Introduction
Horseweed [Conyza canadensis (L.) Cronquist] is considered a facultative winter annual; germination can occur in the fall when soil temperatures decline or facultatively during other times of the year (Cici and Van Acker Reference Cici and Van Acker2009). However, there are two main C. canadensis emergence timings, April to June (spring) and August to October (fall), that serve as critical C. canadensis control periods (Bhowmik and Bekech Reference Bhowmik and Bekech1993; Buhler and Owen Reference Buhler and Owen1997; Loux et al. Reference Loux, Stachler, Johnson, Nice, Davis and Nordby2006; Main et al. Reference Main, Steckel, Hayes and Mueller2006). Fall-emerging C. canadensis typically form dark-green, lightly haired, basal rosettes that overwinter, while spring-emerging C. canadensis skip or spend a short period of time as a rosette before bolting, forming an upright growth type (Loux et al. Reference Loux, Stachler, Johnson, Nice, Davis and Nordby2006; Regehr and Bazzaz Reference Regehr and Bazzaz1979). In Michigan field-cropping systems, primary C. canadensis emergence has shifted from fall to spring/summer, and therefore from a rosette to an upright growth type. In addition, the rosette and upright growth types have been observed co-emerging during the summer with visual differences in glyphosate tolerance (Schramski et al. Reference Schramski, Sprague and Patterson2021). Schramski et al. (Reference Schramski, Sprague and Patterson2021) found that C. canadensis growth type was not strictly inherited but was instead environmentally controlled and that both growth types could be forced to germinate from seeds from the same parent plant. The upright growth type seems to be environmentally triggered by a vernalization period (4 C) of at least 4 wk following water imbibition, but before germination. Conyza canadensis is primarily a self-pollinating species with ≤10% cross-pollination (Buhler and Owen Reference Buhler and Owen1997; Weaver Reference Weaver2001), thus many C. canadensis biotypes have been evolving independently, and it is likely that agronomic factors such as recurring herbicide applications, lack of herbicide rotation, and no-till regimes have selected for similar traits on various genetic pools of C. canadensis convergently (Dinelli et al. Reference Dinelli, Marotti, Bonetti, Minelli, Catizone and Barnes2006).
Conyza canadensis has documented resistance to at least one herbicide site of action in 18 different countries, including biotypes resistant to acetolactate synthase inhibitors (WSSA Group 2); photosystem II inhibitors (WSSA Groups 5 and 7); glyphosate, the 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) inhibitor (WSSA Group 9); and paraquat, a photosystem I electron diverter (WSSA Group 22) (Heap Reference Heap2022). However, glyphosate-resistant biotypes are the most prevalent. The first confirmed case of glyphosate resistance in any weed was in C. canadensis, identified in Delaware in 2000 (VanGessel Reference VanGessel2001). This occurred after reliance on only glyphosate for weed control for 3 yr in a glyphosate-resistant soybean [Glycine max (L.) Merr.] field. Following the release of Roundup Ready® crops in 1996, glyphosate use increased almost 15-fold (Benbrook Reference Benbrook2016), contributing to increased selection pressure for resistant individuals. By 2021, there were 14 countries with confirmed glyphosate-resistant C. canadensis (Heap Reference Heap2022). Within the United States, glyphosate-resistant C. canadensis is present in 25 states, including Michigan.
