Introduction
Palmer amaranth is a dioecious annual forb (Sauer Reference Sauer1955). It is adapted to intense heat and low rainfall and can germinate rapidly to complete its life cycle in response to available moisture (Ehleringer Reference Ehleringer1983). Palmer amaranth initiates growth each spring from seed present in the seedbank. It is a very prolific seed producer, with a single female plant capable of producing up to 600,000 seeds (Keeley et al. Reference Keeley, Carter and Tullen1987). Its seeds are relatively persistent in the seedbank, but research suggests that the viability of seeds significantly drops after 3 yr in the soil (Sosnoskie et al. Reference Sosnoskie, Webster and Culpepper2013).
Palmer amaranth is recognized as one of the most troublesome agricultural weed species in the United States (Webster Reference Webster2001). It has an aggressive growth habit and is extremely competitive with row crops even at low densities (Rowland et al. Reference Rowland, Murray and Verhalen1999). It has been reported to grow 7.62 cm per day and commonly reaches heights of 1 to 3 m. Studies have shown that Palmer amaranth reduced corn (Zea mays L.) yields 11% to 91% (Massinga et al. Reference Massinga, Currie, Horak and Boyer2001; Massinga and Currie Reference Massinga and Currie2002) and reduced soybean (Glycine max L. Merr.) yields 17% to 68% (Klingaman and Oliver Reference Klingaman and Oliver1994). Palmer amaranth can possess toxic properties that are harmful to livestock including high concentrations of nitrates (Schmutz et al. Reference Schmutz, Freeman and Reed1974) and oxalate (Saunders and Becker Reference Saunders and Becker1984).
Palmer amaranth has evolved resistance to multiple herbicide mechanisms of action including herbicides that inhibit acetolactate synthase, dinitroanilines, triazines, glyphosate, and inhibitors of 4-hydroxphenylpyruvate dioxygenase (Ward et al. Reference Ward, Webster and Steckel2013). Glyphosate was an important tool for managing Palmer amaranth in the late 1990s (Culpepper and York Reference Culpepper and York1998). Some researchers argued that resistance to glyphosate was unlikely due to its unique properties including its mode of action, metabolism, chemical structure, and lack of residual activity in soil (Bradshaw et al. Reference Bradshaw, Padgette, Kimball and Wells1997). However, glyphosate-resistant Palmer amaranth was first discovered in Georgia in 2004 (Culpepper et al. Reference Culpepper, Grey, Vencill, Kichler, Webster, Brown, York, Davis and Hanna2006) and resistant plants have since been identified in other states (Nandula et al. Reference Nandula, Reddy, Kroger, Poston, Rimando, Duke, Bond and Ribeiro2012; Steckel et al. Reference Steckel, Main, Ellis and Mueller2008). Resistance evolved in cropping systems that were exposed to repeated glyphosate use with a lack of diversity in weed management (Culpepper et al. Reference Culpepper, Grey, Vencill, Kichler, Webster, Brown, York, Davis and Hanna2006). Palmer amaranth has become one of the most economically damaging glyphosate-resistant weeds in the United States (Beckie Reference Beckie2011). Protoporphyrinogen oxidase (PPO) inhibitors became a popular option for controlling glyphosate-resistant Palmer amaranth biotypes (Owen and Zelaya Reference Owen and Zelaya2005). Similar to the development of glyphosate resistance, repeated use of PPO-inhibiting herbicides on Palmer amaranth has resulted in the evolution of resistance, which was first reported in Arkansas (Salas et al. Reference Salas, Burgos, Tranel, Singh, Glasgow, Scott and Nichols2016).
A critical step in managing Palmer amaranth is early identification. However, it can be difficult to correctly identify the plant early in the season because it looks very similar to other seedling Amaranth species including redroot pigweed (Amaranthus retroflexus L.), smooth pigweed (A. hybridus L.), Powell amaranth (A. powellii S. Watson ssp. powelii), and common waterhemp (A. rudis Sauer). Despite these similarities, Palmer amaranth has distinctive features at later growth stages, but these features are often visible well after the window of opportunity for successful herbicide management and may go unnoticed until after seed maturation and dispersal. The seed head of Palmer amaranth is generally much longer than other pigweed species, and female seed heads have distinctively sharp bracts. Terminal seed heads can reach up to 0.5 m in length (Elmore Reference Elmore1990). The leaves, stems, and petioles are completely hairless. Leaves are oval to diamond-shaped and are arranged in a rosette-like appearance. The most consistent and reliable characteristic that differentiates Palmer amaranth from other pigweed species is the petiole length. The petioles, particularly on older leaves, will exceed the length of the leaf blade.
Palmer amaranth is a successful invasive species, which is evidenced by its expansion in North America and overseas (Mosyakin and Robertson Reference Mosyakin and Robertson2003). Palmer amaranth is native to the southwestern United States and northwestern Mexico. Population spread beyond its natural range started in the early 20th century and has been attributed to human activity transporting seeds and creating new habitats through agricultural expansion (Sauer Reference Sauer1957). Palmer amaranth seeds can also be distributed through irrigation waters (Wilson Reference Wilson1980), wind (Menges Reference Menges1987), and agricultural practices (i.e., plowing, herbicide application, mowing, harvesting, etc.; Norsworthy et al. Reference Norsworthy, Griffith, Scott, Smith and Oliver2008). It was first reported outside of its established native range in Virginia in 1915 but was not considered to be problematic (Sauer Reference Sauer1957). Eventually, it was reported in Oklahoma in 1927, and North Carolina in 1957 (Culpepper et al. Reference Culpepper, Webster, Sosnoskie and York2010; Sauer Reference Sauer1957). It continued to spread and became the most troublesome weed of cotton in both North Carolina and South Carolina by 1995 (Dowler Reference Dowler1995). In 2009, Palmer amaranth was considered the most troublesome weed of cotton in the southern United States (Webster and Nichols Reference Webster and Nichols2012). Palmer amaranth has since spread to several other regions including the U.S. Corn Belt.
