Age-related cataract and maculopathy are the leading causes of blindness among an elderly population worldwide(Reference Chiu and Taylor1). It is predicted that the number of people with age-related eye diseases and subsequent visual disorders will increase dramatically during the next 20 years in US populations and in the Western world as well, because of increasing life expectancy(Reference Congdon, O'Colmain and Klaver2). For instance, prevalence of cataract in the Finnish population is 10 % for ages < 30 years and 34 % for >30 years(Reference Laitinen, Laatikainen and Harkanen3).
Cataracts can be defined as clouding of the lens in the eye due to clumping of lens protein and coloration of the lens to a brownish shade because of age, smoking, sunlight exposure, use of oral corticosteroids, oestrogen replacement therapy and diabetes(4). Nuclear cataract is the most common type of cataract among the older population and especially women(Reference Vashist, Talwar and Gogoi5). The pathogenesis of nuclear cataract is caused by the accumulated stressors resulting from the inability to sufficiently defend against or repair the damage due to a variety of environmental stressors, including photochemical formation of free radicals(Reference Moeller, Voland and Tinker6). Reactive oxygen species can damage lens proteins and fibre cell membranes, leading to cataract formation(Reference Spector7). Lutein and zeaxanthin are the most abundant carotenoids that accumulate in the lens of the eye, where they possibly filter phototoxic blue light and neutralise reactive oxygen species(Reference Yeum, Shang and Schalch8).
Only two studies have found statistically significant results to support the hypothesis that increased β-carotene intake or supplement use is related to diminished risk of nuclear cataract(Reference Mares-Perlman, Brady and Klein9, Reference Jacques, Chylack and Hankinson10). In addition, an inverse relationship between α-carotene intake or plasma concentrations and the risk of nuclear cataract has been observed(Reference Mares-Perlman, Brady and Klein9, Reference Gale, Hall and Phillips11). Also, high plasma concentrations of lycopene may protect against age-related cataract development(Reference Gale, Hall and Phillips11, Reference Dherani, Murthy and Gupta12). Furthermore, significant inverse associations have been found for plasma α- and β-carotene, and β-cryptoxanthin(Reference Dherani, Murthy and Gupta12).
In a previous cross-sectional study, risk of posterior subcapsular cataract was lowest in those with highest concentrations of lutein(Reference Gale, Hall and Phillips11). Inverse associations have been found between lutein and zeaxanthin concentrations and cataracts (nuclear and cortical) in a North Indian population(Reference Dherani, Murthy and Gupta12). Both plasma and diet rich in lutein and zeaxanthin were associated with a decreased prevalence of nuclear cataract among older American women(Reference Moeller, Voland and Tinker6) and the Australian population(Reference Vu, Robman and Hodge13). In a longitudinal study, plasma zeaxanthin was significantly associated with a reduced risk of nuclear cataract and any cataract(Reference Delcourt, Carriere and Delage14). However, some other previous studies based on dietary intake or blood concentrations of lutein and/or zeaxanthin have not supported the protective role against cataracts (nuclear, cortical and posterior subcapsular cataract)(Reference Gale, Hall and Phillips11, Reference Valero, Fletcher and De Stavola15–Reference Lyle, Mares-Perlman and Klein17).
There are no intervention studies that looked at the effect of lutein and zeaxanthin supplementation and cataract incidence. There are only cross-sectional and longitudinal studies that looked at dietary intake of lutein and zeaxanthin or plasma lutein and zeaxanthin concentrations and cataract incidence. The Food and Drug Administration determined that scientific conclusions about the relationship between lutein or zeaxanthin intake and the risk of cataracts could not be drawn from these twelve studies(Reference Trumbo and Ellwood18).
The aim of the present study was to examine whether plasma concentrations of lutein and zeaxanthin are associated with the risk of age-related nuclear cataract in an Eastern Finnish elderly population as shown previously.
