whole-body plethysmograph (Buxco Electronics, Inc., Troy, NY, USA) as described in the manual provided. Airway resistance was expressed as Penh (enhanced pause). Increasing doses of methacholine were administered by nebulisation for 3 min, and Penh was measured over the subsequent 3 min. Mice were killed with an overdose of sodium pentobarbitone. The trachea was exposed, cannulated, and BAL was performed with two 1-ml aliquots of saline. A total of 1·8–1·9 ml of BAL fluid was consistently recovered by this technique. The total cells in cytocentrifuge preparations of BAL fluid were stained with Liu's stain for differential cell counts.
Lung histochemistry
The lungs of mice were first inflated with 0·5 ml of formalin (Sigma-Aldrich) via trachea, then excised and fixed in 4 % buffered formalin overnight at 4°C. Tissues were embedded in paraffin, cut into 5-μm sections and stained with haematoxylin and eosin. Inflammatory infiltrates and lung architecture were assessed using light microscopy.
Total IgE and Dermatophoides pteronyssinus-specific IgG1 and IgG2a/2b antibodies
Serum samples were collected from the retro-orbital venous plexus 3 d after the intra-tracheally allergen challenge. The total IgE, Der p-specific IgG1 and IgG2a/2b serum antibody titres were determined by ELISA, with the required reagents and monoclonal antibodies purchased from BD PharMingen, San Jose, CA, USA.
Splenocytes culture
The spleens were removed from three mice for each experiment, and the pooled splenocytes (106 cells) were incubated with transforming growth factor (TGF)-β (2 ng/ml) plus IL-6 (20 ng/ml) at 37°C for 72 h in 1 ml of Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10 % fetal bovine serum (FBS), 10 μm-2-mercaptoethanol, 10 mm-HEPES, penicillin and streptomycin. Various doses of Der p extract (1 μg/ml or 10 μg/ml) and heat-killed L. gasseri (107 bacteria, multiplicity of infection = 10 or 106 bacteria, multiplicity of infection = 1) were added to the culture according to experimental condition. Cell cultures were set up in duplicate or triplicate in six-well flat-bottom polystyrene microtitre plates. A culture without the addition of TGF-β plus IL-6 or heat-killed bacteria and Der p was included as a control. Culture supernatants were harvested and stored at − 80°C until assayed for cytokines, as described next.
Assay for cytokines
The amounts of thymus and activation-regulated chemokine (TARC), IL-10, IL-12, IL-17A, IL-23 and TNF-α in the BAL fluid supernatants and IL-17F in culture supernatants were determined by ELISA kits (R&D Systems, Minneapolis, MN, USA). The minimum detectable doses were 2, 7, 4, 1·5, 5 and 2 pg/ml, respectively.
Isolation of alveolar macrophages from lung
The lung tissues of mice were digested with collagenase and hyaluronidase and minced. After lysis of erythrocytes, the dissociated cells were underlaid with 5 ml of lymphocyte separation solution (Mediatech, Herndon, VA, USA) and centrifuged at 2200 rpm for 20 min. The mononuclear cells in the middle layer were collected for flow cytometric analysis. The neutrophils were collected from the bottom of the tube. For lung macrophage isolation, the aforementioned cells were incubated in a six-well plate for 2 h and the adherent cells were harvested as lung macrophages.
Flow cytometric analysis
The BAL fluids cells from mice were incubated with 1 μg/106 cells of Fc receptor-blocking antibody (Ab) (clone 24G2; American Type Culture Collection, Manassas, VA, USA), and then stained with phycoerythrin (PE)-Cy3-conjugated anti-mouse CD3, allophycocyanin-cy5-conjugated anti-mouse CD11b, PE-conjugated anti-mouse DX5 (pan-NK cells), allophycocyanin-conjugated anti-mouse c-Kit and PE-conjugated anti-mouse F4/80 Abs. After cell-surface staining, cells were washed, fixed and permeabilised with Fix-Perm solution for intracellular staining with fluorescein isothiocyanate-conjugated anti-mouse IL-17 Ab (clone eBio17B7, eBioscience, San Diego, CA, USA). All fluorophore-conjugated Abs and the corresponding isotypes were purchased from eBioscience. The stained cells were used for flow cytometric analysis (BD LSR II).
