Dietary fibres describe a range of indigestible carbohydrates with distinct physicochemical properties(1). These properties (e.g. number and type of constituent molecules), in turn, define the specific functional effects each fibre exerts upon the gastrointestinal tract, from forming viscous gels to stool bulking and laxation, and fermentation(Reference Eswaran, Muir and Chey2,Reference O’Grady, O’Connor and Shanahan3) . The therapeutic value of specific fibres is determined, in part, by these functional properties; for example, bulking fibres are useful in constipation(Reference Buttriss and Stokes4,Reference McRorie and McKeown5) . An understanding of the functional and subsequent therapeutic effects provided by specific fibres is required to guide their clinical and nutraceutical use.
Of these functional effects, the fermentation characteristics of specific fibres are of key importance in clinical medicine. Upon reaching the colon, certain fibres are hydrolysed by the microbial population through fermentation(Reference Cummings6), providing energy to promote bacterial proliferation whilst generating metabolites, such as SCFA, which have multiple health-promoting effects as reviewed elsewhere(Reference Gill, van Zelm and Muir7) and gases, which, among other effects, occupy a volume and have the potential to distend the intestinal lumen. Such distension stretches the intestinal wall and may trigger mechanoreceptors to cause sensations of bloating and abdominal pain, especially relevant in patients with irritable bowel syndrome (IBS) who commonly have heightened sensitivity to such stimulation(Reference Gibson and Shepherd8–Reference Ong, Mitchell and Barrett10). Readily fermentable fibres, notably fructans such as those present in wheat bran, have been identified as triggers for these symptoms in IBS(Reference Eswaran, Muir and Chey2,Reference Hernot, Boileau and Bauer11) , alongside other short-chain carbohydrates collectively termed FODMAP (Fermentable Oligo-, Di- and Mono-saccharides and Polyols)(Reference Gibson and Shepherd8,Reference Shepherd, Lomer and Gibson12) . Hence, the extent or rate of fermentation of fibres may have a role in symptom control.
The suitability of many types of fibres in patients with IBS, fermentable or otherwise, is unclear. To date, the only fibres that have been repeatedly investigated in the context of IBS are fructans (fructo-oligosaccharides (FOS) and inulin), partially hydrolysed guar gum (PHGG), psyllium and wheat bran(Reference Bijkerk, de Wit and Muris13–Reference Wilson, Rossi and Dimidi17). Of these, PHGG and psyllium have been shown to be tolerable in a higher proportion of patients(Reference Bijkerk, de Wit and Muris13–Reference Russo, Andreozzi and Zito16), while fructans and wheat bran led to worsened symptoms(Reference Bijkerk, de Wit and Muris13,Reference Ford, Moayyedi and Chey14,Reference Wilson, Rossi and Dimidi17) . Fructans and wheat bran are both readily fermentable (wheat bran by virtue of its fructan fraction), while psyllium is minimally fermented and PHGG has few data on this(Reference Hernot, Boileau and Bauer11,Reference Nilsson, Dahlqvist and Nilsson18,Reference Yao, Rotbart and Ou19) . It may be then that an understanding of the fermentation characteristics of specific types of fibre can be used to identify and predict their suitability for patients with IBS.
Traditionally, in vitro fermentation models have been used to evaluate interactions between fibres and the colonic microbiota(Reference Williams, Walton and Jiang20). These models range from simple batch cultures used to simulate a single, specific region of the colon over a maximum of 72 h, to artificial digestion simulators possessing the capacity to replicate digestion from stomach to distal colon(Reference Williams, Walton and Jiang20,Reference Van de Wiele, Van den Abbeele, Ossieur, Verhoeckx, Cotter and López-Expósito21) . While these models are capable tools used for determining specific changes resulting from these interactions(Reference Carlson, Erickson and Hess22–Reference Reichardt, Vollmer and Holtrop24), limitations exist precluding their clinical applicability to IBS and as screening tools. First, as with most in vitro models, the physiological relevance is limited, as most models are unable to stimulate the absorptive and detoxification systems occurring in vivo (Reference Williams, Walton and Jiang20). Second, traditional models are typically bulky, expensive and run for extended durations in excess of 24 h and generally lack clinical relevance to IBS where symptoms are attributed more to the rapid generation of gases over a short period rather than the total production of gases over an extended duration(Reference Murray, Wilkinson-Smith and Hoad9,Reference Major, Pritchard and Murray25) .
Recently, a rapid in vitro screening model has been developed possessing the capacity to detect changes in gas production over a 4-h period(Reference Yao, Rotbart and Ou19,Reference Rotbart, Yao and Ha26) . This batch fermentation model enables assessment of gas production kinetics and profiling of fermentation metabolites believed to be important to gastrointestinal health, positioning it as a convenient assay system capable of characterising the fermentative activities of specific fibres while complementing data derived from traditional models. This study aimed to use this in vitro model to evaluate and compare the fermentation characteristics of a comprehensive range of established and novel fibres, in order to identify specific fibres that may be suitable for patients with IBS.