Experimental methods
Study population
The subjects of the present study were participants of the Kuopio Ischaemic Heart Disease Risk Factor (KIHD) Study. The KIHD Study is an ongoing population-based, cohort study that was designed to investigate risk factors for CVD and other degenerative disease in a sample of middle-aged men and women in the city of Kuopio, Finland, and its rural communities(Reference Salonen19). The study protocol was approved by the Research Ethics Committee of the University of Kuopio and each participant gave written informed consent.
In the present study, 2340 eligible subjects were screened (1557 men and 783 women), of which 396 refused to participate and seventy could not be contacted. Of the remaining 1874 participants, concentrations of carotenoids were available for 1689 subjects (559 women and 1130 men). Number of women and men were not equal in the present study, because smaller numbers of women were recruited at the baseline study that was conducted during the years 1998–2001. The present study was carried out during the years 2005–2008. Nuclear cataracts diagnosed during the study were included in the statistical analysis.
Sample collection and laboratory analyses
Subjects fasted for 12 h and refrained from consuming alcohol for 2 d before blood sampling. Fasting venous blood samples were collected during the years 2005–2008 into vacuum tubes (Terumo, Leuven, Belgium) without tourniquet, after the subject had rested in the laying position for 5 min. The samples were collected during the years 2005–2008. The diagnoses of cataract were made during the follow-up years. Blood for carotenoids was collected in lithium heparin tubes and plasma was separated immediately after centrifugation in dark screw-capped tubes and stored at − 70°C until analysis.
Concentrations of plasma α-tocopherol, retinol, lycopene, α-carotene, β-carotene lutein, zeaxanthin and β-cryptoxanthin were analysed with the reversed-phase HPLC method(Reference Karppi, Nurmi and Olmedilla-Alonso20). The frozen Li-heparin plasma samples were thawed at room temperature, homogenised and 200 μl were pipetted into borosilicate glass tubes. Then, 500 μl of ethanol (Altia Corporation, Helsinki, Finland) and 0·01 % (w/v) butylated hydroxytoluene (Fluka Corporation, Buchs SG, Switzerland) containing α-tocopherol acetate (Sigma-Aldrich Corporation, St Louis, MO, USA) and β-apo-8′-carotenal (Fluka Corporation) as internal standards were added to the samples. The volume was diluted to 1 ml with ultra pure water (Millipore Corporation, Milford, MA, USA). After mixing with a vortex, 2 ml of hexane (Rathburn Chemicals Limited, Walkerburn, Scotland) and 0·01 % (w/v) butylated hydroxytoluene solution were added to the samples and mixed. After extraction, 500 μl of ultra pure water were added into the samples. The samples were centrifuged at 1500 g for 5 min at 4°C and frozen at − 70°C. After separation of the hexane layer, the samples were evaporated to dryness using a Techne Sample Concentrator (Techne, Cambridge, UK) under a gentle stream of N2 at room temperature. The dried residue was reconstituted in 200 μl of the mobile phase and a volume of 50 μl was injected into a pair of Synergy Hydro-RP 80A 4 μm (150 × 4·6 mm) columns (Phenomenex, Torrance, CA, USA) on a Shimadzu system (Shimadzu Corporation, Kyoto, Japan) and a Beckman 168 diode-array detector (Beckman Coulter, Inc., Fullerton, CA, USA). The inter-assay CV were 5·8 % for lutein and 7·6 % for zeaxanthin (n 107). Limits of detection were 0·01 μmol/l for both carotenoids. Values below the limit of detection of the method were marked as 0·00 in the statistical analysis.
Concentrations of serum total cholesterol, LDL-cholesterol and TAG were analysed with enzymatic methods (Thermo Fisher Scientific, Vantaa, Finland). Serum HDL-cholesterol was measured from the supernatant after magnesium chloride dextran sulphate precipitation with enzymatic methods (Thermo Fisher Scientific)(Reference Rissanen, Voutilainen and Nyyssönen21).