Statistical analysis
Mediator contents in culture supernatants and antibody production values were analysed by one-way ANOVA, followed by a post hoc comparison between groups. The cellular accumulation and mediator contents in BALF and serum Ab titre were analysed by using the Mann–Whitney U test. Results are expressed as means with their standard errors. A P value < 0·05 was considered significant.
Results
Lactobacillus gasseri suppresses Th2 and Th17 cytokine levels in bronchoalveolar lavage fluids
After the mite-allergen challenge, the levels of TARC, TNF-α, IL-23 and IL-17A were significantly increased in BAL fluid in mite-sensitised mice as compared to saline-challenged mice (Fig. 3). The levels of IL-12 and IL-10 were not significantly changed, compared with saline controls. Oral administration of high dose of L. gasseri in Der p-sensitised and -challenged mice significantly attenuated the increase in TARC, TNF-α and IL-17A but did not affect IL-23 levels. In contrast, the levels of IL-12 and IL-10 were significantly elevated when compared with non-treated, sensitised and challenged mice. Although middle- and low-dose L. gasseri-treated mice also had significantly lower levels of TARC and higher level of IL-12 (middle dose) in BAL fluids, the levels of other cytokines were not significantly altered (Fig. 3).
The change of IgE, IgG1 and IgG2a antibodies in the sera and the cytokines production from splenocytes
To determine whether the attenuated allergen-induced Th2 and Th17 cytokine response was an entirely local effect or a result of systemic immunological changes due to L. gasseri ingestion, we measured the changes in serum antibody concentration and cytokine production from culture supernatants of mite-allergen-stimulated splenocytes. While mite-allergen-sensitised mice produced a large amount of total IgE and Der p-specific IgG1 antibodies, compared to naïve mice, oral administration of L. gasseri did not significantly change these two antibodies, compared to Der p-sensitised mice, except that there was a significant increase in concentration of Der p-specific IgG2a antibody in the serum of high-dose L. gasseri-treated mice (Fig. 4(a)). There was also no significant change of Th1 (interferon-γ) and Th2 (IL-4) cytokines production in the culture supernatant of mite-allergen-stimulated splenocytes from L. gasseri-treated mice as compared to non-treated sensitised mice. In contrast, The IL-10 cytokine production was significantly increased in both high- and middle-dose-treated mice than in non-treated sensitised mice (P < 0·05, Fig. 4(b)).
Oral administration of Lactobacillus gasseri suppresses IL-17 production in murine splenocytes
To know the regulatory effects of L. gasseri on Th17 immune response in mite-allergen-sensitised and -challenged mice, we first examined its effect on IL-17 production in cultured splenocytes. The addition of TGF-β plus IL-6 drastically enhanced IL-17 production in Der p-sensitised mice as compared to naive mice (990·6 (sem 128·9) v. 467·7 (sem 72·3) pg/ml, P < 0·05). Addition of Der p-allergen (10 μg/ml) into the culture condition significantly increased IL-17 production from splenocytes of Der p-sensitised mice (1594·7 (sem 164·5) v. 990·6 (sem 128·9) pg/ml, P < 0·05), but not from naive mice. When heat-killed L. gasseri was added to the culture condition, there was a significant reduction of IL-17 production in both Der p-stimulated and Der p-unstimulated splenocytes (Fig. 5(a)). The suppressive effect of L. gasseri on IL-17 production was also found in the L. gasseri-treated, mite-sensitised and -challenged mice. Splenocytes collected from the high- and middle-dose L. gasseri-treated mice had significantly lower production of IL-17 when stimulated with Der p as well as combined stimulations with heat-killed L. gasseri (Fig. 5(b)).
