Term infants born small-for-gestational age (SGA; >37 weeks gestation and <10th percentile) often show accelerated postnatal weight gain( Reference Kerkhof and Hokken-Koelega 1 , Reference Lei, Chen and Ye 2 ), which is associated with an increased risk of non-communicable diseases later in life( Reference Ong, Ahmed and Emmett 3 – Reference Singhal, Kennedy and Lanigan 5 ). Adverse sequelae include visceral fat mass accumulation( Reference Ay 6 ), hypertension( Reference Ben-Shlomo, McCarthy and Hughes 7 , Reference Zamecznik, Niewiadomska-Jarosik and Wosiak 8 ), CVD( Reference Barker, Osmond and Forsen 9 ), the metabolic syndrome( Reference Khuc, Blanco and Burrows 10 ) and type 2 diabetes mellitus( Reference Milovanovic, Njuieyon and Deghmoun 11 ).
In line with the Developmental Origins of Health and Disease hypothesis( Reference Vickers 12 ), intra-uterine deficiency during specific developmental periods leads to intra-uterine growth restriction (IUGR) and affects lifelong metabolic health by modification of organ structure and function, as well as epigenetic programming( Reference Zamecznik, Niewiadomska-Jarosik and Wosiak 8 , Reference Heijmans, Tobi and Stein 13 , Reference Painter, Roseboom and Bleker 14 ). We demonstrated that experimental utero-placental insufficiency by uterine vessel ligation in pregnant rats results in fetal programming of impaired glucose tolerance, hyperinsulinaemia, hyperlipidaemia and hyperleptinaemia, as well as altered gene and protein expressions in the offspring( Reference Nüsken, Dötsch and Rauh 15 – Reference Nüsken, Wohlfarth and Lippach 17 ).
Despite some inconclusive evidence( Reference Horta and Victora 18 ), breast-feeding and breast-feeding duration have been associated with a moderate, yet consistent, reduction of childhood obesity risk and later metabolic disease( Reference Arenz, Ruckerl and Koletzko 19 – Reference Anderson 24 ). In breast-fed SGA infants, enhanced early growth( Reference Lucas, Fewtrell and Davies 25 ) and lower circulating adiponectin and insulin-like growth factor 1 (IGF-1)( Reference de Zegher, Sebastiani and Diaz 26 , Reference Diaz, Bassols and Sebastiani 27 ) concentrations also hint at some protective effect compared with infant milk formula (IF) feeding.
In both human milk (HM) and IF, dietary lipids are predominantly presented in the form of TAG. However, there are distinct differences in phospholipid and other polar lipid content. Furthermore, a major difference is the supramolecular structure of the milk fat globule (MFG)( Reference Delplanque, Gibson and Koletzko 28 ). The raw human MFG is on average 4 μm in diameter( Reference Delplanque, Gibson and Koletzko 28 , Reference Michalski, Briard and Michel 29 ), and the TAG core is surrounded by a triple-layered physiological membrane( Reference Michalski, Briard and Michel 29 , Reference Martini, Salari and Altomonte 30 ). This complex supramolecular structure has been shown to affect digestion, absorption kinetics and utilisation of dietary lipids( Reference Bourlieu and Michalski 31 , Reference Michalski 32 ). In contrast, IF lipids mostly originate from plant oil blends and are not structured as MFG, but typically consist of small fat droplets (average diameter of 0·5 μm). Dietary lipids affect growth and development not only as energy source, but also as cellular constituents, signalling molecules and transcription factors( Reference Koletzko, Agostoni and Bergmann 33 ). To be able to investigate the physiological effects of the supramolecular lipid structure in milk, a complex lipid matrix (CLM) IF concept (Nuturis®; Nutricia Research B.V.) was developed by adding dairy-derived phospholipids (PL) in MFG membrane (MFGM) fragments (Fonterra Co-operative Group Limited) in comparable amount and profile as HM, in addition to applying an altered processing procedure to obtain PL-coated lipid droplets larger than those in standard IF. Briefly, the polar PL were added to the aqueous phase and blended with the lipid phase containing the neutral vegetable lipids to generate a PL coating around the lipid droplets. Homogenisation pressure during processing was adjusted to not only secure a homogeneous mixture of ingredients but also to retain a large lipid droplet size( Reference Gallier, Vocking and Post 34 , Reference Van Den Brenk, Van Dijke and Van Der Sten 35 ). To investigate whether the supramolecular structure of lipids in early-life diet affects long-term metabolic health, recently, healthy preweaning mice were exposed to the new CLM IF concept diet. Indeed, fat accumulation was reduced, and metabolic profile was improved in adult CLM mice challenged by a moderate Western-style diet (WSD) from adolescence onwards( Reference Oosting, Kegler and Wopereis 36 – Reference Baars, Oosting and Engels 38 ).
Our aim was to ameliorate programmed metabolic disease in an ‘at-risk’ population by early nutrition. We hypothesised that known metabolic long-term sequelae in our established IUGR rat model( Reference Nüsken, Dötsch and Rauh 15 – Reference Nüsken, Wohlfarth and Lippach 17 ) would be alleviated by feeding intervention with a diet containing large lipid droplets (CLM diet)( Reference Oosting, Kegler and Wopereis 36 – Reference Baars, Oosting and Engels 38 ) during development in early life. The diet intervention was started on postnatal day (PND) 15 when the offspring starts intake of solid food, corresponding to the complimentary feeding period in humans, and was continued until late adolescence( Reference Evans 39 ).
Methods
Animals and surgical procedures
This study was conducted in accordance with institutional guidelines for the care and use of laboratory animals established by the Ethics Committee for Animal Experimentation of the University of Cologne and the German Government (AZ 2011.A248), in full compliance to the European Directive 2010/63/EU for the use of animals for scientific purposes.
Time-mated female Wistar rats (age 3 months) were obtained on post-conceptual day (PCD) 13 or 14 (plug day is PCD 1) of their first pregnancy from Charles River Wiga Deutschland GmbH. Dams were housed individually under standard conditions with free access to water and semi-synthetic rodent diet (American Institute of Nutrition (AIN)-93G; ssniff Spezialdiäten GmbH).