The goal of this paper is to document the prompt actions taken by many different stakeholders that prevented the spread of Palmer amaranth in Minnesota and minimized the negative impacts on agricultural and natural environments once it was introduced into the state. Establishing the appropriate regulatory framework, providing funding, fostering collaboration between partners, and actively responding to new infestations were critical to the success of combatting Palmer amaranth (Figures 1 and 2).
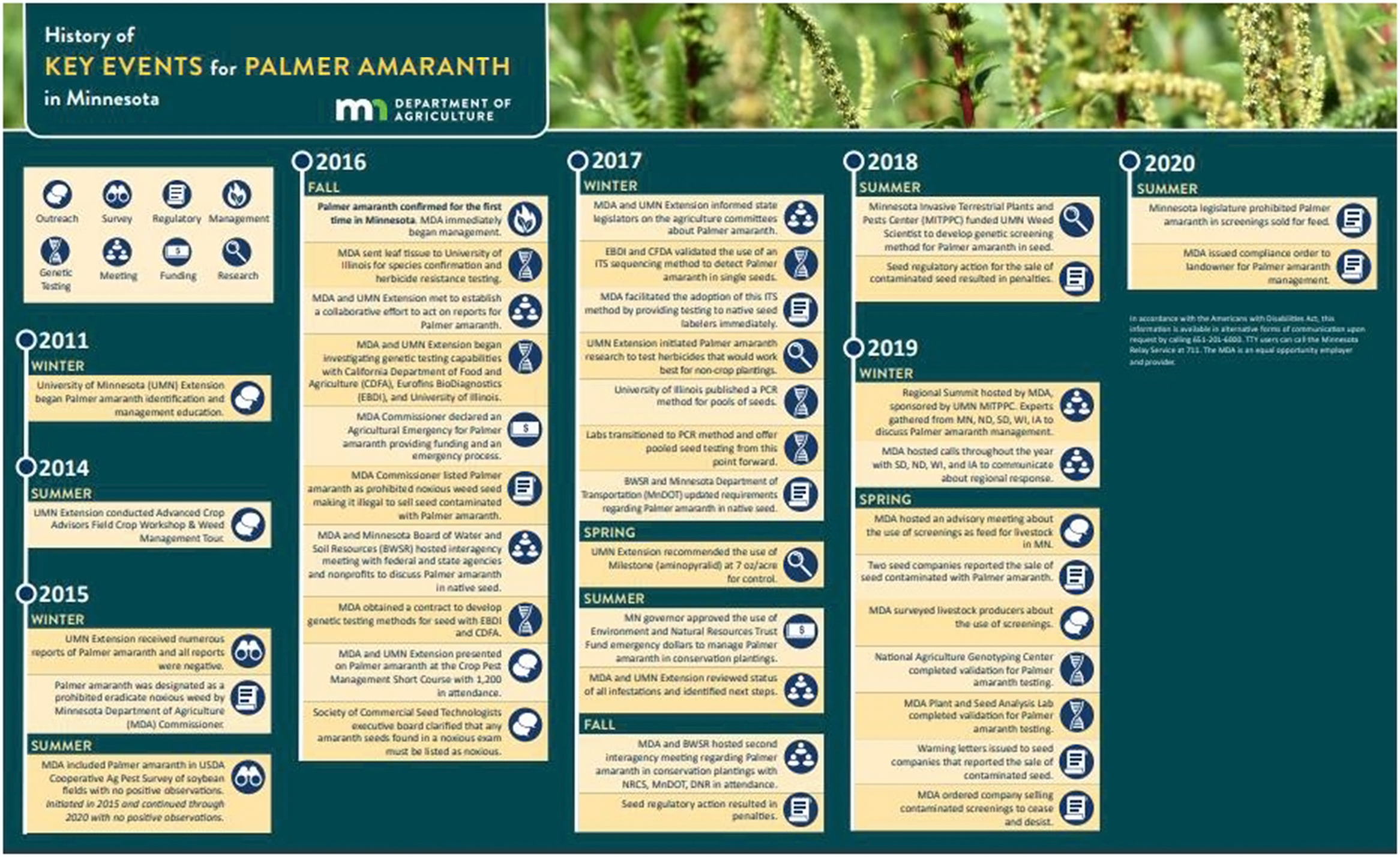
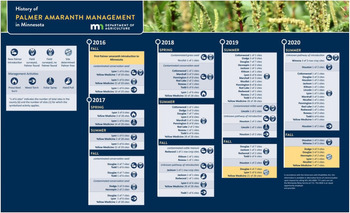
Figure 1. History of key events for Palmer amaranth in Minnesota.