Common mechanisms of herbicide resistance in weeds include an altered target site, reduced absorption, reduced translocation to the target site, or rapid metabolic detoxification (Koger and Reddy Reference Koger and Reddy2005). The primary mechanism of glyphosate resistance in C. canadensis was reported to be rapid glyphosate sequestration into the vacuole, resulting in reduced translocation to the target tissue (i.e., the meristem) (Dinelli et al. Reference Dinelli, Marotti, Bonetti, Minelli, Catizone and Barnes2006; Feng et al. Reference Feng, Tran, Chiu, Sammons and Heck2004; Ge et al. Reference Ge, d’Avignon, Ackerman and Sammons2010; González-Torralva et al. Reference González-Torralva, Rojano-Delgado, Luque de Castro, Mülleder and De Prado2012; Koger and Reddy Reference Koger and Reddy2005; Moretti and Hanson Reference Moretti and Hanson2016; Nandula et al. Reference Nandula, Reddy, Duke and Poston2005). Ge et al. (Reference Ge, d’Avignon, Ackerman and Sammons2010, Reference Ge, d’Avignon, Ackerman, Duncan, Spaur and Sammons2011, Reference Ge, d’Avignon, Ackerman and Sammons2014) further investigated this resistance mechanism through 31P-nuclear nuclear magnetic resonance performed in vivo and speculated that a putative tonoplast pump in glyphosate-resistant C. canadensis is responsible for the rapid vacuolar sequestration, thus shielding the chloroplast and making limited translocation to sink tissues a secondary response. However, many of these studies were performed on rosette C. canadensis plants. Recently, the first documented case of target site–mediated glyphosate resistance in C. canadensis in the United States was observed in biotypes from Ohio and Iowa with resistance from 20 to 40 times the field use rate (1× = 840 g ae ha−1) (Beres et al. Reference Beres, Giese, Mackey, Owen, Page and Snow2020). A proline to serine mutation at position 106 of EPSPS2 was detected, the same target-site mutation identified in 21 glyphosate-resistant C. canadensis accessions from Canada (Page et al. Reference Page, Grainger, Laforest, Nurse, Rajcan, Bae and Tardif2018).
Schramski et al. (Reference Schramski, Sprague and Patterson2021) reported that the upright type from two glyphosate-resistant biotypes were 3- and 4-fold less sensitive to glyphosate than their rosette siblings; however, these differences were not observed in the susceptible biotype (Schramski et al. Reference Schramski, Sprague and Patterson2021). The level of resistance in the rosette and upright growth types were 84- to 386-times and 26- to 97-times, respectively. The upright type had a significantly higher ED50 value than its rosette siblings across both glyphosate-resistant biotypes (Schramski et al. Reference Schramski, Sprague and Patterson2021). Similarly, Shrestha et al. (Reference Shrestha, Hembree and Va2007) reported increased levels of glyphosate resistance with increasing growth stage, determined by the number of leaves per plant, within the susceptible and resistant C. canadensis populations. Additionally, glyphosate tolerance increased when plants began to grow upright in the resistant and susceptible populations. In contrast, Koger et al. (Reference Koger, Poston, Hayes and Montgomery2004) found no differences in glyphosate tolerance among growth stages in the rosette growth types. Based on these findings, our main objective was to determine whether differential glyphosate sensitivity between the rosette and upright C. canadensis plants with known glyphosate resistance was due to higher glyphosate interception and retention, absorption, and/or translocation.
Materials and Methods
Growth Parameters
Seed from the same parent plants of the glyphosate-resistant (MSU-18 or R) and glyphosate-susceptible (S-117 or S), C. canadensis biotypes studied in Schramski et al. (Reference Schramski, Sprague and Patterson2021) were used for this experiment. To generate the upright growth type, ∼0.6 g of seed from each biotype was surface planted in 30 by 30 cm flats filled with potting media (Suremix Perlite, Michigan Grower Products, Galesburg, MI) and imbibed with water. These flats were placed in a vernalization room set to 4 C with an 8-h photoperiod for 4 wk, then moved to a greenhouse. At that time, flats with seed to produce rosette siblings were planted using the same method, without a vernalization period. Flats were placed in the greenhouse at 25 ± 5 C and a total midday light intensity of 1,000 μmol m−2 s−1 photosynthetic photon flux with 16-h days. After 3 wk, seedlings were transplanted, 1 plant pot−1, to 10 by 10 by 12 cm pots filled with potting media. Plants were watered and fertilized as needed to promote optimum plant growth. Individual plants were grown to an average rosette size of 10-cm wide and an upright size of 7-cm tall (approximately 42-d old).