Similar to that described before( Reference Nüsken, Dötsch and Rauh 15 – Reference Nüsken, Wohlfarth and Lippach 17 ), IUGR was induced either by performing a bilateral ligation (LIG) of uterine arteries and veins caudally of the most caudal vessel branch running to the most caudal feto-placental unit or by sham operation (SOP) – that is, the suture material was not fixed but removed before closing the abdomen. All surgeries were performed under isoflurane anaesthesia and metamizol analgesia. Total duration of each intervention was 15–20 min. All fetuses were counted intra-operatively. LIG dams (n 8) carried eight to fourteen and SOP dams (n 7) eight to thirteen living fetuses. Dams started to drink water (containing tramadol for 24 h after surgery) 5–10 min after surgery, and their behaviour recovered fully within 1 h. As both LIG and SOP (i.e. intra-uterine stress) offspring show IUGR and metabolic programming( Reference Nüsken, Dötsch and Rauh 15 – Reference Nüsken, Wohlfarth and Lippach 17 ), offspring of non-operated (NOP) dams (n 16) served as controls for both groups. All dams delivered spontaneously after approximately 22·5 PCD within a time frame of 12 h. Litters comprised between seven and fifteen living pups, except one LIG litter of four (six fetuses were resorbed; three of the remaining pups were included in the study). On PND 2, all offspring were weighed, measured and determined for sex visually. The body weight of pups born alive was as follows: NOP (n 160), 6·84 (sem 0·06) g; LIG (all pups, n 71), 5·73 (sem 0·08) g; LIG (selected light pups, n 36, see below for explanation), 5·40 (sem 0·10) g; and SOP (n 75), 6·28 (sem 0·08) g. Thus, the mean percentage growth restriction was 16·2 % for all LIG pups, 21·1 % for selected light LIG pups and 8·2 % for SOP pups (P<0·001 for comparisons LIG–NOP, SOP–NOP and LIG–SOP). The error of our visual sexing method is approximately 1 % in our laboratory; hence, we did not perform sex determining region Y (SRY) PCR as previously reported( Reference Nüsken, Schneider and Plank 16 , Reference Nüsken, Wohlfarth and Lippach 17 ). Immediately afterwards, postnatal environmental conditions were equalised in all groups as we wanted to clearly restrict the exposure to deficiency in groups LIG and SOP to late pregnancy. Thus, all offspring were transferred to foster dams originating from group NOP to ensure similar postnatal handling; all foster dams received AIN-93G diet until PND 14, and all foster litters were adjusted to five male and one female offspring (males originating from at least two different litters). From LIG dams, either three (in case of litter size below 10) or six lightest pups of each original litter were selected and randomly reassembled to four foster dams. From SOP and NOP dams, offspring were randomly selected and reassembled to four foster dams for SOP and eight foster dams for NOP offspring. The whole selection and transfer procedure took 5–10 min for each litter and was performed in a similar way in all litters. Group names were assigned to the litters/pups on PND 2: two foster litters each either containing NOP, LIG or SOP offspring heading for ‘normal matrix’ control diet (CTRL) from PND 15 to 42 and moderate WSD thereafter (NOP-CTRL; LIG-CTRL and SOP-CTRL); four foster litters containing NOP offspring heading for CLM diet from PND 15 to 42 and WSD thereafter (NOP-CLM); and two foster litters each either containing LIG or SOP offspring heading for CLM diet from PND 15 to 42 and WSD thereafter (LIG-CLM; SOP-CLM). In addition, two foster litters containing NOP offspring heading for CTRL diet from PND 15 to 42 and AIN-93M diet (i.e. no WSD challenge) thereafter (group ‘REF’) were used as comparators to healthy control rats (group NOP-CTRL) exclusively to illustrate the metabolic effects of WSD in healthy rat offspring (for data see online Supplementary Table S1). Group ‘REF’ was not compared with the other groups and not used as a statistical control group. For full experimental setup, see Fig. 1.

Fig. 1 Experimental setup. A timeline is provided at the bottom; each line represents 1 d. LIG, experimental utero-placental insufficiency by bilateral ligation of the uterine arteries and veins on postconceptional day (PCD) 19; SOP, sham operation (i.e. intra-uterine stress) on PCD 19; NOP, no operation (normal pregnancy); CTRL, infant formula-based ‘normal matrix’ control diet; CLM, infant formula-based intervention diet containing a complex lipid matrix; AIN-93G, American Institute of Nutrition standard growth diet; AIN-93M, American Institute of Nutrition standard maintenance diet; ‘REF’, background reference group, which was used as a comparator to healthy control rats (group NOP-CTRL) exclusively to illustrate the metabolic effects of Western-style diet in healthy rat offspring. Group REF was neither compared with other groups nor used as a statistical control group. PND, postnatal day; μCT, micro computer tomograph.
Table 1 Diet compositionFootnote *
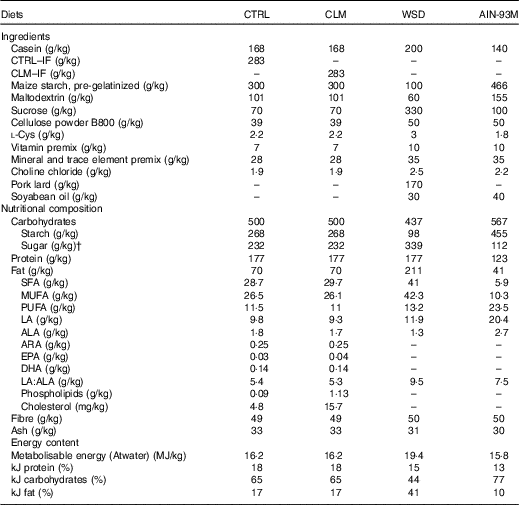
CTRL, normal matrix control diet (infant milk formula based); CLM, complex lipid matrix intervention diet (infant milk formula based); WSD, Western-style diet; AIN-93M, American Institute of Nutrition standard maintenance diet; IF, infant milk formula; LA, linoleic acid; ALA, α-LA; ARA, arachidonic acid.
* There was no difference in nutrient amounts between CTRL and CLM diets except increased phospholipid and cholesterol content in CLM necessary to form large lipid droplets.
† Total sugar: including lactose, glucose and sucrose.