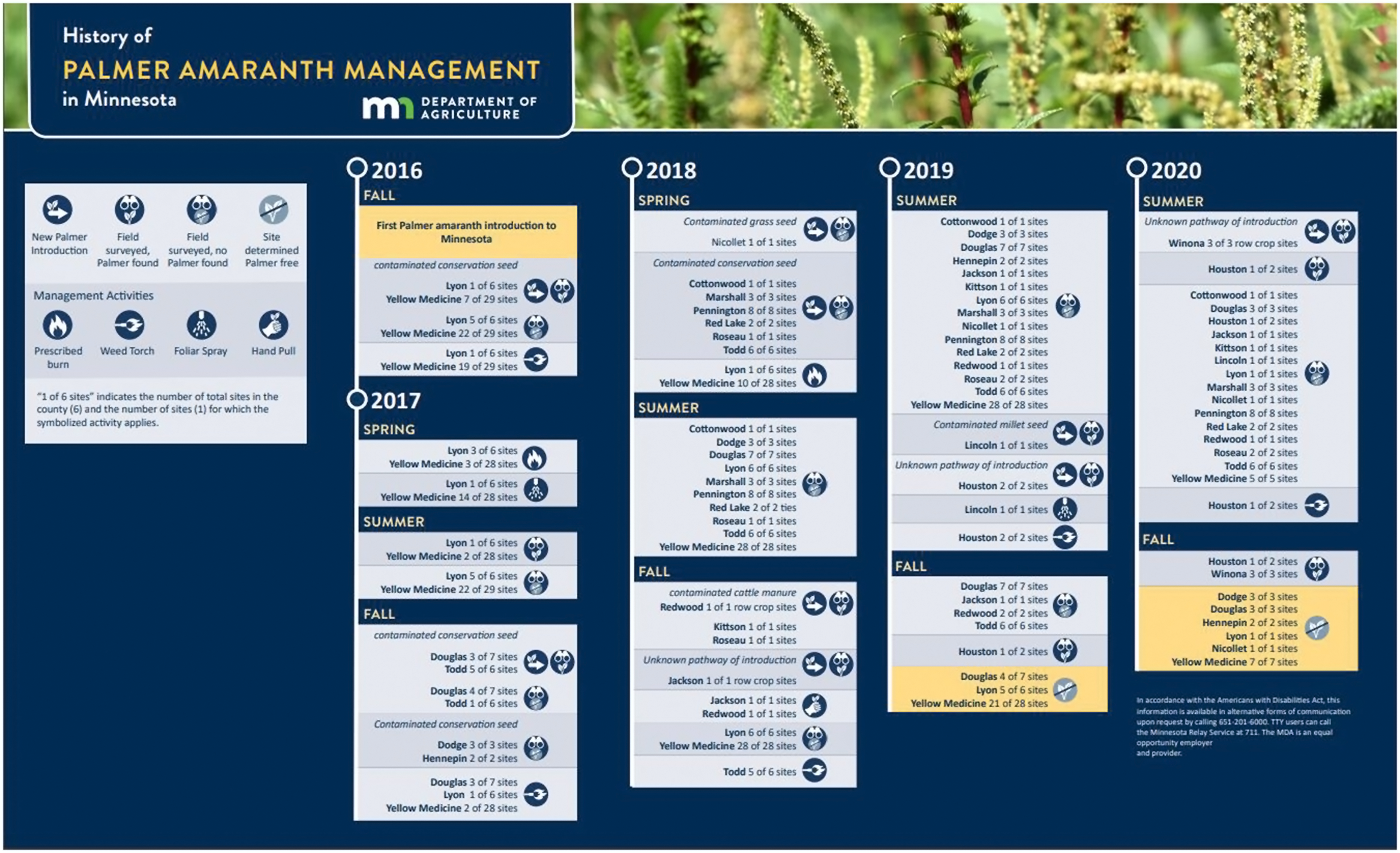
Figure 2. History of Palmer amaranth management in Minnesota.
Legal Status of Palmer Amaranth in Minnesota
By 2014 Palmer amaranth had spread northward from southern states, and was confirmed in Illinois, Iowa, Nebraska, South Dakota, and Wisconsin. The spread into the upper Midwest is believed to have occurred through a variety of pathways including movement of contaminated livestock feed and equipment, planting seed from infested regions, and other dispersal methods (Anderson Reference Anderson2015; Anderson et al. Reference Anderson, Hartzler and Jha2020; Hoppe et al. Reference Hoppe, Ikley, Jenks and Keena2020). Resistance to herbicides commonly used for management of weeds in corn and soybeans has also contributed to the spread of Palmer amaranth (Legleiter and Johnson Reference Legleiter and Johnson2013). In 2014, the Minnesota Department of Agriculture (MDA) and Minnesota Noxious Weed Advisory Committee (NWAC) made a recommendation to designate Palmer amaranth as a Prohibited Eradicate Noxious Weed after it was first reported in southern Iowa (Hartzler and Pope Reference Hartzler and Pope2013). In 2015, Palmer amaranth was officially designated as Prohibited Eradicate, meaning all aboveground and belowground parts of the plant must be destroyed. Additionally, no transportation, propagation, or sale of this plant is allowed. The intent of the Minnesota Noxious Weed Law is to target the species at the beginning of its invasion curve before eradication becomes difficult or impossible. Fortuitously, Palmer amaranth was added to the Minnesota Noxious Weed List 1 yr before Palmer amaranth was documented in the state. This listing provided awareness to farmers and landowners regarding the seriousness of Palmer amaranth and allowed for a quick response by state regulatory officials to manage infestations. In November 2016, the Commissioner of Agriculture listed Palmer amaranth as a Prohibited Noxious Weed Seed in agricultural, vegetable, flower, tree, shrub, native grass, and forb seeds sold in Minnesota after contaminated Conservation Reserve Program (CRP) seed mixes were the source for the first documented Palmer amaranth infestations in the state. It is not legal to sell a seed lot containing or contaminated with Palmer amaranth. In 2020, the Minnesota Screenings Act was updated by the legislature to prohibit Palmer amaranth and other noxious weeds in screenings sold for feed.