Retention
Glyphosate interception and retention was examined by applying 1.27 kg ae ha−1 of glyphosate (Roundup PowerMax®; Bayer CropScience, St. Louis, MO) plus ammonium sulfate (AMS) (Actamaster®; Loveland Products, Greeley, CO) at 2% w/w with Chicago Sky Blue dye (2.5 g L−1; Chem-Impex International, Wood Dale, IL) to both the R rosette and upright growth types at plant sizes as previously described at the same time. The method used was modified from the technique described by Boldt and Putnam (Reference Boldt and Putnam1980). Herbicide applications were made with a single-track sprayer (Generation 4, DeVries Manufacturing, Hollandale, MN) equipped with an 8001E TeeJet® flat-fan nozzle (TeeJet Technologies, Wheaton, IL) calibrated to deliver 187 L ha−1 at 193 kPa of pressure.
Immediately after herbicide application, plants were excised at the soil surface, and the retained dye was collected by a 30-s agitated rinse of the plant in 10 ml of a nonionic surfactant at 0.25% v/v with water solution. An additional 5 ml of the nonionic surfactant–water solution was used to collect the remaining retained dye. A 1-ml aliquot of the Chicago Sky Blue dye rinsate was used to measure absorbance with a spectrophotometer at 625 nm. Dye retention was calculated by comparing sample absorbance values with those from a standard curve. The technique was similar to that used by Sprague et al. (Reference Sprague, Penner and Kells1999). Conyza canadensis plants were dried at 60 C for 7 d and weighed to determine aboveground biomass.
Before spray application, exposed leaf area (cm2) from above was measured. All plants were photographed using an iPhone X® (Apple, Cupertino, CA) with a white background and a ruler as a size reference, and the photos were manually selected to obtain the leaf area using ImageJ software (National Institutes of Health, Bethesda, MD; University of Wisconsin Laboratory for Optical and Computation Instrumentation, Madison, WI). The average distance between the camera and the plant was 30 cm. There were 20 replications of rosette and upright plants, and the study was repeated in time.
Absorption and Translocation
The uppermost fully developed leaf of the R and S rosette and upright growth types at 10-cm wide and 7-cm tall, respectively, was targeted for radiolabeled [14C]glyphosate application. These leaves were covered with aluminum foil, and the remainder of the plant was sprayed with unlabeled glyphosate at 1.27 kg ha−1 plus AMS at 2% w/w. Spray applications were made as previously described in the retention study. The aluminum foil was removed immediately after spray application. Each plant was treated with 1.67 kBq of [14C]glyphosate (50 mCi mmol−1 specific activity, 99% purity). The spotting solution contained the appropriate amounts of [14C]glyphosate, unlabeled glyphosate, AMS, and water to give the same concentration as in the 1.27 kg ha−1 application of glyphosate. Each treated leaf was spotted on the adaxial leaf surface with 10 1-μl droplets and placed in a growth chamber maintained at 25/20 C day/night temperature with a 16-h photoperiod (1,000 μmol m−2 s−1).
Plants were harvested at 0, 12, 24, 72, and 168 h after treatment (HAT). At harvest, each plant was divided into treated leaf, above treated leaf, below treated leaf, and roots. Unabsorbed [14C]glyphosate was removed by placing the treated leaf in a 20-ml scintillation vial containing 3 ml of a methanol:water (1:9 v/v) solution and agitating it for 30 s followed by a 1-ml rinse with the methanol:water (1:9 v/v) solution as the treated leaf was removed from the scintillation vial. The samples for each plant were immediately placed in the freezer and stored at −30 C until further analysis. Each plant part was combusted in a biological sample oxidizer for 2 min. The 14CO2 released from the biological oxidizer was trapped in 20 ml of scintillation fluid (Carbo-Sorb® E:Permafluor® E+, 1:1 v/v; PerkinElmer, Groningen, Netherlands) and the radioactivity was quantified using a liquid scintillation counter (Tricarb 4910TR Liquid Scintillation Analyzer; PerkinElmer, Boston, MA). Radioactivity in the 4-ml leaf wash solution was quantified with the addition of 16 ml of Ultima Gold™ scintillation fluid (PerkinElmer, Groningen, Netherlands). The technique was similar to that used by Sprague et al. (Reference Sprague, Penner and Kells1999). Each study had five replications and was repeated in time.