Feeding interventions (programming diets and Western-style diet challenge)
On PND 15, the feeding intervention was started in eight foster litters for exposure to CLM diet and in eight foster litters for exposure to CTRL diet (Fig. 1). Pups had free access to maternal milk, water and their dams’ respective diets. On PND 21, pups were weaned, females were excluded and males continued to receive the respective intervention diets until PND 42. For the dietary challenge in early adulthood (PND 43–98), animals of all experimental groups were switched to a moderate WSD. The additional ‘REF’ group (for further explanation of this group see above) received AIN-93M diet from PND 43 to 98. WSD was included in all experimental groups to provide a moderately nutritionally challenging environment corresponding to the human situation with a Western lifestyle. Food and water were available ad libitum.
Anthropometrics and body composition
Weight of the offspring was determined at PND 2, 4, 7 and weekly thereafter. Crown-rump length was measured bi-weekly until week 3 using a measuring ruler, and on PND 40 and 92 by means of micro computer tomograph (μCT).
A LaTheta LCT-100 (Aloka Co. Ltd) µCT was used for morphometry and whole-body scan (excluding tail) under isoflurane anaesthesia. The X-ray source tube voltage was set at 50 kV with a constant 1 mA current using a holder with inner diameter of 120 mm, resulting in pixel resolutions of 250 μm. Pitch size was 2·0 mm and scan speed was 4·5 s/image. The LaTheta software version 2.10 was used to measure body length (tip of the nose to base of the tail) and to determine volumes of whole-body fat, visceral and subcutaneous adipose tissue compartments. Different densities on the X-ray images were used to differentiate bone, air, adipose tissue and the remainder. Visceral and subcutaneous adipose tissue was determined by using abdominal scans between vertebrae L4 and L6 using the ‘Visceral Fat Measurement’ function of the software. Hereby, the abdominal muscle layer was used to differentiate visceral fat located inside the abdominal muscle from subcutaneous fat (area outside the abdominal muscle).
Termination and dissection
Animals were killed by cervical dislocation at PND 98 under deep isoflurane anaesthesia after overnight fasting. Organs (kidneys, liver, pretibial muscle and various fat depots (inguinal, mesenteric, retroperitoneal and perirenal)) were harvested immediately, weighed, snap-frozen in liquid N2 and stored at –80°C or fixed in 4 % paraformaldehyde and embedded in paraffin for later analyses.
Metabolic readouts and tissue analyses
At the age of PND 35–42 and 88–95, sixty out of eighty randomly selected offspring animals were housed in metabolic cages (Tecniplast) for 24 h to record food and water intake, as well as urine and faeces excretion. Blood was collected retro-orbitally after fasting overnight on PND 42 and 95 after isoflurane anaesthesia for 3 min. Fasted blood glucose and Hb concentrations were analysed immediately using 150 µl of blood (ABL 800 FLEX; Radiometer GmbH). The remaining blood was used to obtain EDTA-blood, EDTA-plasma and serum. Rat leptin, adiponectin and insulin concentrations in serum were determined using ELISA kits (Millipore) according to the manufacturer’s instructions. Similar to previous reports( Reference Nüsken, Dötsch and Rauh 15 ), Hb concentrations were determined by blood count; glycosylated Hb (HbA1c; as marker for long-term circulating glucose concentrations), total cholesterol, HDL-cholesterol (calculated as percentage of total cholesterol), TAG and total protein concentrations were measured by clinical routine laboratory procedures and corticosterone and 11-dehydrocorticosterone (11-DHC) concentrations by tandem MS. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using the formula HOMA-IR=(fasting insulin (mU/l) × fasting glucose (mmol/l)/22·5.
Quantitative real-time PCR
In mesenteric fat, retroperitoneal fat and liver and skeletal muscle, gene expressions of ‘metabolic’ genes were analysed by quantitative RT-PCR as previously described( Reference Nüsken, Wohlfarth and Lippach 17 ). We performed a thorough evaluation of reference genes in all tissues and finally used ribosomal protein S29 (RPS29) as the housekeeping gene, because it was most stable between different tissues and groups. Mitogen-activated protein kinase kinase kinase 12 (MAP3K12), heat-shock 70-kDa protein 1 (HSPA1), NADH:ubiquinone oxidoreductase subunit A5 (NDUFA5), uncoupling protein 2 (UCP2) and uncoupling protein 3 (UCP3) gene expressions were measured in skeletal muscle, PPARα, IGF-1, IGF-2 and insulin-like growth factor binding protein (IGFBP)-3 gene expressions in liver and leptin (LEP) gene expression in mesenteric and retroperitoneal fat. For primers and probes, see the online Supplementary Table S2.
Table 2 Anthropometric data and food intake(Mean values with their standard errors)

NOP, offspring of no operation (control) dams; LIG, offspring of ligated dams; SOP, offspring of sham-operated dams; PND, postnatal day; CTRL, normal matrix control diet (infant milk formula based); CLM, complex lipid matrix intervention diet (infant milk formula based); KW-test, Kruskal–Wallis test; LIG-CTRL v. NOP-CTRL, Mann–Whitney test comparing the groups LIG-CTRL and NOP-CTRL; LIG-CTRL v. LIG-CLM, Mann–Whitney test comparing the groups LIG-CTRL and LIG-CLM.
Experimental diets
The early programming CTRL and CLM diets contained 28·3 % w/w standard IF powder or Nuturis® IF powder (stage 1 IF recipe for infants aged between 0 and 6 months), respectively. The standard stage 1 IF powder was manufactured according to current standard processing procedures (Nutricia) yielding small non-coated fat droplets (mode diameter based on volume 0·4 (sem 0·0) μm( Reference Gallier, Vocking and Post 34 )) with a plant oil core consisting predominantly of rapeseed, sunflower, fish, coconut and palm oil. For the manufacturing of Nuturis® concept IF, processing procedures were applied as previously reported( Reference Van Den Brenk, Van Dijke and Van Der Sten 35 ). These modifications yielded large fat droplets with a vegetable oil core and a mode diameter based on volume of 4·3 (sem 0·1) μm( Reference Gallier, Vocking and Post 34 ). By adding bovine MFGM fragments (Fonterra Co-operative Group Ltd to a level of 1·5 wt% PL based on total fat, yielding 0·54 g/l reconstituted powder) during the manufacturing process, these large fat droplets were coated with a simplified layer of PL and other membrane components such as glycoproteins and cholesterol( Reference Gallier, Vocking and Post 34 ). These IF powders provided the complete fat fractions of the diets, which were then complemented with nutrients meeting requirements defined by the American Institute of Nutrition for the semi-synthetic AIN-93G laboratory rodent diet( Reference Reeves, Nielsen and Fahey 40 ). The moderate semi-synthetic WSD used as challenge for adult rodents contained 20 % (w/w) fat and 33 % sucrose. All diets were prepared by ssniff Spezialdiäten GmbH. An overview of diet composition is given in Table 1.