Partnerships
Minnesota was prepared for the first discovery of Palmer amaranth in the state due to the network of private and public stakeholders. In 2011, the University of Tennessee presented the challenges of identifying and managing Palmer amaranth to the University of Minnesota (UMN) Extension, which initiated the first of many presentations regarding Palmer amaranth identification and management. By late 2013, UMN Extension members who served on the NWAC advocated for listing Palmer amaranth. In 2015, UMN Extension received numerous reports of Palmer amaranth in crop production fields, and MDA surveyed 467 soybean fields with no detections. Also in 2015, the Minnesota Department of Transportation (MnDOT) added Palmer amaranth to the Minnesota Noxious Weeds book (Hanson Reference Hanson2020).
MDA worked to manage numerous invasive plants prior to the discovery of Palmer amaranth in the state. Previous work on priority species such as Oriental bittersweet (Celastrus orbiculatus Thunb.), Japanese hops (Humulus japonica Siebold & Zucc.), common teasel (Dipsacus fullonum L.), and cut-leaf teasel (D. laciniatus L.) led to the protocol that MDA implemented for early detection and eradication of Palmer amaranth in the state.
Resources were made possible by the Minnesota Environment and Natural Resources Trust Fund, as recommended by the Legislative-Citizens Commission on Minnesota Resources (LCCMR), and the immediate availability of MDA emergency funds. Additionally, MDA was able to offer financial support to UMN Extension, which enabled the co-development of management and containment strategies.
In 2017, MDA participated in industry meetings in Iowa where Palmer amaranth had also been introduced in CRP seed plantings. In 2017 and 2018, MDA facilitated meetings with the UMN, MnDOT, Minnesota Department of Natural Resources, Minnesota Board of Water and Soil Resources, and United States Department of Agriculture (USDA) Natural Resources Conservation Service and Farm Service Agency to ensure that state and federal agencies were addressing the risks and identifying pathways of introduction for Palmer amaranth into the state. Through these efforts, MDA, UMN Extension, USDA, Conservation Corps Minnesota and Iowa (CCMI), and landowners throughout the state work cooperatively to detect and eradicate Palmer amaranth infestations in Minnesota before they spread to new areas.
In 2019, the Minnesota Invasive Terrestrial Plants and Pests Center (MITPPC) and MDA co-sponsored a joint Palmer Summit at the UMN. University, government, and industry experts gathered from Iowa, North Dakota, South Dakota, and Wisconsin to discuss Palmer amaranth detection and management. Communication between these experts continued throughout the year with bimonthly meetings.
MDA lead numerous management efforts for Prohibited Eradicate species, including Palmer amaranth, with grant funding. In general, Minnesota landowners are responsible for the labor and financial burden of managing noxious weeds on their properties and a penalty may result if left untreated. Fortunately, MDA has had access to state general funds and received funding from LCCMR to scout, monitor, and treat high-priority noxious weeds with cooperation from landowners.
Genetic Testing
Adding Palmer amaranth to both the Noxious Weed and Noxious Seed lists gave MDA probable cause to investigate seed sold in Minnesota, which has led to the discovery of several contaminated seed lots and screening shipments. Prior to Minnesota’s emergency declaration, a seed analyst visually identified noxious weed seeds in purity and noxious tests. In December 2016, the Society of Commercial Seed Technologists Executive Board issued a statement clarifying that seed analysts would not be able to visually identify Palmer amaranth. Therefore, all amaranth seeds found in testing should be listed as noxious in the absence of other information and acknowledged the potential development of genetic testing for identification.
A genetic method was developed, validated, and became available in a short time frame using MDA’s emergency fund. In 2017, two laboratories, Eurofins BioDiagnostics and the California Department of Food and Agriculture, sent MDA a validation report for an internal transcribed spacer (ITS) sequencing method that could identify Palmer amaranth with single seeds. This method amplified and sequenced the ITS region (18S–26S nuclear ribosomal DNA). Seeds were pulverized for DNA extraction, then DNA was amplified using polymerase chain reaction (PCR), followed by sequencing the amplified region (Price Reference Price2016). MDA allowed native seed companies to submit amaranth seeds for genetic testing to facilitate compliance and prevent Palmer amaranth from being established through planting of a variety of seed mixes and individual seed lots. MDA’s swift and aggressive response to Palmer amaranth led to the first genetic tests available on a larger scale for detection in seed and ultimately changed the way seed is tested and labeled.