Lactobacillus gasseri decreased IL-17-producing alveolar macrophages in allergic lung inflammation
Previously, it has been suggested that macrophages rather than Th17 cells are the main producers of IL-17 in allergic inflammation related to asthma(Reference Song, Luo and Lei25). Therefore, it is imperative to know whether the ability of L. gasseri to decrease IL-17 cytokine production in BAL fluid in mite-sensitised and -challenged mice was related to the change of IL-17-producing alveolar macrophages (AM). Fig. 6(a) shows the levels of cytokine production of stimulated and non-stimulated AM collected from mite-sensitised and -challenged mice. Der p-allergen-stimulated AM produced increasing amounts of IL-17 and IL-23p19 and decreased levels of IL-10 than non-stimulated AM did. In contrast, heat-killed L. gasseri (multiplicity of infection 10) significantly increased production of IL-12 and decreased amounts of IL-17 and IL-23p19 as compared to Der p-stimulated AM. Moreover, we found a significantly increased percentage of IL-17-producing AM, which showed CD11b+/IL-17A+ in cytometry analysis (Fig. 6(b)), in BAL fluid in mite-sensitised and -challenged mice as compared to naive mice (Fig. 6(c)). In contrast, oral administration of high dose of L. gasseri significantly decreased the percentage of IL-17-producing AM in BAL fluid as compared to non-treated sensitised and challenged mice (Fig. 6(c)).
Discussion
In the present study, we hypothesised that L. gasseri is able to attenuate allergen-induced airway inflammation as well as decrease Th17 pro-inflammatory response in the murine model of asthma, which may explain the beneficial response of probiotics supplement in the prevention of allergic diseases and asthma. Accumulating data suggest that IL-17 or Th17 plays an important role in the development of various allergic diseases that have classically been considered to be Th1- or Th2-mediated disorders(Reference Oboki, Ohno and Saito26). Although it was speculated that IL-17 may have a negative effect on the established allergic asthma(Reference Schnyder-Candrian, Togbe and Couillin27), IL-17/IL-17R signalling has been proved to be a critical requirement for the development of allergic asthma(Reference Nakae, Komiyama and Nambu28). The concentration of IL-17 was significantly increased in BAL fluids, sputum and blood from patients with asthma(Reference Molet, Hamid and Davoine29, Reference Barczyk, Pierzchala and Sozanska30). In a mouse model of allergic airway inflammation, systemic blockade of IL-17 inhibits the allergen-induced accumulation of neutrophils in the airway(Reference Hellings, Kasran and Liu31). These findings suggest that IL-17 plays an important role in the pathophysiological process of allergic asthma. Despite the important role of IL-17 in allergic asthma, the development and function of CD4+Th17 cells and its relationship with Th2 cytokine response in allergic asthma have not yet been fully identified, hindering the further elucidation of the mechanisms underlying the onset of allergic asthma(Reference Schmidt-Weber, Akdis and Akdis32).
Our data demonstrated that oral administration of L. gasseri is able to alleviate allergen-induced airway inflammation in mite-sensitised and -challenged mice. The L. gasseri-treated mice resulted in significant attenuation of inflammatory cell influx to the lung and airway responsiveness to methacholine (Fig. 1). The decreases in cellular influx and airway hyper-responsiveness were associated with reduced levels of inflammatory, Th2 chemokine (TNF-α, TARC) and Th17 cytokine (IL-17A), as well as increased production of Th1 (IL-12) and inducible regulatory T cell response (IL-10) in BAL fluids (Figs. 2 and 3). These findings are similar to recent studies showing that LAB decrease the Th2 type CD4+T cell responses to sensitised allergen in a mouse model of asthma(Reference Feleszko, Jaworska and Rha33–Reference Forsythe, Inman and Bienenstock35). However, the immunomodulation mechanism or the immunological target cells of LAB for the beneficial effect on allergic disease model animals is apparently not confined to the simple notion of the restoration of the Th1/Th2 balance. Particularly, our study of oral administration of L. gasseri did not result in a decrease of serum total IgE and IL-4 levels or an increase of interferon-γ production from Der p-stimulated splenocytes collected from L. gasseri-treated mice (Fig. 4).
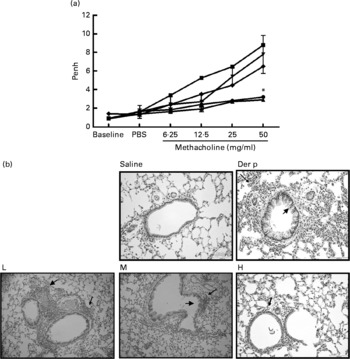
Fig. 1 (a) The airway hyper-responsiveness (AHR) in Dermatophoides pteronyssinus (Der p, ![]() )-sensitised mice treated with different doses of Lactobacillus gasseri. Measurements of AHR were using whole-body plethysmography in unrestrained mice after airway exposure to increased concentrations of methacholine. Values are means with their standard errors represented by vertical bars (n 6). * Mean values were significantly different from those of the Der p group (P < 0·05).