Other measurements
BMI was calculated as weight (kg)/height (m2). Resting blood pressure was measured in the morning by a trained nurse with a random-zero mercury sphygmomanometer (Hawksley, Lancing, UK). After the subjects had rested for 5 min, three measurements were taken at 2 min intervals while the subjects were sitting. The mean of all three measurements was used to calculate systolic and diastolic blood pressure. Alcohol consumption was assessed with a structured quantity-frequency method on drinking behaviour over the previous 12 months. The lifelong exposure to smoking (cigarette pack-years) was estimated as the product of the number of smoking years and the number of tobacco products smoked daily at the time of examination(Reference Laukkanen, Laaksonen and Niskanen22). Education was coded into three categories based on years of education (less than 6 years (n 295), 7–11 years (n 1007) and 12 or more years (n 385)). Subjects were grouped based on educational levels (high school or less, high school and college or postgraduate). Medications were asked by a self-administered questionnaire, and checked by the interviewer. Hypertension was confirmed by current use of antihypertensive medication and/or systolic blood pressure >165 mmHg and/or diastolic blood pressure >95 mmHg. Diabetes mellitus was assessed by previous diagnosis or fasting blood glucose concentration >6·7 mmol/l(Reference Laukkanen, Laaksonen and Lakka23).
Cataract diagnoses
The cataract diagnoses (lens photography and grading) were made by an ophthalmologist. All cataract diagnoses that occurred between study entry and 31 December 2008 were included. Data on cataract events were obtained by record linkage from the national computerised hospitalisation registry, which covers every hospitalisation in Finland. Data on vital status were obtained from Statistics Finland.
Statistical methods
Descriptive data are presented as means and standard deviations. Means of the subjects were compared using ANOVA. Correlations were assessed using Pearson's correlation coefficients. Linear trends were performed using the means option. Subjects were classified into tertiles according to their plasma concentrations of lutein and zeaxanthin. The relative risks (RR) and 95 % CI for age-related nuclear cataract in tertiles of plasma concentrations of lutein and zeaxanthin were estimated by using the Cox proportional hazards model. The following two different sets of covariates were used: (1) age and examination year; (2) age, examination year, sex, BMI, smoking, alcohol consumption, serum LDL-cholesterol, serum HDL-cholesterol, years of education, use of oral corticosteroids, history of diabetes and history of hypertension with current use of antihypertensive medication. Tests for statistical significance were two-sided and differences with P < 0·05 were considered statistically significant. Statistical analyses were performed using SPSS software (version 14.0; SPSS, Inc., Chicago, IL, USA).
Results
Subject characteristics
A total of 113 cases of incident age-related cataracts were confirmed, of which 108 cases were nuclear cataracts and the rest were unclassified incipient cataracts among 61–80-year-old subjects. Subjects with nuclear cataract were older, had lower education, consumed less alcohol, and their plasma zeaxanthin and lycopene concentrations were significantly lower compared with subjects without nuclear cataract. Other characteristics did not differ between the groups (Table 1). The 4-year nuclear cataract incidence among 1689 subjects was 6·4 % in the present study population. The mean plasma lutein and zeaxanthin concentrations were 0·24 and 0·034 μmol/l in subjects with nuclear cataract and 0·25 and 0·039 μmol/l in subjects without nuclear cataract, respectively. (mean 0·25 (sd 0·10) μmol/l for lutein and 0·038 (sd 0·018) μmol/l for zeaxanthin, in the whole population). Nuclear cataracts occurred significantly more in males (7 %) than in females (4 %) (P = 0·013). Concentrations of carotenoids, fat-soluble vitamins (retinol and α-tocopherol), total cholesterol, LDL-cholesterol, HDL-cholesterol and BMI were significantly higher in females than in males. Females had higher education (P < 0·001), smoked more (P = 0·001) and consumed alcohol less (P < 0·001) than males.
Table 1 Characteristics of the study population with and without age-related nuclear cataract (n 1689 men and women)
(Mean values and standard deviations)

* P for differences between those with and without nuclear cataract (one-way ANOVA).