The CTRL and CLM diets were prepared as fresh dough by mixing 150 g of powder with 30 ml of water in a plastic bag and squeezed gently to retain the fat architecture. Once prepared, the dough ball with a weight of 60 g per animal was placed in the cages and replaced every 48 h. The remaining dough was stored in the plastic bag at 4°C for a maximum of 3 d. AIN-93G, WSD and the reference diet AIN-93M were stored at 4°C as pellets, directly placed in the cage and replaced at least once per week.
Statistical analysis
Sample size was calculated by G*Power Software version 3.1 before the start of the study. The calculation was designed to detect relevant differences either in blood parameters (e.g. leptin, insulin, glucose, TAG and cholesterol) or gene expression (e.g. LEP, IGF-1) or fat mass (e.g. visceral fat mass measured by µCT scan; weights of fat compartments). We aimed to detect a 50 % difference at 25 % sd with a power (1–β error) of 0·8 and a significance level (α error) of <0·05 in a two-sided Mann–Whitney test. Accordingly, sample size was calculated to be at least eight in each group.
All data were tested for outliers by Grubb’s test, and an outlier was excluded from some data sets. Thereafter, each parameter was analysed by a Kruskal–Wallis test including the groups LIG-CTRL, LIG-CLM, SOP-CTRL, SOP-CLM, NOP-CTRL and NOP-CLM. In case of significance, single-group comparisons were carried out by Mann–Whitney test (LIG-CTRL v. LIG-CLM, SOP-CTRL v. SOP-CLM, NOP-CTRL v. NOP-CLM, LIG-CTRL v. NOP-CTRL, LIG-CTRL v. SOP-CTRL, SOP-CTRL v. NOP-CTRL, LIG-CLM v. NOP-CLM, LIG-CLM v. SOP-CLM, SOP-CLM v. NOP-CLM). The significance level for the P-values generated by both tests was defined as P≤0·01 to avoid over-interpretation and account for multiple testing. All data are shown as means and standard error of the mean. Statistical analysis was performed using Graph Pad Prism Software version 6.
Results
Ligation and sham operation induced a phenotype of intra-uterine growth restriction
During the course of the experiment, one LIG-CTRL animal had to be excluded owing to lack of weight gain in the neonate, one LIG-CLM owing to weight loss after blood sampling and one LIG-CLM animal owing to significant lower weight gain throughout life. The remaining group sizes were as follows: NOP-CTRL, n 10; LIG-CTRL, n 9; SOP-CTRL, n 10; NOP-CLM, n 20; LIG-CLM, n 8; SOP-CLM, n 10; and ‘REF’, n 10.
At birth (PND 2), male LIG and SOP offspring were significantly (P≤0·01) lighter than NOP offspring regardless of the future assigned diet group. Length measured on PND 3 also was significantly reduced in LIG and SOP (Table 2). Body weight gain was similar in all groups except SOP-CLM compared with NOP-CLM during lactation and similar in all groups during the intervention period until PND 42 (online Supplementary Table S3). During WSD challenge, however, the LIG-CLM group gained 23 g less weight compared with LIG-CTRL (P=0·070), which in turn gained as much weight as the control group NOP-CTRL (Fig. 2). Comparisons of body length gain did not show significant differences. However, length was 0·5 cm less in LIG-CLM compared with LIG-CTRL on PND 92 (Table 2). BMI and food intake were similar in all groups, except a slightly increased food intake relative to body weight in group LIG-CLM v. LIG-CTRL at the second measurement (Table 2).

Fig. 2 Body weight gain in the ligation (LIG)-complex lipid matrix (CLM) (n 8) group compared with LIG-CTRL (n 9) during nutritional challenge by a moderate Western-style diet (WSD). The control group no operation (NOP)-normal matrix control diet (CTRL) (n 10) also is shown to illustrate weight gain during nutritional challenge after normal pregnancy and CTRL in early life. LIG animals fed CTRL diet showed full weight gain, resulting in a body weight similar to the healthy controls at postnatal day (PND) 89. LIG animals fed CLM before WSD exposure consistently accumulated less weight, but still narrowly missed statistical significance on PND 89 ((P=0·070); LIG-CLM compared with LIG-CTRL between PND 42 and PND 98). Values are means, with their standard errors. ![]() , NOP-CTRL;
, NOP-CTRL; ![]() , LIG-CTRL;
, LIG-CTRL; ![]() , LIG-CLM.
, LIG-CLM.
Table 3 Weights of organs and organ compartments (Mean values with their standard errors)
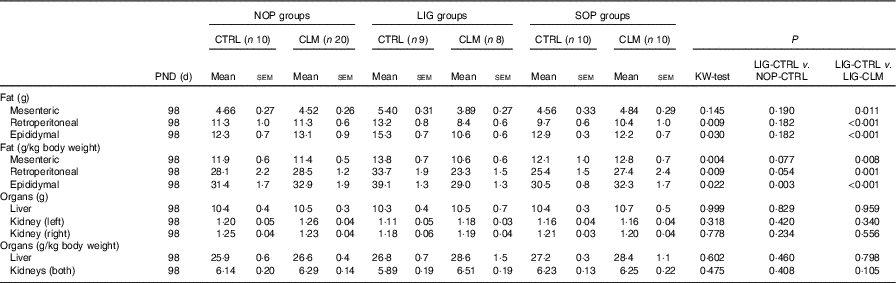
NOP, offspring of no operation (control) dams; LIG, offspring of ligated dams; SOP, offspring of sham-operated dams; PND, postnatal day; CTRL, normal matrix control diet (infant milk formula based); CLM, complex lipid matrix intervention diet (infant milk formula based); KW-test, Kruskal–Wallis test; LIG-CTRL v. NOP-CTRL, Mann–Whitney test comparing the groups LIG-CTRL and NOP-CTRL; LIG-CTRL v. NOP-CLM, Mann–Whitney test comparing the groups LIG-CTRL and LIG-CLM.