Methods
Participants
Faecal samples were collected from healthy adults with no known gastrointestinal diseases or significant co-morbidities. Participants were excluded if, in the month preceding sample collection, they had consumed antibiotics, prebiotics, probiotics or fibre supplements. Once enrolled into the study, participants were instructed not to alter their diets during the collection period. All participants provided written informed consent prior to sample collection. In order to obtain more accurate results, a minimum of three participants were sought(Reference McBurney and Thompson27). Ethical approval was obtained from Monash University Human Research and Ethics Committee (ID: 11491) to undertake this study. This study was conducted according to the guidelines laid down in the Declaration of Helsinki.
Fibre substrates
A range of different purified fibre substrates, from those well characterised in terms of their fermentation to novel fibres, were evaluated (see Table 1). FOS and methylcellulose were included as fibres that had been thoroughly investigated(Reference Ferguson and Jones28,Reference Meyer and Stasse-Wolthuis29) , serving as readily fermentable ‘positive control’ and minimally fermentable ‘negative control’, respectively. Novel fibre types investigated were: xylo-oligosaccharide (XOS) derived from almond shells; carrot fibre derived from carrot peel (a composite fibre primarily composed of cellulose, hemicelluloses and pectin); sugarcane bagasse (a composite fibre of cellulose, hemicelluloses and lignin); as well as two derivatives of Hylon VII, a type of resistant starch 2 (RS2), esterified with SCFA molecules (Hylon VII Acetate (acetylated RS2) and Hylon VII Butyrate (butyrylated RS2)). The following fibres were included as ones that had been somewhat investigated to date, though not to the extent of FOS and methylcellulose: acacia gum, maize-derived XOS, long-chain inulin, medium-chain inulin, PHGG, two RS2 derivatives of the commonly used Hi-Maize RS2 (Hi-Maize 260 and Hi-Maize 1043) and RS4 (see Table 3). Aside from fructans (FOS and inulin), the fermentation characteristics of these fibres had not been investigated in the context of IBS.
Table 1. Fibre types used as substrates for the anaerobic in vitro fermentation procedure with their respective commercial names and suppliers
DP, degrees of polymerisation.
Table 2. Total gas production (ml/g), pH, concentrations of total SCFA (μmol/g), concentrations of ammonia (μmol/g) and the ratio of SCFA:branched-chain fatty acids (BCFA) in the supernatants after 4-h incubation* (Mean values with their standard errors)
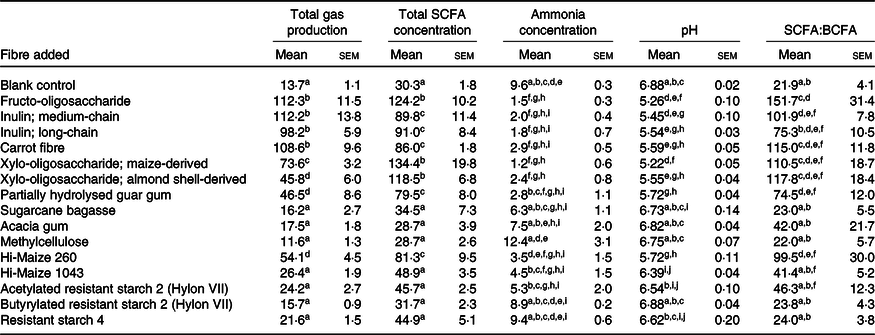
a,b,c,d,e,f,g,h,i,j Significant differences (P < 0·05; Fisher’s least significant difference) between fibres are highlighted with the use of superscript letters: values that share superscript letters within a column are not significantly different.
* Differences between fibres were evaluated using mixed-model ANOVA. The results are shown from three samples provided by three independent participants.
Table 3. SCFA and branched-chain fatty acid concentrations* (μmol/g) in the supernatants after 4-h incubation (Mean values with their standard errors)

a,b,c,d,e,f,g,h,i Significant differences (P < 0·05; Fisher’s least significant difference) between fibres are highlighted with the use of superscript letters: values that share superscript letters within a column are not significantly different.
* Differences between fibres were evaluated using mixed-model ANOVA. The results are shown from three samples provided by three independent participants.
In vitro fermentation procedure
The in vitro fermentation protocol was adapted from the published methodology(Reference Yao, Rotbart and Ou19). Participants collected freshly passed faecal samples in plastic containers, which were sealed immediately afterwards and transported to the laboratory. Upon arrival, the container carrying the sample was gassed with oxygen-free nitrogen (N2) for 2 min, resealed and kept at 37°C in a water bath until preparation of the faecal inoculum. The total weight of each sample provided, as well as consistency as assessed by the Bristol Stool Chart, was documented.