Glyphosate absorption was calculated as the sum of the total 14C in the plant parts divided by the total 14C recovered, including the treated leaf wash. The amount of 14C present in the leaf wash and the plant sections was considered as total 14C recovered, which averaged 90% of applied [14C]glyphosate. 14C translocation out of the treated leaf was calculated by taking the amount of 14C absorbed in the untreated plant parts divided by the total 14C absorbed in the plant.
Statistical Analysis
Retention and translocation data were analyzed using PROC GLIMMIX in SAS OnDemand (SAS Institute Cary, NC, USA) at α = 0.05. The statistical model included the main effect of growth type and growth type within biotype for the retention and translocation experiments, respectively. Data were combined over repetition in time, and replication was treated as a random effect. Normality of residuals was examined using PROC UNIVARIATE (α ≤ 0.05). Squared and absolute value residuals were examined with Levene’s test to confirm homogeneity of variances (α ≤ 0.05). Treatment means were separated using Fisher’s protected LSD at α ≤ 0.05 when ANOVA indicated a significant main effect.
Absorption and translocation over time were analyzed using the drc package in R v. 4.0.2 (R Development Core Team 2020). Three-parameter log logistic models (Equation 1) were fit for the rosette and upright growth types within each biotype as selected by the drc modelFit function using the lack-of-fit test. The effective time to reach 50% absorption was determined using the ED function for each biotype and growth type.
In Equation 1, y is the percent absorption; x is the time (HAT); c and d are the lower and upper limits, respectively; b is the relative slope around e; and e is the ED50 (Streibig Reference Streibig1988). Relative differences in ED50 values by growth type within each biotype (based on a t-statistic with α ≤ 0.05) were compared using the EDcomp function.
Results and Discussion
Interception and Retention
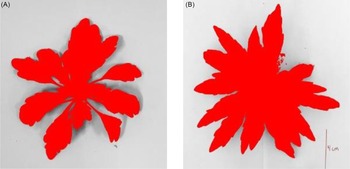
At 42 d after planting, the average height of the upright plants was 7 cm and the average diameter of the rosette plants was 10 cm (data not shown). The upright growth type accumulated 29% more biomass (358 mg plant−1) and had 20% more leaf area exposed (89 cm2 plant−1) compared with the rosette growth type (Table 1; Figure 1A and B). Total glyphosate interception and retention was not different among the rosette and upright growth types and ranged from 0.77 to 0.78 g ae of glyphosate per plant (Table 2). However, the upright growth type retained 21% less glyphosate on a per-weight (0.0022 g mg−2) and 18% less on a per-area bases (0.0088 g cm2) than the rosette type (Table 2). In theory, the upright growth type should have intercepted more glyphosate per plant, because leaf area was higher; however, differences in leaf arrangement likely altered spray interception. Previous research has not found differences in total glyphosate retention between glyphosate-susceptible and glyphosate-resistant biotypes (Feng et al. Reference Feng, Tran, Chiu, Sammons and Heck2004; González-Torralva et al. Reference González-Torralva, Rojano-Delgado, Luque de Castro, Mülleder and De Prado2012). Based on these results, reduced glyphosate retention on a per-weight and per-area bases in the upright growth type may result in a more diluted concentration of glyphosate inside the plant compared with the rosette growth type. This may contribute to differences in sensitivity between the rosette and upright growth types with known glyphosate resistance. Similarly, Schuster et al. (Reference Schuster, Shoup and Al-Khatib2007) reported reduced glyphosate injury as common lambsquarters (Chenopodium album L.) height increased was partially due to reduced glyphosate accumulation per unit of plant tissue. However, we believe this relatively small change is unlikely to be the only or primary mechanism responsible for the 3- to 4-fold difference in sensitivity we have observed between rosette and upright R plants.