Genetic testing technology and costs have changed over time with a shift to PCR methods (Murphy et al. Reference Murphy, Plewa, Phillippi, Bissonnette and Tranel2017). PCR methods are advantageous because they allow the testing of multiple seeds at a time, thus increasing efficiency and lowering cost per sample. Five laboratories currently offer genetic testing services for Palmer amaranth enabling seed industry compliance. Minnesota updated the All-States Noxious Weed Seed List to clearly indicate genetic testing is required on any amaranth contaminant in seed being sold in the state.
In 2018 a collaborative research project at the UMN led to the development of a genetic test based on novel, species-specific, single-nucleotide polymorphisms (SNPs) from genotype by sequencing data. The study used three SNP-based genetic tests for identifying Palmer amaranth alone or in a mixed pool of Amaranthus spp. and is applicable to both leaf tissue and pools of seeds (Brusa et al. Reference Brusa, Patterson, Gaines, Dorn, Westra, Sparks and Wyse2021). The project was funded by MITPPC. These new markers are not yet available in a commercial setting.
Management Strategies and Tools
Early detection and rapid response to Palmer amaranth infestations was critically important for preventing its spread in the state. MDA and UMN Extension put a great deal of effort into educating landowners and the public about the threat and identification of Palmer amaranth. Crop consultants and commodity organizations have added significantly to this effort. As a result, the number of reports submitted to MDA and UMN Extension from concerned landowners has significantly increased. MDA also made it easy for Minnesota residents to report suspect invasive species, such as Palmer amaranth, through MDA’s Arrest the Pest program. MDA followed up on all reports that were suspected to be Palmer amaranth. After visual confirmation of Palmer amaranth plants at a given site, samples of all suspected plants were submitted for genetic testing for species determination. MDA and UMN immediately worked with landowners, crop consultants, and county agricultural inspectors to survey sites where Palmer was confirmed and roughly a 5-mile (8-km) circumference of the surrounding areas. Management activities across all sites included either prescribed burns, weed torching, foliar sprays, hand pulling, or a combination of activities.
Burning
Most Palmer amaranth sites were found during the late summer and fall after female plants had formed mature seed heads. Therefore, burning was the most efficient management tool available to destroy plant populations, including the seed. MDA used two burning strategies: prescribed burn and the use of propane torches to incinerate small populations of Palmer amaranth plants (Figure 3). MDA coordinated these efforts with landowners and CCMI, which then conducted the prescribed burns and torching. Each site with confirmed Palmer amaranth plants was considered for either a full burn of the site, or spot treatment. Sites that had a continuous thatch layer across the field, without gaps of bare soil, were completely burned. Sites that did not meet this criterion were torched in specific areas where Palmer amaranth plants were mapped and/or flagged.
After the initial finds in 2016 we learned that both prescribed fire and torching can be an excellent management technique. The lack of emergence of Palmer amaranth seedlings suggested that the propane torches, which produced at least 1,093 C, provided a heat treatment hot enough to kill Palmer amaranth seeds on the ground.

Figure 3. (A) CCMI crew members burning a Palmer plant in Lyon County using propane torches. (B) Scorched ground after Palmer incineration using propane torches.
Herbicide Application
In addition to prescribed burns and torching, a foliar herbicide was applied in 2017 on a majority of the 2016 infested sites. This was a response to the large acreage sown with contaminated seed mixes and to provide extra protection against any seed that germinated after the fall of 2016 and spring of 2017 burning events.
Although many herbicide formulations are labeled for Palmer amaranth control in cropping systems, little research had occurred on herbicide control of Palmer amaranth in non-crop areas. Soon after the initial discovery of Palmer amaranth in CRP plantings in 2016, the UMN Extension, with support from MDA’s Agricultural Emergency Funds, began greenhouse studies to define the efficacy of herbicides on Palmer amaranth. These herbicides were labeled for non-crop use, so they could be applied on CRP lands.
The UMN Extension screened a variety of herbicide products that were labeled for non-crop use to assess pre- and post-emergent options for Palmer amaranth control (R Becker, personal communication). The main objective was to find a product that would be highly effective on Palmer amaranth yet minimize injury to desirable forbs in conservation plantings. From these trials, the UMN Extension concluded that aminopyralid (Milestone®, Corteva Agriscience, Wilmington, DE) had less impact on forbs than other equally efficacious products such as 2,4-D or dicamba. Clopyralid (Transline®, Corteva Agriscience, Wilmington, DE), commonly used for thistle control in native forb rich plantings, was not efficacious on Palmer amaranth. Based on these results, MDA used a POST broadcast application of Milestone® at 200 g acre−1 at most affected sites. Although the treatment killed most of the weeds, including Palmer amaranth, it also affected other broadleaf species. Further assessment in following years showed that even though the herbicide applications negatively affected some of the native forbs establishing on site, a good mixture of native broadleaf plants and grasses have since established, maintaining a competitive vegetative cover against Palmer amaranth and other weedy species. Milestone®, at 200 g acre−1, appears to be a viable choice for landowners who want to manage Palmer amaranth in mixed plant communities while facilitating recovery of desirable forbs and shrubs.
Pathways
MDA investigated potential sources for all Palmer amaranth infestations. If suspected weeds were found in a field that was recently planted, MDA investigated the seed planted to identify possible sources of the infestation. If the seed tested positive for the presence of Palmer amaranth, the labeler was ordered to provide a complete set of records for the lot including a list of customers who purchased the contaminated seed. MDA investigated where the seed was sold and whether the seed was planted. Sites where contaminated seed was sown were surveyed and monitored by MDA. Similarly, traceback investigations were initiated to determine other potential sources of Palmer amaranth infestation, including contaminated screenings, manure, forage, and equipment.