Pearson's correlations
Association with plasma lutein, zeaxanthin and risk factors was evaluated. Lutein and zeaxanthin correlated inversely with age ( − 0·05 and − 0·11), alcohol intake ( − 0·08, not statistically significant for zeaxanthin), BMI ( − 0·23 and − 0·13), diabetes ( − 0·15 and − 0·12), hypertension ( − 0·10 and − 0·08), pack-years smoking ( − 0·13 and − 0·08) and serum TAG ( − 0·09 and − 0·06). Correlations were positive with years of education (0·09 and 0·12), use of oral corticosteroids (0·06 and 0·05), serum total cholesterol (0·28 and 0·22), serum HDL-cholesterol (0·26 and 0·21) and LDL-cholesterol (0·21 and 0·17). Carotenoids correlated significantly with each other and with α-tocopherol. Correlations varied from 0·14 to 0·79. Smokers had significantly lower concentrations of plasma carotenoids except for lycopene than non-smokers (lutein: r − 0·13; zeaxanthin: r − 0·11). BMI was inversely related to these carotenoids (P < 0·001) and total cholesterol (P = 0·003), and positively to serum TAG (P < 0·001) and alcohol intake (P = 0·011). Alcohol intake was positively correlated with smoking (P < 0·001). All correlations were statistically significant.
Relationship between nuclear cataract and risk factors
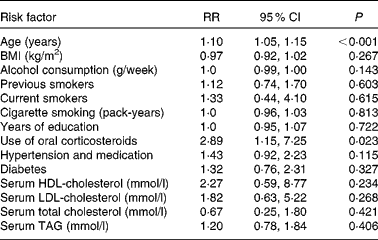
Relationships between nuclear cataract and risk factors are described in Table 2. Age increased the risk of nuclear cataract by 10 % (P < 0·001). Age was positively associated with nuclear cataract. Use of oral corticosteroids was the most important risk factor, since it increased the risk nearly 3-fold (P = 0·023). Other factors had no effect on the risk of nuclear cataract.
Table 2 Risk factors of age-related nuclear cataract using the Cox proportional hazards model
(Relative risks (RR) and 95 % confidence intervals, n 1689)
Association between plasma lutein and zeaxanthin and nuclear cataract
The risk estimates of nuclear cataract in relation to plasma lutein and zeaxanthin are described in Table 3. After adjustment for age, examination year, sex, BMI, smoking, alcohol consumption, serum LDL-cholesterol, serum HDL-cholesterol, years of education, use of oral corticosteroids, history of diabetes and history of hypertension with current use of antihypertensive medication, subjects in the highest tertiles of plasma concentrations of lutein and zeaxanthin had 42 and 41 % lower risks of nuclear cataract compared with those in the lowest tertiles (RR = 0·58, 95 % CI 0·35, 0·98; P = 0·041 for lutein and RR = 0·59, 95 % CI 0·35, 0·99; P = 0·046 for zeaxanthin).
Table 3 Age-related nuclear cataract according to tertiles of plasma concentrations of lutein and zeaxanthin using the Cox proportional hazards model, the Kuopio Ischaemic Heart Disease Risk Factor Study
(Relative risks (RR) and 95 % confidence intervals, n 1689)
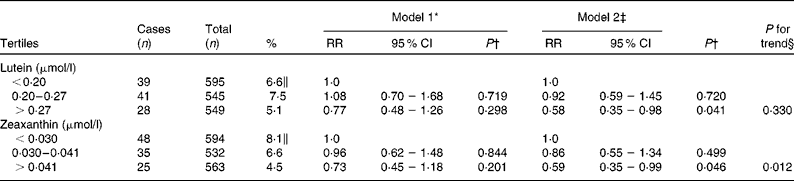
* Adjusted for age and examination year.
† P for association of the highest tertile compared with the lowest tertile.
‡ Adjusted for age, examination year, sex, BMI, smoking, intake of alcohol, serum LDL-cholesterol, HDL-cholesterol, years of education, use of oral corticosteroids, diabetes and hypertension with current use of antihypertensive medication.
§ P for trend across tertiles.
∥ Proportion of cases in each tertile.
Discussion
The present cross-sectional study showed an inverse association between concentrations of plasma lutein and zeaxanthin and the risk of nuclear cataract in an elderly population. This finding was consistent with previous epidemiological studies(Reference Moeller, Voland and Tinker6, Reference Dherani, Murthy and Gupta12–Reference Delcourt, Carriere and Delage14).