Complex lipid matrix diet reduced visceral fat mass in ligation both in early and later life
Most interestingly, the data from μCT scans indicated that CLM diet significantly (P≤0·01) reduced visceral fat mass in LIG offspring as early as PND 40 (LIG-CLM v. LIG-CTRL; Fig. 3(a)). On PND 92, this significant effect was even more pronounced, and LIG-CTRL animals now showed significantly more visceral fat mass compared with SOP-CTRL and NOP-CTRL (Fig. 3(b)). The gain of visceral and total fat mass between PND 40 and 92 also was less in LIG-CLM v. LIG-CTRL, and increased in LIG-CTRL v. SOP-CTRL (online Supplementary Table S4). For representative µCT scans, see Fig. 3(c) (LIG-CLM; PND 92) and 3(d) (LIG-CTRL; PND 92). In line with the µCT findings, absolute and relative weight of mesenteric, retroperitoneal and epididymal fat depots were significantly reduced in the LIG-CLM group compared with the other groups at PND 98. Group LIG-CTRL showed significantly increased retroperitoneal and epididymal fat weights compared with SOP-CTRL and increased epididymal fat weight compared with NOP-CTRL. Kidney and liver weights did not differ significantly (Table 3).
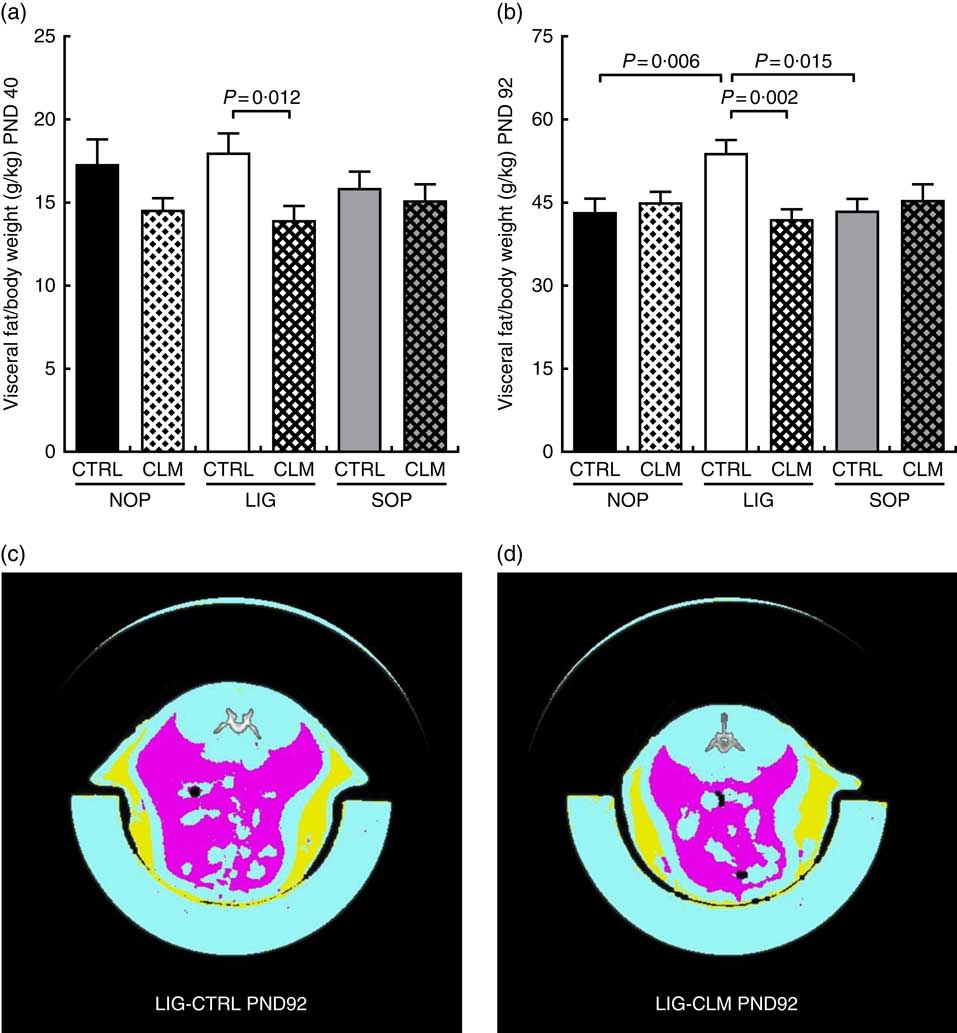
Fig. 3 Body composition before (a) and at the end of Western-style diet challenge (b) measured by micro computer tomograph. Example scans of the abdominal cavity, offspring from groups LIG-CTRL (c) and LIG-CLM (d) are shown. (c) and (d) show subcutaneous fat in yellow and visceral fat in magenta. NOP, offspring of no operation (control) dams; LIG, offspring of ligated dams; SOP, offspring of sham-operated dams; CTRL, normal matrix control diet (infant milk formula based); CLM, complex lipid matrix intervention diet (infant milk formula based); PND, postnatal day. Values are means, with their standard errors.
Table 4 Blood parameters (fasted) on postnatal days (PND) 42 and 96(Mean values with their standard errors).