)-sensitised mice treated with different doses of Lactobacillus gasseri. Measurements of AHR were using whole-body plethysmography in unrestrained mice after airway exposure to increased concentrations of methacholine. Values are means with their standard errors represented by vertical bars (n 6). * Mean values were significantly different from those of the Der p group (P < 0·05). ![]() , Saline. (b) Histopathologic examination of representative lungs taken from mice treated with different doses (low (L,
, Saline. (b) Histopathologic examination of representative lungs taken from mice treated with different doses (low (L, ![]() ), 1 × 106 colony-forming units (CFU); medium (M,
), 1 × 106 colony-forming units (CFU); medium (M, ![]() ), 2 × 106 CFU; high (H,
), 2 × 106 CFU; high (H, ![]() ), 4 × 106 CFU) of L. gasseri. Mice were killed 3 d after Der p challenge. Lung sections were prepared and stained with haematoxylin and eosin (400 × magnification). Arrows indicate inflammatory cells infiltrated in the parenchyma and arrowheads represent damaged epithelial cells in the bronchi.
), 4 × 106 CFU) of L. gasseri. Mice were killed 3 d after Der p challenge. Lung sections were prepared and stained with haematoxylin and eosin (400 × magnification). Arrows indicate inflammatory cells infiltrated in the parenchyma and arrowheads represent damaged epithelial cells in the bronchi.
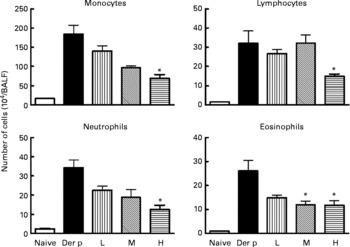
Fig. 2 Differential cell counts of bronchoalveolar lavage fluid (BALF) from different doses (low (L), 1 × 107 colony-forming units (CFU); medium (M), 1 × 108 CFU; high (H), 1 × 109 CFU) of Lactobacillus gasseri-treated and non-treated Dermatophoides pteronyssinus (Der p)-sensitised and -challenged mice. The cells in BALF were collected 3 d after intra-tracheal challenge of 50 μl (1 mg/ml) Der p. Values are means with their standard errors represented by vertical bars (n 6). * Mean values were significantly different from those of the Der p group (P < 0·05).
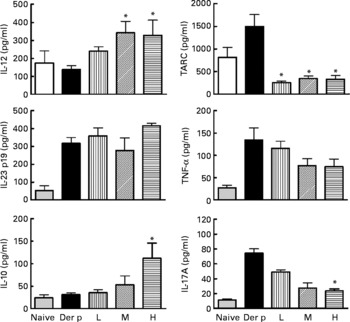
Fig. 3 Cytokines production in bronchoalveolar lavage fluid (BALF) of naive and different doses of Lactobacillus gasseri-treated and non-treated Dermatophoides pteronyssinus (Der p)-sensitised and -challenged mice. BALF was collected 3 d after Der p challenge and cytokine levels were measured using the respective ELISA kits. Values are means with their standard errors represented by vertical bars (n 6). * Mean values were significantly different from those of the Der p group (P < 0·05). TARC, thymus and activation-regulated chemokine; L, low; M, medium; H, high.
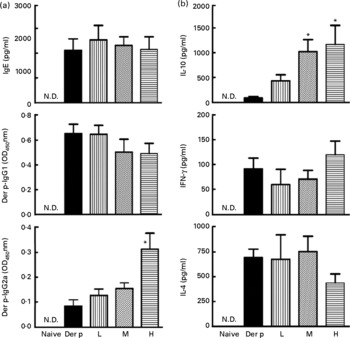
Fig. 4 (a) Total IgE, Dermatophoides pteronyssinus (Der p)-IgG1 and Der p-IgG2a in sera from naive mice and Der p-sensitised mice treated with different doses of Lactobacillus gasseri. Sera from all groups were collected 3 d after allergen challenge and IgE and IgG concentrations were detected by ELISA. Values are means with their standard errors represented by vertical bars (n 6). * Mean values were significantly different from those of the Der p group (P < 0·05). (b) Interferon-γ (IFN), IL-4 and IL-10 productions from Der p-stimulated splenocytes of mice treated with different doses of L. gasseri. Mice splenocytes were collected 3 d after Der p challenge and incubated with 10 μg/ml Der p for 48 h. Culture supernatants were assayed by ELISA. Values are means with their standard errors represented by vertical bars (n 6). * Mean values were significantly different from those of the Der p group (P < 0·05). L, low; M, medium; H, high; N.D., not detectable.