Within 30 min of arrival in the laboratory, a faecal inoculum was prepared by mixing the faecal sample with sodium phosphate buffer (pH 7·0) to make up a 16 % faecal slurry (weight/volume), which was then homogenised in a generic blender (Adobe Glass Blender XJ-10402; Adobe Appliances) for 2 min. Prior to the experiment, the sodium phosphate buffer had been boiled at 100°C for 15 min to ensure that the solution was anaerobic. The slurry was then filtered through four layers of muslin cloth to remove particulate materials. Aliquots (50 ml) of faecal slurry were transferred to 250 ml bottles containing either 10 ml of sodium phosphate buffer alone, as the blank control, or 10 ml of sodium phosphate buffer spiked with 1 g of fibre substrate. The dose of fibre substrate evaluated is within a range that could reasonably be expected to be encountered, per meal, in vivo (Reference Varney, Barrett and Scarlata30). The headspace of the bottles (described below) was then gassed with N2 prior to incubation and sealed. Each bottle was incubated in a shaking water bath at 37°C, shaking at 50 strokes/min, for 4 h.
Experiments were undertaken using faecal samples collected from the three independent participants in order to capture potential individual variations in faecal bacterial metabolism, with a total of three replicate experiments performed for each fibre evaluated (one experiment per independent participant). RS4 was the only fibre fermented in duplicate using samples collected from two independent participants. Each experiment performed included one blank control and between 1 and 3 fibres, with 7–9 total experiments conducted per participant (see online Supplementary Table S1).
Prior to and immediately following the 4-h incubation, 25 ml aliquots of faecal slurry were transferred to sterile faecal containers and frozen immediately at –80°C for analysis of ammonia and SCFA content. At baseline and following fermentation of either the blank or a fibre substrate, the pH of the faecal slurries from each participant was measured using a pH probe (Mettler-Toledo Five-Go pH meter and pH electrode LE427; Mettler-Toledo) prior to freezing.
Total gas production
Total gas production from the faecal slurries in response to the fibre substrates over the 4-h incubation period was measured via automated headspace sampling using a pressure sensor (ANKOMRF Gas Production System; ANKOM Technology), with samples recorded at 15-s intervals. Gas production was adjusted for total headspace volume, converted from pressure (psi) into volume (ml) using the ‘ideal’ gas and Avogadro’s laws and expressed as volume (ml/g fibre). Cumulative gas production at the end of the 4-h incubation period was used to assess the total extent of fermentation. Variations in total gas production between individual bottles were minimal over the 4-h experimental period, demonstrated in separate experiments involving triplicate fermentations evaluating the same substrate (FOS) and slurry from a single participant (CV < 5 %) (see online Supplementary Fig. S1).
SCFA and branched-chain fatty acid concentrations
Frozen samples of faecal slurry were defrosted overnight at 4°C. A 5 ml aliquot of the defrosted slurry was spiked with internal standard (1·68 mm heptanoic acid) at 1:3 (sample to internal standard, volume/volume), homogenised and then centrifuged (2000 g , 10 min, 4°C) as previously described(Reference Humphreys, Conlon and Young31). Following centrifugation, 300 µl of supernatant was added to a 0·2 µl filter vial containing 10 µl of 1m phosphoric acid. The vials were then analysed for SCFA and branched-chain fatty acid (BCFA) content via GC.
All samples were analysed using an Agilent GC6890 coupled to a flame ionisation detector with helium used as the carrier gas. An Agilent FFAP column (30 m × 0·53 mm (internal diameter) × 1·00 µm (film thickness)) was installed for analysis, with a constant flow rate of 4·0 ml/min. A splitless injection technique was used, with 0·2 µl of sample injected. The oven was held at 90°C for 1 min and then raised to 190°C at 20°C/min and held for 3 min. Samples were run in triplicate, with a CV < 15 % within triplicate samples used for quality control.
Ammonia concentrations
Frozen samples of faecal slurry were defrosted overnight at 4°C. A 1·5 ml aliquot of the defrosted slurry was spiked with an equal volume of 1m perchloric acid (Sigma Aldrich (catalogue no. 311421)), homogenised and then centrifuged (2000 g , 10 min, 4°C). Following centrifugation, the supernatant was transferred and centrifuged again (13 000 g , 5 min, 4°C), after which 500 µl of supernatant was transferred and neutralised with 215 µl of 1 m potassium hydroxide, and then filtered through a nylon syringe filter (25 mm, 0·22 μm). The recovered supernatant was analysed in duplicate for ammonia content via spectrophotometer following the manual assay procedure according to the manufacturer’s instructions (Ammonia Assay Kit; Megazyme).
Dietary intake
Participants completed 24-h food records on the day preceding each sample collection. A research dietitian (D. S.) provided each participant with instructions on maintaining the food record and subsequently assessed each completed record for completeness prior to data entry. These records were analysed for energy, macronutrients, sugars and fibre via FoodWorks 10 software (Xyris Software Pty. Ltd).
Statistical analysis
Statistical analyses were performed using GraphPad Prism (version 8.4.3; GraphPad Software) and R statistical software (version 4.0.2; R Foundation for Statistical Computing). Study outcomes, namely, differences in concentrations of each metabolite, were compared between the fibres investigated (including same fibres from different source as well as blank controls) within participants using mixed-model ANOVA, with multiple comparisons performed using Fisher’s least significant difference. Correlation analysis between the fermentation end points, faecal characteristics and indices of dietary intake was performed using repeated-measures correlation(Reference Bakdash and Marusich32), with a P value ≤ 0·05 considered significant. Data are presented as means with their standard errors unless otherwise specified.