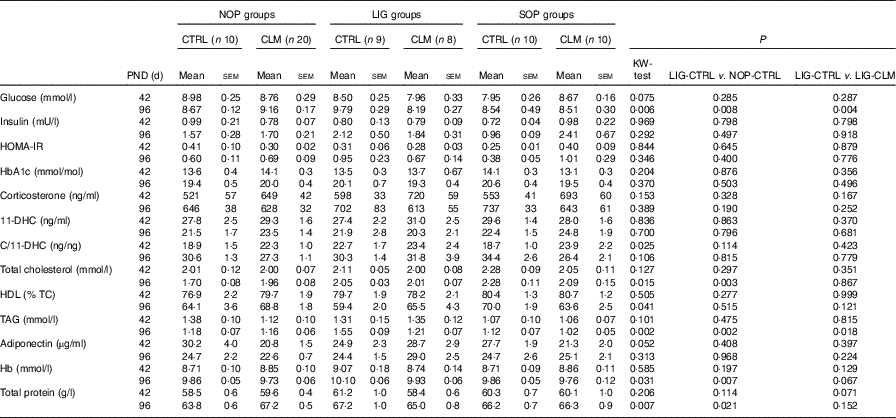
NOP, offspring of no operation (control) dams; LIG, offspring of ligated dams; SOP, offspring of sham-operated dams; CTRL, normal matrix control diet (infant milk formula based); CLM, complex lipid matrix intervention diet (infant milk formula based); KW-test, Kruskal–Wallis test; LIG-CTRL v. NOP-CTRL, Mann–Whitney test comparing the groups LIG-CTRL and NOP-CTRL; LIG-CTRL v. NOP-CLM, Mann–Whitney test comparing the groups LIG-CTRL and LIG-CLM; HOMA-IR, homoeostatic model assessment of insulin resistance; HbA1c, glycosylated Hb; 11-DHC, 11-dehydrocorticosterone; TC, total cholesterol.
Complex lipid matrix prevented leptin resistance and reduced basal glucose and TAG in later life
At the end of programming diet intervention (PND 42), serum leptin concentrations were comparable between groups (Fig. 4(a)). After the WSD challenge (PND 96), however, serum leptin was more than twice as high in LIG-CTRL compared with LIG-CLM, SOP-CTRL and NOP-CTRL animals (all significant (P≤0·01); Fig. 4(b)). Leptin relative to total body fat showed a similar pattern (Fig. 4(c)). The LIG-CTRL group was the only group to show a significant increase in leptin to total body fat ratio between PND 42 and 96 (Fig. 4(d)). In line with circulating leptin concentrations, LIG-CLM showed 3·2-fold lower LEP gene expression compared with LIG-CTRL (P=0·013) in mesenteric fat (Fig. 4(e)). This CLM effect was not present in retroperitoneal fat (Fig. 4(f)). Unlike in other tissues, however, the reference gene RPS29 showed some variation between the groups in fat, slightly limiting the significance of these results.
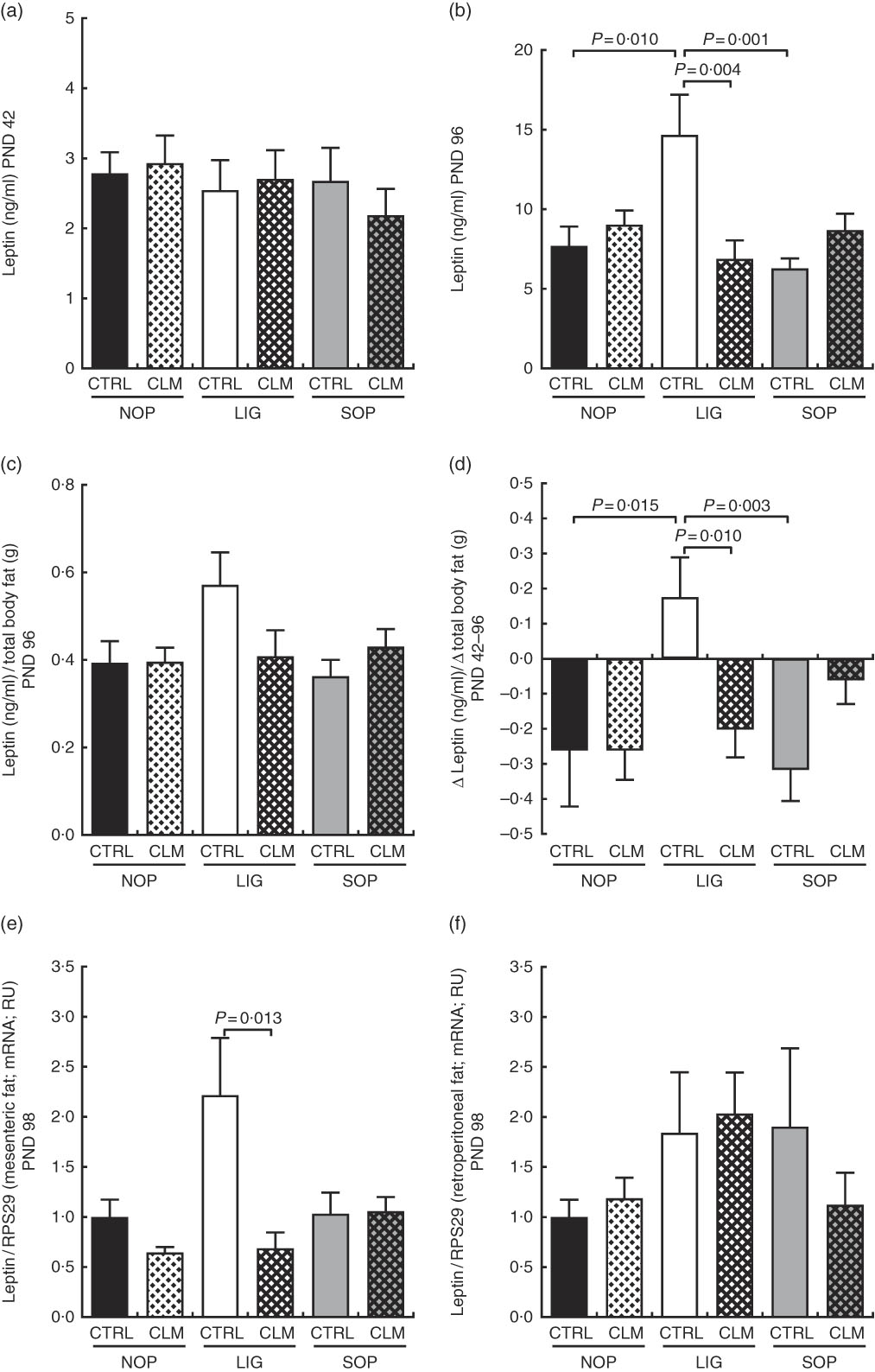
Fig. 4 Circulating leptin concentrations in serum at the end of the diet intervention period on postnatal day (PND) 42 (a) and at the end of the experiment on PND 96 (b); circulating leptin concentrations in relation to total body fat (micro computer tomograph) on PND 96 (c); delta of circulating leptin concentrations in relation to delta of total body fat between PND 42 and PND 96 (d); leptin gene expression in mesenteric (e) and retroperitoneal (f) fat compartments on PND 98. NOP, offspring of no operation (control) dams; LIG, offspring of ligated dams; SOP, offspring of sham-operated dams; CTRL, normal matrix control diet (infant milk formula based); CLM, complex lipid matrix intervention diet (infant milk formula based); RPS29, ribosomal protein S29; RU, relative units. Values are means, with their standard errors represented by vertical bars.