Previous studies(Reference Reid, Jass and Sebulsky36, Reference Matsuzaki, Hashimoto and Yokokura37) have shown strong immunomodulatory properties of lactobacilli, which include anti-inflammatory and anti-tumour activity, modulation of autoimmune diseases and prevention of infections. LAB was also demonstrated in the treatment of inflammatory disorders like pouchitis(Reference Mimura, Rizzello and Helwig38), ulcerative colitis(Reference Bibiloni, Fedorak and Tannock39) and experimental arthritis in mice(Reference Soa, Lee and Kwon40), which are hallmarks of Th17-mediated inflammatory diseases. Our data have clearly demonstrated that live L. gasseri suppressed IL-17 production both in TGF-β and in IL-6-stimulated splenocytes collected from Der p-sensitised mice (Fig. 5) and decreased IL-17-producing AM in the lungs of treated mice (Fig. 6). Th17 cells are generated from naive CD4+T cells, and Th17 cell differentiation is driven by stimuli such as TGF-β plus IL-6(Reference Oboki, Ohno and Saito26). Indeed, allergen-specific stimulation alone was not enough to induce splenocytes from allergen-sensitised mice to produce IL-17 cytokine (data not shown). In contrast, TGF-β plus IL-6 combined with Der p-allergen induced a remarkable enhancement of IL-17 production in Der p-sensitised murine splenocytes (Fig. 5), representing the ongoing systemic Th17 immune response that resulted in allergen-induced airway inflammation in sensitised and challenged mice.
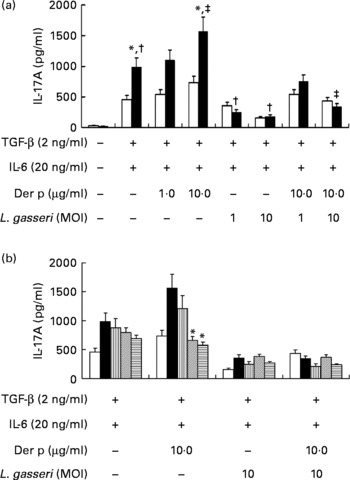
Fig. 5 (a) Effects of Lactobacillus gasseri on IL-17A production in murine splenocytes. Spleens were removed from naive (□) and Dermatophoides pteronyssinus (Der p, ■)-sensitised and -challenged mice (n 6), and the pooled splenocytes (1 × 106 cells) were incubated with transforming growth factor (TGF)-β (2 ng/ml) plus IL-6 (20 ng/ml). Different doses of Der p and heat-killed L. gasseri were added, respectively, or combined into culture conditions. Culture supernatants (n 3) were harvested, pooled and assayed for IL-17A concentrations using an ELISA kit. * Mean values were significantly different from those of the conditional medium without Der p or L. gasseri (P < 0·05). † Mean values were significantly different from those of the conditional medium without Der p or L. gasseri (P < 0·05). ‡ Mean values were significantly different from those of the conditional medium with Der p alone (P < 0·05). (b) Effects of L. gasseri on IL-17A production in murine splenocytes removed from naive and different doses (low (L), ![]() ; medium (m),
; medium (m), ![]() ; high (h),
; high (h), ![]() ) of L. gasseri-treated or non-treated Der p-sensitised and -challenged mice (n 6). * Mean values were significantly different from those of the Der p group (P < 0·05). MOI, multiplicity of infection.
) of L. gasseri-treated or non-treated Der p-sensitised and -challenged mice (n 6). * Mean values were significantly different from those of the Der p group (P < 0·05). MOI, multiplicity of infection.