Results
Participants
Three healthy adults (all male; aged 23, 25 and 27 years) provided faecal samples for the study. Their mean consumption of energy, macronutrients and total dietary fibre in the 24 h preceding sample collection was as follows: 9985 kJ energy, 115 g protein, 275 g carbohydrate, 89 g fat and 31 g dietary fibre. Total carbohydrate consumption was significantly different across the participants preceding each experiment (online Supplementary Table S2), but no significant differences in intake of total energy, protein, fat or dietary fibre were observed.
Total gas production
The total amounts of gas produced in response to the fibre substrates over the 4-h incubation period are shown in Fig. 1 and Table 2. In descending order, total gas production in response to fermentation was greatest for FOS, followed by medium-chain inulin, carrot fibre, long-chain inulin, maize-derived XOS, PHGG, almond shell-derived XOS and Hi-Maize 260. The total gas produced in response to the fermentation of these fibres was significantly greater compared with the blank control. Compared with the blank control, there were no significant differences in mean gas production in response to the fermentation of acacia gum, Hi-Maize 1043, methylcellulose, acetylated RS2, butyrylated RS2, RS4 and sugarcane bagasse (see Table 2).
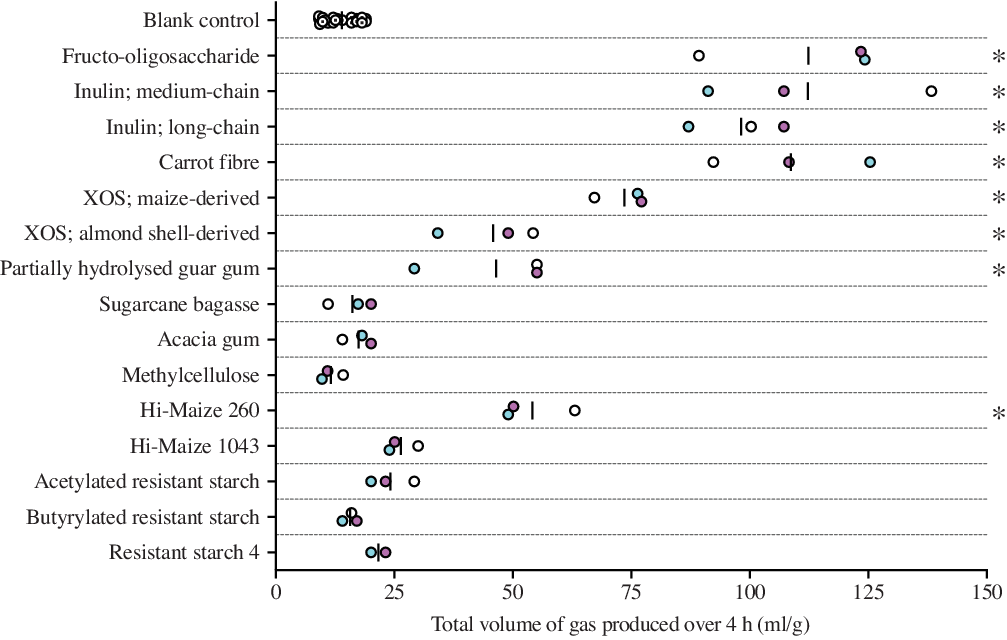
Fig. 1. Total gas produced (ml/g) across fibres investigated as a result of fermentation. The values displayed represent the individual results per experiment, across the three replicate experiments conducted using samples provided by three independent participants. Results per individual participants are colour coded. Differences between fibres were evaluated using one-way ANOVA. * Significant differences (P < 0·05; Fisher’s least significant difference) between fibres and the blank control. XOS, xylo-oligosaccharide.
Based on the total volume of gas produced over the incubation period, the fibres evaluated could be grouped into three distinct categories: rapidly fermented fibres that produced ≥60 ml/g mean gas production over 4 h (the three fructans, carrot fibre and maize-derived XOS), mildly fermented fibres generating 30–60 ml/g gas (almond shell-derived XOS, Hi-Maize 260 and PHGG) and minimally fermentable fibres whose gas production was not significantly different compared with blank controls (acacia gum, acetylated and butyrylated RS2, Hi-Maize 1043, methylcellulose, RS4 and sugarcane bagasse).
Multiple comparison analyses highlighted specific differences and similarities in total gas production between fibre substrates sharing similar classifications. No significant differences in gas production were found across fructans of three chain lengths investigated, or between the Hylon VII RS2 derivatives: acetylated RS2 and butyrylated RS2. Comparatively, total gas production was significantly different between the following fibres with similar properties: maize compared with almond shell-derived XOS and Hi-Maize 260 compared with Hi-Maize 1043.