Blood glucose concentrations were similar in all groups on PND 42, but significantly reduced in LIG-CLM compared with both LIG-CTRL and NOP-CLM, and significantly increased in LIG-CTRL compared with NOP-CTRL animals on PND 96. Serum insulin and HOMA-IR, however, were comparable between groups (Table 4). Fasted serum TAG concentrations were similar in all groups on PND 42. On PND 96, TAG concentrations were lower in LIG-CLM compared with LIG-CTRL (P=0·018) and significantly increased in LIG-CTRL compared with both SOP-CTRL and NOP-CTRL (Table 4). Circulating total cholesterol concentrations were comparable between groups on PND 42, but significantly increased in both LIG-CTRL and SOP-CTRL compared with NOP-CTRL on PND 96. However, there was no significant CLM effect (Table 4). Circulating HbA1c, corticosterone, 11-DHC, HDL-cholesterol and adiponectin concentrations were similar in all groups. Hb and total protein concentrations showed some small differences between specific groups (Table 4).
Complex lipid matrix reduced liver insulin-like growth factor 1 and attenuated increased skeletal muscle uncoupling gene expression in the ligation group
Expression levels of other ‘metabolic’ genes were measured in liver and skeletal muscle to gain first insight in molecular mechanisms of reduced fat accumulation in the LIG-CLM group (Table 5). In the liver, IGF-1, IGF-2 and IGFBP-3 gene expressions were measured to evaluate genes encoding growth factors. IGF-1 was significantly (P≤0·01) reduced in both LIG-CLM compared with LIG-CTRL (1·4-fold) and SOP-CLM compared with SOP-CTRL (1·3-fold). IGF-2 gene expression was significantly reduced in LIG-CLM v. NOP-CLM only (1·5-fold), but similar compared with LIG-CTRL. We did not find other significant differences in liver gene expressions in any group of animals, including PPARα, which was measured to ensure similar energy status and lipid metabolism in all groups.
Table 5 Gene expression data in muscle and liver on postnatal day (PND) 98 (Mean values with their standard errors).
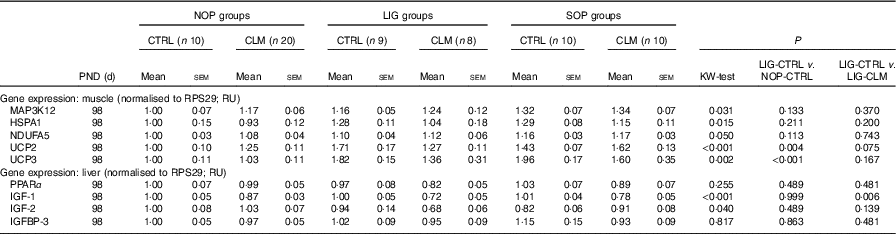
NOP, offspring of no operation (control) dams; LIG, offspring of ligated dams; SOP, offspring of sham-operated dams; CTRL, normal matrix control diet (infant milk formula based); CLM, complex lipid matrix intervention diet (infant milk formula based); KW-test, Kruskal–Wallis test; LIG-CTRL v. NOP-CTRL, Mann–Whitney test comparing the groups LIG-CTRL and NOP-CTRL; LIG-CTRL v. NOP-CLM, Mann–Whitney test comparing the groups LIG-CTRL and LIG-CLM; RPS29, ribosomal protein S29; RU, relative units; MAP3K12, mitogen-activated protein kinase 12; HSPA1, heat-shock 70 kDa protein 1; NDUFA5, NADH:ubiquinone oxidoreductase subunit A5; UCP, uncoupling protein; IGF, insulin-like growth factor; IGFBP, insulin-like growth factor binding protein.
In skeletal muscle, MAP3K12, HSPA1 and NDUFA5 gene expressions were measured to exclude locomotor activity-related gene regulation. There were some significant, however, small differences in SOP-CTRL compared with NOP-CTRL only (differences≤1·3-fold; Table 5), indicating similar locomotor activity in all groups. UCP2 and UCP3 gene expressions were measured to evaluate mitochondrial function. For UCP2, we found significant inductions in both LIG-CTRL (1·7-fold) and SOP-CTRL (1·4-fold) v. NOP-CTRL, as well as in SOP-CLM v. NOP-CLM (1·3-fold). Comparison between LIG-CLM and LIG-CTRL merely showed a trend for decreased UCP2 gene expression in LIG-CLM (P=0·075). For UCP3, there were significant inductions in both LIG-CTRL v. NOP-CTRL (1·8-fold) and SOP-CTRL v. NOP-CTRL (2·0-fold; Table 5), but no significant reduction in LIG-CLM compared with LIG-CTRL.
Discussion
There is a growing body of evidence that quantity and quality of early-life nutrition has long-lasting effects on health and disease risk throughout life. Providing an optimal diet in infancy and childhood is a key factor in the primary prevention of disease( 41 ). HM provided by breast-feeding is established as the preferred infant feeding and serves as normative standard for infant nutrition( Reference Eidelman 42 ). Accumulating evidence shows that breast-feeding protects against adiposity later in life( Reference Yan, Liu and Zhu 43 ), although the exact underlying mechanisms are not sufficiently understood.