Risk of Spread through Livestock Feed
In late 2018, MDA identified manure as a pathway for introduction to the state when contaminated sunflower screenings were fed to cattle, and Palmer amaranth seed germinated when that manure was spread onto cropland. MDA obtained several samples of sunflower hull screenings from a sunflower processing company in North Dakota and confirmed the presence of Palmer amaranth in the screenings through genetic testing. After interviewing feedlot owners that fed cattle the sunflower screenings, MDA and UMN Extension personnel were able to tie manure application from one of the feedlots to the Redwood County soybean field introduction in 2018. This began a research partnership with UMN Extension to study the effects of contaminated feed and Palmer amaranth seed viability.
In late June 2019, MDA and UMN Extension sampled manure from three of the four feedlots that received contaminated screenings from the sunflower processing company. In July 2019, a subsample of the manure was sent to Illinois Crop Improvement Assoc., Inc. They placed manure samples into separate flats and allowed seeds in the samples to germinate. No amaranth seedlings germinated during the 6-wk course of this experiment. By 2020, UMN Extension developed a process to sieve the manure for amaranth seeds. The seeds extracted from the manure were visually identified as amaranth seeds by a seed analyst, but the seeds appeared to be brittle and falling apart. There was not enough DNA present for PCR testing these seeds. It was undetermined whether the seeds decayed in the digestive tract, manure, or in the steps taken to extract the seeds from the manure. The UMN Extension is continuing to refine their methods and has received a permit to work directly with Palmer amaranth seeds in manure.
The discovery of this pathway led to sampling and monitoring numerous screenings throughout the state. Some screenings contained as many as 250 Palmer amaranth seeds per 450 g of screenings. It was evident that Palmer seeds can remain intact and viable in cattle. In rumen animals, which includes cattle, 27% of amaranth seed remained viable after digestion (Blackshaw and Rode Reference Blackshaw and Rode1991), whereas the digestive system of poultry can be highly effective at destroying weed seeds; for example, only 3.5% of Palmer amaranth seeds fed to ducks were recovered and found viable (Farmer et al. Reference Farmer, Webb, Pierce and Bradley2017). Properly composting manure kills most weed seeds, including Palmer amaranth. Keeping the compost at 60 C for 3 d while maintaining a minimum of 35% moisture will eliminate seed viability (Wiese et al. Reference Wiese, Sweeten, Bean, Salisbury and Chenault1998). Another study found that it took between 21 and 50 d of composting with proper management to eliminate amaranth seed (Larney and Blackshaw Reference Larney and Blackshaw2003). Although composting drastically reduces weed seed viability, there is still potential for seed survival, which can be attributed to cooler pockets that do not sustain high temperatures for long enough to destroy the seed (Grundy et al. Reference Grundy, Green and Lennartsson1998).
Monitoring and Recording
All sites that had contaminated seed mix sown were monitored at a minimum of two visits per growing season. Monitoring surveys consisted of visiting the infested fields and performing intensive scouting to determine the extent and status of the locations based on treatment success, spread, or other important observations. Surveys of the surrounding infested areas were also performed within a 5-mile (8-km) circumference. Crop fields, roadsides, field entrances, and non-crop areas were included in these surveys. A site is considered eradicated after three consecutive years with no new finds. Although MDA has been able to visit all Palmer amaranth sites for multiple year monitoring to date, priority has been given to locations where Palmer was most recently identified and treated.
All monitoring and management activities involving Palmer amaranth were entered into the Invasive Species Management Tracking System within the Early Detection and Distribution Mapping System (EDDMapS 2020). To date, MDA surveyed a total of 78 sites in 17 counties, of which 25 sites were confirmed to have Palmer amaranth in nine counties (Table 1; Figure 4A). Eight counties had with sites where contaminated seed mix was sown, but there were no positive finds (Figure 4B).

Figure 4. (A) Minnesota counties confirmed with Palmer amaranth. (B) Minnesota counties with sites where contaminated seed was sown, but no plants were found at inspection.
Table 1. Total number of sites monitored for Palmer amaranth in Minnesota.
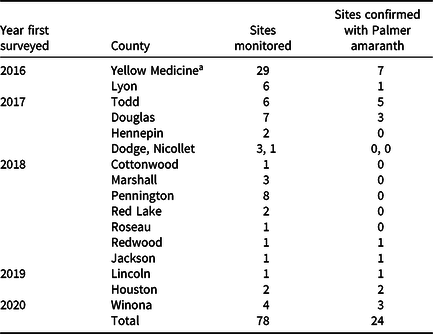
a Follow-up management was carried out by the landowner at one of the 29 sites in Yellow Medicine County.
In 2019, MDA and UMN made a collaborative effort to conduct aerial surveys using drones equipped with multispectral cameras to detect Palmer amaranth. Sites with confirmed Palmer amaranth infestations were visited and aerial images were captured. Spectral images were used to attempt identification of spectral signatures of Palmer amaranth from surrounding vegetation. MDA and UMN continue to work on making aerial surveys a feasible option to scout for Palmer amaranth.