Fig. 6 (a) Cytokine production in Dermatophoides pteronyssinus (Der p) or Lactobacillus gasseri (L.g.)-stimulated alveolar macrophages (AM). The AM were isolated and pooled from Der p-sensitised and -challenged mice (n 6), and analysed as described in the Materials and methods section. Mononuclear cells from bronchoalveolar lavage fluid were stained for the analysis of CD3, CD11b, F4/80 and IL-17. The CD3− cells were gated. Among them, the CD11b+IL-17+cells were further gated for the analysis of F4/80. The result of (b) flow cytometric analysis and the (c) percentage of IL-17A-producing macrophages in AM of different doses of L.g.-treated or non-treated mice were shown. * Mean values were significantly different from those of the Der p group (P < 0·05). MOI, multiplicity of infection.
In general, Th17 cells are abundant at steady state in gut-associated tissues, particularly in the small-intestinal lamina propria. In the absence of a luminal commensal microbiota, the numbers of Th17 cells are significantly reduced(Reference Ivanov, Rde and Manel41). Recently, it has been found that IL-17, a cytokine required for the initiation of allergic inflammation in a mouse model mimicking allergic asthma, was mainly produced by CD11b+AM, rather than Th17, natural killer (NK) cells or others(Reference Song, Luo and Lei25). Our present study also confirmed that there was a significant increase of production of IL-17 as well as cell number of CD11b+/IL-17+AM (Fig. 6), but not CD4+Th17 cells (data not shown), in the BAL fluids of sensitised and challenged mice. The decrease of IL-17-producing AM in L. gasseri-treated mice was also accompanied with the increase of IL-10 production in the culture supernatant of stimulated AM, which is suggested to be a negative regulator for IL-17-producing AM(Reference Song, Luo and Lei25). Nevertheless, there was also a possibility for L. gasseri to interact with antigen-presenting cells in the intestine, then regulating the development of natural regulatory CD4+Foxp3+T cells that may limit the expansion of Th17 cells(Reference Cho, Choi and Chung42, Reference Karimi, Inman and Bienenstock43).
Finally, it should be noted that regulation of IL-17 or Th17 immune response by LAB, particularly in allergic inflammation, is complicated by the involvement of many different immune cells and mediators. One study showed that ATP produced by commensal bacteria can drive the differentiation of Th17 cells(Reference Atarashi, Nishimura and Shima44). In the large intestine, commensal-dependent expression of IL-25 by intestinal epithelial cells limits the expansion of Th17 cells(Reference Zaph, Du and Saenz45). Another investigation found that mice deficient in toll-like receptor 9, a receptor that can interact with commensal DNA, have reduced numbers of IL-17-producing and interferon-γ-producing effector T cells(Reference Hall, Bouladoux and Sun46). Many mechanisms of the inhibitory effect of probiotics for IL-17 cytokine response still remain to be discovered.
In conclusion, we have found that oral administration of L. gasseri in Der p-allergen-sensitised and -challenged mice resulted in significant attenuation of major characteristics of an asthmatic response, including airway inflammatory cell influx, local cytokine responses and airway hyper-reactivity. Our results also indicate that the immune-regulatory effects of oral treatments with L. gasseri are confined not only to the restoration of Th1/Th2 immune balance, but also to the inhibitory effects of systemic IL-17 response and local IL-17-producing AM. However, further studies are required to explore the mechanisms of anti-allergic effects of specific strains of LAB and to identify specific components of bacteria–host interaction that confer potential beneficial immune response, which may aid in the design or therapeutic use of clinically effective agents in the prevention of allergic asthma.
Acknowledgements
The present work was supported by Chi-Mei Hospital and National Cheng Kung University Collaboration Project, grants nos. CMNCKU-9812, -9905, -10014 and National Science Councils, Taiwan, grant no. NSC 98-2622-8-006-003-C1. R.-L. J., K.-C. Y. and J.-Y. W. designed the research; K.-C. Y., M.-H. H., Y.-L. L. and H.-F. K. conducted the research; R.-L. J. and Y.-S. C. analysed the data; R.-L. J., P.-H. L. and J.-Y. W. wrote the paper; J.-Y. W. had primary responsibility for the final content. All authors read and approved the final manuscript. The authors declare that there are no conflicts of interest.