In this study, we demonstrate in an ‘at-risk’ population that the supramolecular structure of dietary lipids in early postnatal nutrition is a novel aspect of nutrient quality that has to be considered in the context of primary prevention of obesity and metabolic disease. Human MFG are large and their membranes are complex( Reference Martini, Salari and Altomonte 30 ). By nutritional intervention with a CLM diet containing artificial large lipid droplets coated with bovine MFGM fragments (Nuturis®) during rat development from the beginning of complimentary feeding until late adolescence( Reference Evans 39 ), we were able to alleviate the well-known metabolic long-term sequelae in our established IUGR rat model. The main feature of the CLM diet is the supramolecular lipid structure inspired by the model of HM droplets. In addition, minor differences between CLM and standard IF exist in the amount of PL and cholesterol( Reference Gallier, Vocking and Post 34 ). A recent study investigating the contribution of the different features of the altered physical structure, for example, droplet size and MFGM addition, showed that only the complete concept resulted in the long-term protection for body composition development( Reference Baars, Oosting and Engels 38 ). In our study, CLM diet significantly reduced visceral fat mass in LIG offspring as early as PND 42. In addition, gain of visceral fat mass was reduced in LIG-CLM compared with LIG-CTRL offspring between PND 42 and 98. The metabolic profile was improved despite equal diet and comparable food intake. Furthermore, metabolic outcome in LIG-CLM offspring was improved despite postnatal transfer of all LIG offspring to healthy foster mothers, which also may ameliorate metabolic sequelae( Reference Siebel, Gallo and Guan 44 ). These results indicate a significant impact of complex lipid globules in early-life nutrition on metabolic outcome in our LIG animals. Although LIG-CLM animals tended towards slower body weight and length gain, other organ weights were not affected. In contrast to previous findings in mice( Reference Oosting, Kegler and Wopereis 36 – Reference Baars, Oosting and Engels 38 ), there is a certain resistance to WSD-induced fat accumulation in our non-IUGR rats as described before( Reference Oosting, Kegler, van de Heijning, Verkade and van der Beek 45 ). The limited effect of CLM in healthy rats compared with mice may be owing to relatively normal body composition in rats despite WSD challenge, which leaves ‘little window of opportunity to ameliorate the outcome’( Reference Oosting, Kegler, van de Heijning, Verkade and van der Beek 45 ). Thus, the possibility to draw conclusions about the protective effect of CLM in healthy individuals may be limited in rat studies that try to exacerbate the phenotype by WSD.
A reduction of adult fat accumulation and an improved metabolic profile has already been shown in healthy yet WSD-challenged mice that were fed CLM diet postnatally( Reference Oosting, Kegler and Wopereis 36 , Reference Oosting, van Vlies and Kegler 37 ). To date, acquisition of clinical data on possible effects of the supramolecular lipid structure (Nuturis®) on growth, body composition and long-term metabolic health in infants is ongoing (clinical trial registry NCT01609634 and the Dutch Trial Register NTR3683). A low-energy, low-protein and bovine MFGM fragment containing IF in healthy term infants resulted in cholesterol concentrations and cognitive development more similar to those of breast-fed infants at 1 year of age compared with standard IF( Reference Oosting, Kegler, van de Heijning, Verkade and van der Beek 45 ), suggesting that dietary MFGM fragments provide health benefits( Reference Timby, Domellof and Hernell 46 , Reference Spitsberg 47 ). However, both energy and protein content were also modified, and long-term data were not recorded. Mice showed long-term metabolic improvement only when MFGM were present at the surface of large lipid droplets( Reference Baars, Oosting and Engels 38 ). Thus, long-term metabolic benefits may only arise when the MFGM-derived PL are present as a coating on large-sized lipid droplets.
The adult phenotype of increased fat mass, hyperglycaemia, hypertriglyceridaemia and hyperleptinaemia in LIG offspring has been reported before by our group( Reference Nüsken, Dötsch and Rauh 15 ). Circulating metabolic markers in this study confirmed that IUGR animals born after utero-placental insufficiency are at risk for an adverse metabolic profile. To obtain first insights into the underlying molecular mechanisms of metabolic protection by the supramolecular structure of dietary lipids, we analysed the expression of ‘metabolic’ genes in fat, muscle and liver. In mesenteric fat, a reduction of LEP gene expression by CLM diet in the LIG group suggested that CLM did not only reduce circulating leptin concentration by reducing fat mass, but also by affecting leptin production in an abdominal fat compartment that may affect energy homoeostasis( Reference Guo, Halo and Leibel 48 ). In the liver, we showed a moderately reduced IGF-1 gene expression in LIG-CLM compared LIG-CTRL offspring, which may have contributed to the reduced gain of fat mass and altered growth( Reference Socha, Grote and Gruszfeld 49 ). Our observations are in line with findings reported in term SGA infants, in which breast-feeding was associated with reduced IGF-1 and glucagon-like peptide-1 levels compared with formula feeding( Reference Diaz, Bassols and Sebastiani 27 ). Similar hepatic PPAR α gene expression reflects compensated energy metabolism in all groups. Increased uncoupling protein (UCP) gene expressions in LIG and SOP offspring on CTRL indicate less-efficient mitochondrial β-oxidation, probably increasing energy expenditure and preventing additional fat deposition( Reference Busiello, Savarese and Lombardi 50 ). However, UCP gene expression was not further elevated in the CLM intervention groups.
In conclusion, this study provides clear evidence that a complex supramolecular structure of lipids in the early-life diet has a beneficial re-programming effect on long-term visceral fat accumulation and metabolic health in animals formerly exposed to utero-placental insufficiency. In rats, however, CLM seems to predominately mitigate metabolic sequelae caused by an adverse fetal environment, without modifying the metabolic profile of healthy individuals. The structure of dietary lipids is a novel aspect of nutrient quality that should be considered in the context of primary prevention of obesity and metabolic disease in ‘at-risk’ populations.
Acknowledgements
The authors thank Diane Kegler for her guidance on experimental design and animal welfare, Mona Mischke for her assistance in gene expression techniques and Andrea Kodde for her support in mRNA isolation from WAT and her expertise in housekeeping gene expression in different tissues. The authors gratefully acknowledge the expert technical assistance of Jens Alber in processing of µCT scans.
J. D. and K. D. N. received a restricted research grant from Nutricia Research B.V., Utrecht, The Netherlands, to perform the project.
I. C. T., A. O., E. M. V. d. B., J. D. and K. D. N. were involved in concept development and in experimental design; H. H.-K., H. B., P. N.-H., G. L., M. W. and K. D. N. were involved in conduction of experiments; I. C. T., H. H.-K., H. B., P. N.-H., M. R., G. L., M. W. and K. D. N. were involved in sample analysis, data analysis and interpretation; and all authors were involved in manuscript development.
I. C. T., A. O. and E. M. v. D. B. are employees of Nutricia Research B.V., Utrecht, The Netherlands.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518001988