Timeline of Palmer Amaranth Introductions and Eradication Activities
2016
In September 2016, UMN Extension notified MDA of the first suspected Palmer amaranth in Minnesota. Palmer amaranth seed was a contaminant of a CRP seed mix that was planted at 35 sites in Lyon and Yellow Medicine counties, with eight of these sites being confirmed to be infested with Palmer amaranth. MDA worked with 14 landowners and located each site that had a potentially contaminated seed mix sown. MDA surveyed each site and marked areas for Palmer amaranth management throughout October and November. In November and December, Palmer amaranth plants were torched.
2017
In 2017, MDA continued to monitor sites that had been sown with 2016 contaminated seed mixes. In April, sites in Lyon and Yellow Medicine counties with sufficient thatch were burned (Figure 5). In June, a foliar herbicide was applied to most sites in both counties. Monitoring continued throughout the summer into late fall, and only a few Palmer amaranth plants were found at two of the sites identified in 2016.

Figure 5. Crews burn CRP pollinator plantings sowed in 2016 with Palmer amaranth contaminated seeds in Lyon County, MN. All sites surveyed and monitored in Yellow Medicine County and Lyon County which was a result from the first Palmer amaranth find in Minnesota.
In October, MDA was notified about a plant suspected to be Palmer amaranth in a Todd County CRP planting. An MDA representative visited the site with the landowner and a county agricultural inspector to visually confirm that the plant was Palmer amaranth, which was later genetically confirmed. The landowner had retained bags of seed mix used for CRP planting. The remnant seed was confiscated and tested. One of the native grass mixtures planted was contaminated with Palmer amaranth seed. MDA obtained records for sites in Douglas, Dodge, and Hennepin counties that were also planted with the contaminated seed mix. Surveying all sites where the mix was planted resulted in confirmed Palmer amaranth plants at sites in Douglas County. No Palmer amaranth was found at the sites in Hennepin or Dodge counties.
2018
In 2018, the MDA Seed Regulatory Program uncovered four seed lots contaminated with Palmer amaranth during routine seed inspections. The most significant was a CRP seed mixture from a vendor in northwestern Minnesota that tested positive for Palmer amaranth. The vendor provided sales records for this lot that showed it was planted in 2017 on more than 1,500 acres in Marshall, Pennington, Red Lake, and Roseau counties. The second inspection found two seed lots of buffalograss [Bouteloua dactyloides (Nutt.) J.T. Columbus] that were contaminated. The vendor cooperated and returned these lots to the state of origin, Nebraska, and provided a single site in Nicollet County where that seed had been planted as part of a native seed mix. Continued mowing at that site has prevented the establishment of Palmer amaranth. A native seed mix planted on an MnDOT right-of-way also tested positive for Palmer amaranth in Cottonwood County. MDA aggressively monitored all these sites in 2018, 2019, and 2020 and has not found any Palmer amaranth plants at any site.
In September, a landowner from Redwood County posted an online message about possibly having Palmer amaranth plants on his land. MDA and UMN Extension representatives met with the landowner and visually confirmed the plants to be Palmer amaranth followed by confirmation by genetic testing leaf tissue. All plants were hand pulled and destroyed before seeds matured. This was the first documentation of Palmer amaranth in a row crop field in Minnesota. The source was investigated, and MDA found that the weed found its way into a soybean field through cattle manure. The cattle had been fed contaminated sunflower screenings that were traced back to a food manufacturing company in North Dakota. MDA sampled screenings throughout the state and Palmer amaranth seed was found repeatedly in sunflower screenings sold as animal feed from the same identified source in North Dakota.
In December 2018, a wheat screening sample from Roseau County submitted to MDA’s laboratory was positive for the presence of Palmer amaranth at a very low level. The wheat screening was traced back to a facility in Wisconsin. The wheat screenings were transferred to another cattle feedlot in Kittson County. Due to the initial positive test, feedlots were surveyed where cattle had been fed the contaminated wheat screening and areas where manure from these cattle were spread and no Palmer amaranth plants were found.
In October, MDA was notified of, and genetically confirmed, a Palmer amaranth plant in a Jackson County soybean field. Although the source of this plant could not be determined, the plant was found next to a utility road where gravel is regularly brought in by the utility company. The company in question also has utility contracts in southern regions where Palmer amaranth is well established, so company vehicles could have carried Palmer amaranth seed and accidentally transferred it to the Jackson County site. The single plant was destroyed prior to seed development.
2019
In July 2019, the MDA Seed Regulatory Program received a report from a seed company in Minnesota that it had acquired and sold seed contaminated with Palmer amaranth. The seed company was able to contact their customers and recall the seed prior to planting. The source lot, proso millet (Panicum miliaceum L.), had been sold both as a single kind and a mixture. Both lots were exported back to the vendor in South Dakota. The seed company in South Dakota also self-reported the sale of this contaminated seed. As a result of this report, an additional site where this proso millet had been planted was identified in Lincoln County. An MDA representative scouted the field and genetically confirmed three Palmer amaranth plants were present. The South Dakota seed company applied a herbicide to burn down all vegetation. In addition, the UMN Extension and MDA representatives offered advice on ways to prevent Palmer amaranth establishment in this field. Monitoring in 2019 and 2020 confirmed the absence of Palmer amaranth at this site.