SCFA and branched-chain fatty acid concentrations
The absolute concentration of SCFA found in the slurries generated as a result of the 4-h fermentation, including BCFA, is outlined in Tables 2 and 3. They were concordant with total gas production: the fibres that generated significantly greater amounts of gas (FOS, medium-chain inulin, long-chain inulin, carrot fibre, maize-derived XOS, almond shell-derived XOS, PHGG and Hi-Maize 260) also generated significantly greater concentrations of total and individual SCFA (acetate, propionate and butyrate) compared with the blank control over 4 h (see Tables 2 and 3). Compared with the blank control, concentrations of isovalerate found in the slurries were significantly lower amongst the following fibres compared with the blank control: FOS, medium-chain inulin, carrot fibre, almond shell-derived XOS, PHGG, acacia gum, Hi-Maize 260 and acetylated RS2. There were no differences in concentrations of other BCFA (isobutyrate, valerate and caproate) following 4-h fermentation of the fibres evaluated compared with blank controls.
SCFA and branched-chain fatty acid proportions
The proportions of SCFA generated, relative to total concentrations, are presented in Fig. 2. The following fibres generated significantly greater percentage of SCFA as acetate compared with the blank control: maize-derived XOS, almond shell-derived XOS, carrot fibre, Hi-Maize 260 and acetylated RS2 (see Fig. 2). Compared with the blank control, no fibres generated a greater percentage of SCFA as propionate. Fermentation of the following fibres led to significant reductions to propionate proportions compared with the blank control: FOS, medium-chain inulin, long-chain inulin, maize-derived XOS, almond shell-derived XOS, carrot fibre, Hi-Maize 260 and acetylated RS2. The only fibres that impacted upon the proportion of butyrate via fermentation were the fructans (FOS, medium-chain inulin and long-chain inulin), all of which generated a higher proportion of butyrate compared with the blank control. The ratio of SCFA:BCFA generated as SCFA compared with BCFA was significantly higher for FOS, medium-chain inulin, long-chain inulin, carrot fibre, maize-derived XOS, almond shell-derived XOS, PHGG and Hi-Maize 260 compared with that from the blank control. There were no significant differences in SCFA:BCFA ratios between the other fibres evaluated (acacia gum, Hi-Maize 1043, methylcellulose, acetylated RS2, butyrylated RS2, RS4 and sugarcane bagasse) compared with the blank control (see Table 3).

Fig. 2. SCFA proportions for acetate, propionate, butyrate and other (isobutyrate, isovalerate, valerate and caproate), as a proportion of total SCFA concentrations, across fibres investigated following fermentation. Values shown represent the mean SCFA proportions across three replicate experiments conducted using samples provided by three independent participants. Differences between fibres were evaluated using one-way ANOVA. * Significant differences (P < 0·05; Fisher’s least significant difference) between fibres and the blank control for acetate and butyrate proportions. XOS, xylo-oligosaccharide. ![]() , Butyrate;
, Butyrate; ![]() , acetate;
, acetate; ![]() , propionate;
, propionate; ![]() , other.
, other.
Ammonia concentrations
Concentrations of ammonia found in slurries were significantly lower amongst the fibres whose fermentation generated greater quantities of gas and concentrations of SCFA compared with those from the blank controls (see Table 2). There were no differences in ammonia concentrations following 4-h fermentation of acacia gum, sugarcane bagasse, methylcellulose, butyrylated RS2 and RS4 compared with those from blank controls.
Slurry pH
The mean pH of the faecal slurry at baseline was 6·95. Following the 4-h fermentation period, the pH of the faecal slurries did not drop below 5·0 across each substrate evaluated. The differences in slurry pH at the end of the fermentation period, between the fibres investigated, are presented in Table 2. The fermentation of all fibres, except for acacia gum, methylcellulose, butyrylated RS2 and sugarcane bagasse, significantly reduced pH from baseline, both in absolute pH and the relative change (data not shown).
Correlations between fermentation characteristics
Correlation analysis was undertaken for the primary fermentation characteristics evaluated, across all experiments conducted (Fig. 3). Positive correlations were observed between total gas production and total SCFA concentrations (r 0·80, P < 0·001) as well as the SCFA:BCFA ratio (r 0·78, P < 0·001), while total gas production and total SCFA concentration correlated negatively with ammonia concentrations (r –0·68, P < 0·001; r –0·73, P < 0·001) and post-fermentation pH (r –0·91, P < 0·001; r –0·94, P < 0·001).