In the Carotenoids in Age-Related Eye Disease cross-sectional study, women in the highest quintile of diet or serum lutein and zeaxanthin had a 32 % lower risk for nuclear cataract compared with those in the lowest quintile(Reference Moeller, Voland and Tinker6). In a North Indian cross-sectional study, higher serum zeaxanthin concentration was related to a decreased risk of nuclear cataract(Reference Dherani, Murthy and Gupta12). In the Pathologies Oculaires Liées à l'Age study (longitudinal), an inverse association between plasma concentrations of zeaxanthin and nuclear cataract has been observed(Reference Delcourt, Carriere and Delage14). Furthermore, in the Melbourne Visual Impairment cross-sectional project, an inverse association between high dietary intake of lutein, zeaxanthin and the prevalence of nuclear cataract has been observed(Reference Vu, Robman and Hodge13). Some other cross-sectional and longitudinal studies did not observe any association(Reference Gale, Hall and Phillips11, Reference Valero, Fletcher and De Stavola15–Reference Lyle, Mares-Perlman and Klein17). However, in a recent review, the Food and Drug Administration suggested that there were no intervention or observational studies from which scientific conclusions could be drawn about the association with the intake of lutein or zeaxanthin and the risk of cataracts. Therefore, the Food and Drug Administration concluded that there was no credible evidence to support the protective effect of lutein or zeaxanthin intake and the risk of cataracts(Reference Trumbo and Ellwood18). Lutein and/or zeaxanthin concentrations in the highest tertiles were lower in the present study population compared with France and Spain (inverse associations)(Reference Delcourt, Carriere and Delage14, Reference Olmedilla, Granado and Blanco24) and slightly higher than in the UK (inverse association)(Reference Gale, Hall and Phillips11). Some previous studies have used dietary measures of carotenoids(Reference Valero, Fletcher and De Stavola15, Reference Rodriguez-Rodriguez, Ortega and Lopez-Sobaler25). Therefore, it is difficult to compare the present results with other European studies. However, mixed results of previous studies may not exclusively be explained by the different blood concentrations. Other reasons for inconsistent results may be the study design, population, variations and levels of dietary intake or plasma carotenoid values.
In the present study, carotenoids were analysed from plasma samples. There are several advantages of blood analysis. Measurements provide an objective analysis of carotenoid status and usual problems of diet questionnaires such as recall or biased responses are avoided(Reference Dherani, Murthy and Gupta12). Serum concentrations of lutein and zeaxanthin have been associated with nuclear cataract in longitudinal but not cross-sectional studies(Reference Moeller, Voland and Tinker6). Reasons for this may be errors, and bias related to possibly recent diet changes, because serum carotenoid concentrations reflect intake over a week or two and not for a longer time (e.g. intake over a month)(Reference Moeller, Voland and Tinker6). Carotenoids are eliminated from the blood circulation within several days. For instance, elimination half-time of lutein is about 10 d(Reference de Moura, Ho and Getachew26) and zeaxanthin 12 (sd 7) d, respectively(Reference Hartmann, Thurmann and Spitzer27).
We assume that the low concentrations of plasma lutein and zeaxanthin preceded the development of nuclear cataract. Carotenoid concentrations were measured during the years 2005–2008. It is conceivable that subjects did not change their diet because of knowledge of cataract, since elderly people may have little knowledge about the possible role of diet rich in antioxidants in the prevention of cataract. There were no subjects taking carotenoid supplements in our cohort.
Lutein and zeaxanthin correlated highly with each other. The present results show that the association of plasma lutein was as strong as that of zeaxanthin with the risk of nuclear cataracts. This is consistent with a previous study that looked at the risk between serum zeaxanthin concentration and nuclear cataract(Reference Dherani, Murthy and Gupta12). However, the hypothesis of a more important role of zeaxanthin in lens health is proved by several lines of evidence. The ratio of zeaxanthin:lutein is much higher in the lens(Reference Bernstein, Khachik and Carvalho28) than in the plasma, suggesting that the lens of the eye mainly accumulates zeaxanthin. Both lutein and zeaxanthin protect liposomal membranes from light-induced oxidative stress. Zeaxanthin appears to be a more effective protector against UV light exposure, because lutein and zeaxanthin may be oriented differently in biological membranes(Reference Sujak, Gabrielska and Grudzinski29). In addition, zeaxanthin is also especially very effective in protecting lipid membranes against peroxyl radical oxidation(Reference Woodall, Britton and Jackson30).