In August, MDA was notified of Palmer amaranth in Houston County. The site was a small planting of recently established forage plants along a larger hay field. The site is only a few acres, but the infestation contained the highest density per unit area of Palmer amaranth plants documented in Minnesota to date. The farmer had remnant seed and allowed MDA to collect samples from four seed sources. None of the samples tested positive for Palmer amaranth. The source of this infestation remains unknown. Soon after discovery, the site was mowed by the landowner. CCMI crew members assisted MDA by torching individual Palmer amaranth plants throughout the site. In October, another Houston County landowner found and pulled three suspicious weeds in a soybean field. The plants were given to MDA and genetic testing confirmed that two of the three plants were Palmer amaranth. No seed was produced at this second site.
2020
In 2020, monitoring efforts continued. Despite numerous reports, Palmer amaranth was found in only one new county. All sites from 2016 and 2017 that originally had Palmer amaranth were surveyed and no Palmer amaranth was found. These sites are now considered eradicated. All remaining sites from 2018 and 2019 were surveyed. The only Palmer amaranth plants found were at the first 2019 Houston County site. These sites will be surveyed in 2021 and the 2018 sites will be considered eradicated if no Palmer amaranth is found.
The first Houston County site from 2019 was revisited in June, and Palmer amaranth plants were found. These plants were burned by CCMI crew members. The site was revisited the following week and new plants were discovered that had produced seed. These plants were hand pulled and the site was revisited weekly. A tentative plan for 2021 was developed with the MDA and UMN Extension to ensure that Palmer amaranth would not return. The plan will be to plant corn or soybeans on the site and use pre- and post-emergent herbicides along with monthly scouting to prevent further establishment of Palmer amaranth plants. Efforts will also be incorporated into the plan to ensure that all equipment is cleaned prior to leaving the site after tillage, planting, and applying herbicides.
MDA was notified by a crop consultant about suspected Palmer amaranth in Winona County. There was a total of four fields, and three of the fields had Palmer amaranth plants growing between rows of soybean plants. These plants were flagged, hand pulled, and submitted for genetic confirmation. The source of the plants is currently unknown. It is suspected that manure from local dairy cattle that were fed cottonseed imported into Minnesota may be the source of Palmer infestation.
Lessons Learned
Several lessons were learned through Minnesota’s Palmer amaranth eradication efforts over the past 5 yr. The first lesson was the need for a robust state noxious weed program that is funded by the legislature, overseen by an independent advisory committee (e.g., NWAC) of key stakeholders, and that works closely with county and municipal enforcement agents (county agricultural inspectors and local weed inspectors). Second, the support from the Commissioner of Agriculture, legislative committees, commodity groups, and farmers allowed MDA and UMN Extension to establish an aggressive protocol that not only addressed Palmer amaranth populations on the ground, but also supplied critical information to the larger agricultural community and general public regarding the serious impacts of this plant, identification, and the importance of reporting suspect Palmer amaranth. Finally, lasting success is more likely if surrounding jurisdictions are collaborating. Without buy-in from neighboring states, it becomes increasingly difficult for one jurisdiction to keep up with a problem plant species while another jurisdiction is not managing infestations and spread pathways.
Efforts to prevent the establishment and spread of Palmer amaranth has shown that regional efforts can be successful. We were fortunate to have access to funds early on and have statewide and regional support. Loss of funding for ongoing Palmer amaranth management and research could significantly reduce successful efforts. Communication between states to address spread pathways, testing strategies, ongoing research, and management activities greatly increases the chance of long-term success with this aggressive agricultural pest.
Acknowledgments
We thank David Fredrickson, Thom Peterson, Andrea Vaubel, Whitney Place, Matthew Wohlman, Geir Friisoe, and Mark Abrahamson with the Minnesota Department of Agriculture (MDA);and Curtis Olson, Don Wyse, Anthony Brusa, Bruce Potter, Ryan Miller, Jared Goplen, Lisa Behnken, David Nicolai, Tom Peters, Lizabeth Stahl, Chryseis Modderman, Angie Peltier, Melissa Wilson, and Kelly Duzan at the University of Minnesota for the contributions and support. Dan Shaw, Minnesota Board of Water and Soil Resources; Rob Venette and Heather Koop, Minnesota Invasive Terrestrial Plants and Pests Center; Carissa Spencer, USDA–Natural Resources Conservation Service; Angela Hanson, USDA–Farm Service Agency; Garth Kaste, Kaste Seed Inc.; Kim Alberty, Agassiz Seed & Supply; Bob Hartzler and Meaghan Anderson, Iowa State University; Mark Renz, University of Wisconsin; and Brian Jenks, North Dakota State University, provided invaluable assistance and support. We acknowledge Conservation Corps Minnesota and Iowa for on-the-ground management and survey assistance and the support and guidance of the Minnesota Seed Advisory Committee, Minnesota Noxious Weed Advisory Committee, Minnesota Association of County Agricultural Inspectors, Minnesota Soybean Growers, Minnesota Corn Growers and Minnesota Livestock Association. This work would have been possible without the assistance of crop consultants and farmers throughout the state. This effort was financially supported through a variety of sources: MDA Agricultural Emergency Funds, MDA Noxious and Invasive Plant Program and the Minnesota Environment and Natural Resources Trust Fund as recommended by the Legislative Citizens Commission on Minnesota Resources. No conflicts of interest have been declared.