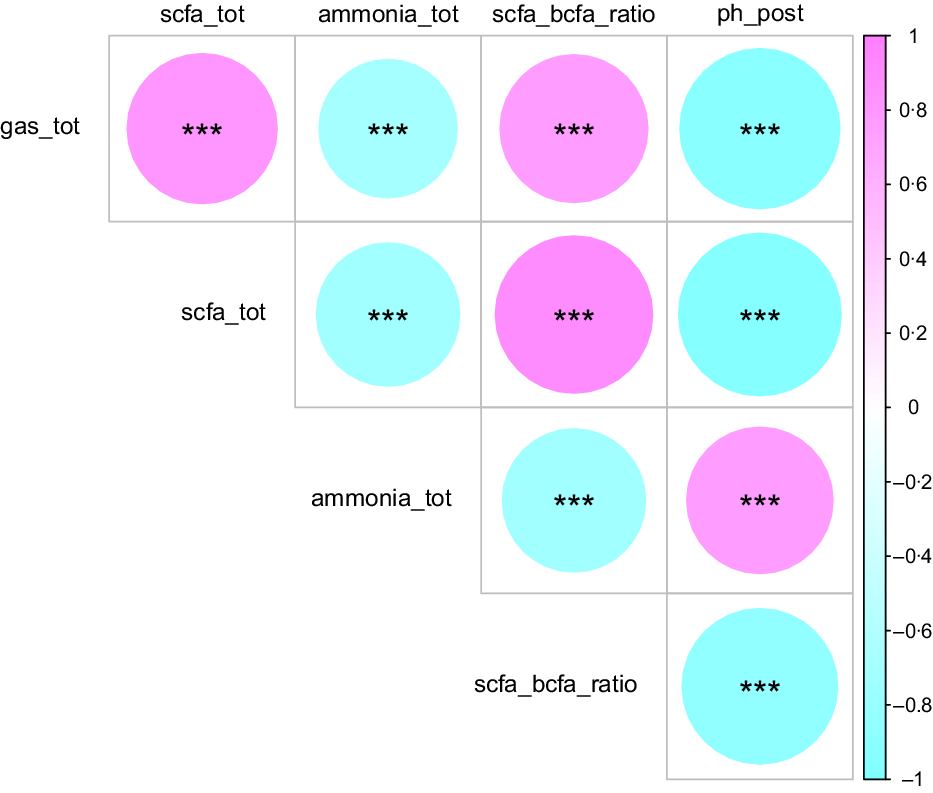
Fig. 3. Repeated-measures correlation matrix highlighting relationships between the following fermentation characteristics assessed: total gas production (gas_tot), total SCFA concentrations (scfa_tot), ammonia concentrations (ammonia_tot), SCFA:BCFA ratio (scfa_bcfa_ratio) and post-fermentation pH (ph_post). Pink colours illustrate positive correlations, while blue illustrate negative correlations. The size of each circle and shade of colour are proportional to each P value, with wider widths and deeper shades indicating higher ρ values. *** Significant correlations (P < 0·001).
Correlations between dietary intake, faecal sample characteristics and the main end points investigated across all experiments conducted as well as for blank controls only are shown in online Supplementary Figs. S2 and S3. For both analyses, no significant correlations were found between dietary components and the primary fermentation characteristics investigated. Across the blank controls, no significant correlations between dietary intake and post-fermentation end points were found. For analyses involving all experiments, significant correlations were found between energy intake and caproate percentage (r 0·36, P = 0·005), carbohydrate intake and acetate percentage (r –0·28, P = 0·04), and protein intake with valerate intake (r 0·41, P = 0·002). No significant correlations were found between faecal sample characteristics and fermentation characteristics investigated.
Discussion
There is growing interest in the utilisation of fibres with specific functional properties to purportedly optimise gut health and function. The physical and functional heterogeneity of fibres is well known, but the limited documentation of the fermentation characteristics of specific fibres is surprising. The current study utilised a rapid in vitro fermentation model to evaluate a comprehensive range of fibres of nutraceutical and clinical interest. Several key findings were evident. First, a heterogeneous response was observed across the fibres evaluated, as evidenced by, for example, the differences in total gas production between fibres. Second, distinct metabolite profiles were produced as a result of fermentation, with strong correlations between metabolites of fibre and protein fermentation demonstrated. Finally, differential effects in terms of fermentative activities were shown between fibres with similar physical and chemical properties. These findings have implications for the understanding of the fermentation characteristics of fibres, informing their potential relevance to IBS.
A heterogeneous response to fermentation was observed using this fermentation model, with several fibres shown to be minimally fermented over 4 h. These fibres may have resisted rapid fermentation through different means. The lack of gas and metabolite production observed with acacia gum, Hi-Maize 1043, RS4 and the Hylon VII derivatives may be reflective of slower, more progressive fermentation than the lack thereof. Investigations of acacia gum, Hi-Maize 1043 and RS4 through 48-h experiments and animal models have previously reported significant increases in SCFA concentrations in comparison with controls over longer durations(Reference Alarifi, Bell and Walton33,Reference Le Leu, Hu and Brown34) . Similarly, while the fermentation characteristics of acetylated and butyrylated RS2 have yet to be assessed, recent work evaluating another Hylon VII derivative, esterified with propionate molecules, also demonstrated minimal fermentation over the initial 4-h period, before reporting multiple-fold increases in both gas and SCFA production as the experiment progressed to 24 h(Reference Xie, Wang and Wang35). The slower rate of fermentation observed with these fibres is likely due to their polysaccharide structures(Reference Cantu-Jungles and Hamaker36). In contrast, methylcellulose and sugarcane bagasse appear to be resistant to fermentation, with no changes in markers of fermentation documented in either this study or in previous 24-h fermentations of methylcellulose or the predominant fibrous constituent of sugarcane bagasse in cellulose(Reference Ferguson and Jones28). The resistance of these fibres to fermentation may be due to the absence of microbes capable of their degradation, which are only present in individuals capable of producing methane(Reference Chassard, Delmas and Robert37).