The rapid and large increase in the risk of cataract has been observed with ageing(4). There is a growing consensus that smoking increases the risk of nuclear cataract. Smoking may increase oxidative stress by decreasing of circulating antioxidant nutrients (e.g. lutein and zeaxanthin) and causing lens damage(4). Use of oral or inhaled corticosteroids is known to increase the risk of nuclear cataract(Reference Wang, Rochtchina and Tan31). Diabetes may cause retinopathy that is associated with the increased risk of cataract. In the present study as in other studies, the relationship persists even after adjustment for potential confounders, such as smoking, alcohol use and diabetes, which have been associated with both educational status and cataract. These results suggest that there are unknown confounding factors associated with both educational level and lens opacity severity(4). The present results show that high concentrations of lutein and zeaxanthin may reduce harmful effects of these risk factors and thereby decrease the risk of nuclear cataract. The relationship between plasma lutein and zeaxanthin and each of the risk factors was examined. It was observed that age, BMI, smoking, alcohol intake and diseases (e.g. diabetes and CVD) decrease the plasma levels of lutein and zeaxanthin because of oxidative stress. Oxidative stress is an imbalance, which produces free radicals that overwhelm the body's antioxidant defences, and high levels of oxidative stress are known to deplete the body's reserves of antioxidants(Reference Coyne, Ibiebele and Baade32). Oxidative stress has been implicated in the pathogenesis of several components of the metabolic syndrome including glucose or insulin abnormalities(Reference Redon, Oliva and Tormos33), hypertension and obesity(Reference Furukawa, Fujita and Shimabukuro34). Lutein and zeaxanthin correlated positively with serum lipoproteins, as in the blood circulation, carotenoids are found primarily in LDL and HDL(Reference Krinsky and Johnson35).
The strength of the study is the precise follow-up system of the Finnish population. Also, we collected information for many confounders. The present study is different from other studies, because it is a large cohort (total n 2691) and contains a low number of patients with nuclear cataract. However, an inverse association between plasma lutein, zeaxanthin and the risk of nuclear cataract was observed. In addition, the KIHD Study was not designed to investigate ophthalmological diseases. One limitation of the present study is that the information on cataract was collected from medical records: the possibility of bias due to attendance at health care should be considered, i.e. people with hypertension are more likely to be frequent attendees and therefore more likely to be referred for cataract. Other limitations of the present study might be that we measured only a single plasma sample, which describes carotenoid concentrations at the time of the blood sampling. Multiple time-point measurements would have resulted in a more precise estimate. The lack of nutrient intake data and the cross-sectional nature of the present findings are also weaknesses. In addition, the measurement of vitamin C was not possible. Nuclear cataract has been observed to be the more common type of cataract in women than in men(Reference Congdon, O'Colmain and Klaver2). However, in the present study, men and women were not analysed separately due to a small number of nuclear cataracts in women (n 24).
In conclusion, we observed that high concentrations of lutein and zeaxanthin were associated with a reduced risk of nuclear cataract in elderly subjects. There may be other protective factors of the diet (e.g. synergism of carotenoids with vitamin C or other antioxidants) that may partly explain the observed results.
Acknowledgements
The authors acknowledge the staff of the Institute of Public Health and Clinical Nutrition at the University of Eastern Finland for helping with data collection. The contribution of each author was as follows: J. K. performed the biochemical and statistical analyses, and contributed to the writing of the manuscript; J. A. L. and S. K. were involved in the commentary of the manuscript and helping with the statistical analyses. Financial support from the University of Eastern Finland is gratefully acknowledged. The authors thank Kimmo Ronkainen for help in the statistical analyses. None of the authors has a personal or financial conflict of interest.