Carrot fibre, the fructans, Hi-Maize 260, PHGG and the two types of XOS were readily fermentable, with the greatest production of gas resulting from the fermentation of fructans and carrot fibre, followed by maize-derived XOS. The rapid generation of gases and SCFA from the breakdown of carrot fibre may have been driven by its pectin fraction(Reference Ross, English and Perlmutter38,Reference Sharma, Karki and Thakur39) , which has previously been shown to be fermented at a rate comparable to fructans(Reference Jonathan, van den Borne and van Wiechen40). Interestingly, while both XOS varieties were expectedly fermentable(Reference Aachary and Prapulla41,Reference Yang, Summanen and Henning42) , there was a significant difference in gas production between the two. The disparity in their gas production kinetics may be the result of their different origins or variations in processing techniques, which may have led to alterations to their chemical composition(Reference Aachary and Prapulla41,Reference Coulon, Page and Raggio43) , as previously speculated(Reference Singh, So and Yao44). Similarly, differences in processing may have contributed to the divergent fermentation characteristics of the Hi-Maize RS2 derivatives (Hi-Maize 260 was readily fermentable, but Hi-Maize 1043 was not), which are produced through different hydrothermal processes(Reference Le Leu, Hu and Brown34,Reference Birt, Boylston and Hendrich45) . Conversely, there was little disparity in the fermentation characteristics of fructans with different chain lengths (FOS and inulins) and Hylon VII RS2 derivatives evaluated within this short time frame, likely due to their similar origins and processing methods: the fructans are all β-2,1 linked fructose polymers differentiated only by the degree of polymerisation(Reference Niness46–Reference Wilson and Whelan48), while acetylated and butyrylated RS2 both share the Hylon VII backbone before esterification of their respective SCFA molecule(49).
Distinct metabolic profiles were produced through the fermentation of the fibres evaluated, highlighting the capacity of specific fibres to selectively promote generation of specific SCFA. The preferential production of butyrate through the fermentation of fructans is consistent with the existing work(Reference Fu, Liu and Zhu50), as is the acetate generated through the fermentation of XOS(Reference Aachary and Prapulla41) and carrot fibre, owing to its pectin fraction(Reference Bang, Kim and Lim51). The lack of selective butyrate production from the fermentation of the RS2 derivatives was unexpected, given the documented butyrogenic effect of the fibre(Reference Birt, Boylston and Hendrich45,Reference Muir, Yeow and Keogh52) . This may be the result of the experimental duration, as highlighted by Xie et al.(Reference Xie, Wang and Wang35). Experimental duration may also have impacted the metabolites produced from the Hylon VII derivatives, as only acetylated RS2 increased proportions of esterified molecule, with no shifts in butyrate observed following the 4-h fermentation of butyrylated RS2.
The low variance in response to the specific substrates between participants was notable, given this study undertook experiments using samples from individual participants to account for heterogeneous responses. The similar age and health status of the three participants, as well as the lack of differences in reported dietary intake, may have contributed to the uniformity of responses observed, despite differences in stool samples provided (see online Supplementary Table S1). Moreover, based on the lack of hierarchical effects in gas production patterns (see Fig. 1), there did not appear to be evidence of responder-specific effects between the participants(Reference Korpela, Flint and Johnstone53). Given dietary trials have previously highlighted the variability of inter-individual responses in microbial taxonomy to alterations in fibre intake(Reference Salonen, Lahti and Salojärvi54,Reference Walker, Ince and Duncan55) , these results suggest that heterogeneity of microbiota composition does not appear to be reflected in metabolite production, at least in the short term. While data on the microbiota profiles may have been helpful to explore whether the uniformity of response across the three individuals was attributable to similarities in microbial composition, it remains unclear whether the colonic microbiota can consistently predict responses to a dietary intervention(Reference Biesiekierski, Jalanka and Staudacher56).
The strong correlations between major fermentation end points highlight the interdependence between a range of metabolic activities performed by the colonic microbiota. The positive correlation between the total gas production and total SCFA concentrations following fermentation (see Fig. 3) reflected their status as the main end products of carbohydrate-based fermentation(Reference Cummings6), corroborated by their negative correlation with pH. Furthermore, the correlations between total gas production and SCFA concentrations with ammonia concentrations and the ratio of SCFA:BCFA suggest the availability of fibres for bacterial degradation may redirect fermentative activities from protein-based compounds. The lack of correlation between dietary parameters, particular of fibre intake, and fermentative end points may be due to the lack of differences in macronutrient intake between participants, the limited number of participants and the use of stool samples representative of distal colon, where residual fibres may have already been fermented, as a proxy for the microbiota.
The rapid in vitro fermentation system used in this study provides information of importance to clinical medicine and the food industry complementary to that provided by traditional fermentation models(Reference Hernot, Boileau and Bauer11,Reference Carlson, Erickson and Hess22–Reference Reichardt, Vollmer and Holtrop24,Reference Xie, Wang and Wang35,Reference Wang, Wichienchot and He57) . The rate of fibre fermentation, as shown by the generation of gas, has proven to be critical in the induction of gut symptoms, such as abdominal pain and bloating, by virtue of the distension of the caecum and proximal colon(Reference Ong, Mitchell and Barrett10). This is one of the major mechanisms by which oligosaccharide FODMAP trigger symptoms in patients with IBS and, most importantly, the alleviation of those symptoms when their intake is reduced(Reference Dionne, Ford and Yuan58,Reference Gibson59) . Likewise, symptoms generated by supplementation of wheat bran are linked in part to the presence of rapidly fermentable components(Reference Nilsson, Dahlqvist and Nilsson18).
This information complements that of longer term fermentation studies, indicating the rate of fibre fermentation and the spectrum of SCFA produced (as discussed above). Delivery of SCFA to the colonic mucosa appears to occur where it is produced. Hence, slowly fermented fibres better deliver SCFA to the distal colon for its many health-promoting effects(Reference Gill, van Zelm and Muir7). Such carbohydrate fermentation is also important in suppressing protein fermentation, which produces potentially harmful metabolites, particularly in the distal colon(Reference Yao, Muir and Gibson60). Furthermore, the fibres that resist fermentation may be of importance in clinical practice to patients with IBS and chronic constipation who cannot tolerate or wish to minimise gas production, as the other functional effects of these fibres may contribute to stool bulking effects without gas production.
There are several limitations to this study in addition to the inherent limitations of in vitro investigations. First, the experiments used faecal samples as a proxy for the colonic microbiota. Therefore, the fermentative interactions and end points assessed are representative of the distal colon, which may not be reflective of the processes that occur more proximally(Reference Cummings6). Second, the lack of microbiota characterisation precluded further exploration of whether differences in microbiota of each participant may have mediated the responses to the fibres evaluated. Greater numbers might have strengthened the notion that the fermentation characteristics are dictated more by the fibre itself than by the individual’s microbial community. However, it should be noted that consistency of results was tight. Third, despite the quantity and concentration of inoculum utilised, the absence of media may have led to the selective stimulation of bacteria capable of utilising the fibres, potentially underrepresenting the cross-feeding interactions that occur in vivo, though the addition of media may also have altered the metabolic activities of the microbiota. Finally, this study used faecal samples donated from healthy individuals rather than IBS patients to conduct these experiments, which has often been critiqued to lack sufficient representation of the clinical response to these fibres. However, colonic gas production is in general similar in patients with IBS with that in healthy subjects(Reference Major, Pritchard and Murray25) and that it is anticipated that any meaningful functional differences, if exists, should be comparable between patients and controls(Reference Pittayanon, Lau and Yuan61).
In conclusion, this study utilised a rapid in vitro fermentation model to demonstrate the heterogeneity of fermentation characteristics across a range of novel and established fibres. These findings have implications for the design of dietary strategies where there is clinical value in manipulating the fermentative activities of dietary components, identifying fibres whose fermentation characteristics and metabolic profiles may be suitable for specific conditions. Several fibres, identified as slowly fermentable and resistant to fermentation, appear to be suitable for patients with IBS. Both types of fibre appear to possess secondary characteristics suggestive of further therapeutic value, with slowly fermentable fibres potentially offering utility in attenuating protein fermentation in the distal colon, while fibres resistant to fermentation may convey secondary functional effects to support bowel function. Future dietary trials are required to translate these in vitro findings to patients.
Acknowledgements
The authors wish to thank Dr Kyle Berean and Dr Christopher Harrison for assisting with the in vitro fermentation systems. Special thanks to Dr Ramkrishna Singh and Kagome Australia for providing novel fibre substrates.
This work was supported by Tamu Innovations through the Graduate Research Industry Partnership programme at Monash University. Tamu Innovations had no role in the design, analysis or writing of this article.
The authors’ responsibilities were as follows – D. S., C. K. Y., P. R. G. and J. G. M. designed the study; D. S., P. A. G. and N. P. carried out the study; D. S. analysed the data; D. S., C. K. Y., P. R. G. and J. G. M. interpreted the findings; D. S. wrote the initial manuscript and C. K. Y., P. A. G., P. R. G. and J. G. M. critically revised the manuscript.
D. S. was supported by a scholarship from Monash University through the Graduate Research Industry Partnership programme. C. K. Y. has received support for investigator-initiated grants from Atmo Biosciences. J. M. was supported by a Research Fellowship from the National Health and Medical Research Foundation of Australia. D. S., C. K. Y., P. A. G., P. R. G. and J. M. also work in a department that financially benefits from the sales of a digital application and booklets on the low FODMAP diet. Funds raised contribute to research of the Department of Gastroenterology and to the University. No author receives personal remuneration. N. P. has no interests to disclose.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520003943