Table 1. Biomass and exposed leaf area (±SE) for rosette and upright Conyza canadensis growth types at herbicide application for interception and retention of glyphosate with Chicago Sky Blue dye. a

a Glyphosate plus Chicago Sky Blue dye applications were made approximately 42 d after planting. Means followed by the same letter within a column are not statistically different at α ≤ 0.05.

Figure 1. Exposed leaf area from above at the time of glyphosate application for (A) the rosette (74 cm2) and (B) upright (89 cm2) Conyza canadensis growth types.
Table 2. Glyphosate retention (±SE) on a per-weight, per-area, and per-plant bases by rosette and upright Conyza canadensis growth types at 42 d after planting. a

a Means followed by the same letter within a column are not statistically different at α ≤ 0.05.
Absorption and Translocation
There were no differences in glyphosate absorption among the rosette and upright growth types across both biotypes. Each growth type by biotype combination reached 50% of its total absorption (ED50) at 11 and 15 HAT (Figure 2). Similarly, past research did not find reduced glyphosate absorption to be a mechanism of resistance in C. canadensis (Dinelli et al. Reference Dinelli, Marotti, Bonetti, Minelli, Catizone and Barnes2006; Feng et al. Reference Feng, Tran, Chiu, Sammons and Heck2004; González-Torralva et al. Reference González-Torralva, Rojano-Delgado, Luque de Castro, Mülleder and De Prado2012; Koger and Reddy Reference Koger and Reddy2005). Maximum glyphosate absorption ranged between 75% and 85%, plateauing around 72 HAT (Figure 2). González-Torralva et al. (Reference González-Torralva, Rojano-Delgado, Luque de Castro, Mülleder and De Prado2012) reported no significant differences in absorption between resistant and susceptible C. canadensis biotypes, with peak absorption occurring at 96 HAT when 71% and 62% of glyphosate was absorbed, respectively. Similarly, Feng et al. (Reference Feng, Tran, Chiu, Sammons and Heck2004) observed no differences in glyphosate absorption among 11 biotypes of susceptible and resistant C. canadensis at 4 to 5 d after treatment. These results suggest that glyphosate absorption does not contribute to differences in glyphosate sensitivity between the upright and rosette growth types or between resistant and susceptible biotypes.

Figure 2. [14C]glyphosate absorption over time in rosette and upright plants from glyphosate-resistant (MSU-18) and glyphosate-susceptible (S-117) Conyza canadensis biotypes.
There was no difference in translocation among the rosette and upright plants within each biotype at any time point (Figure 3). Additionally, translocation was similar between all growth type by biotype combinations at 12 and 24 HAT. However, by 72 to 168 HAT, differences in translocation were observed between the S rosette type and both R growth types (Figure 3). Radioactivity was distributed throughout the plant, with a majority remaining in the treated leaf at all time points, regardless of growth type within biotype. At 168 HAT, 86.8% to 90.7% of the absorbed [14C]glyphosate remained in the treated leaf in both R growth types and the S upright-type, whereas only 76.3% remained in the treated leaf for the S rosette type (Table 3). The amount of [14C]glyphosate translocated out of the treated leaf in the rosette and upright growth types at 168 HAT was 21.8% and 14.7% in the S biotype, respectively. For the R biotype, [14C]glyphosate translocation out of the treated leaf in the rosette and upright plants at 168 HAT was 12.0% and 9.9%, respectively. Interestingly, [14C]glyphosate translocation at 168 HAT was higher in the S rosette compared with the upright and rosette growth types from the R biotype; however, the S upright growth type was similar to both growth types of the R biotype. Translocation to the above and/or below treated leaves was greater compared with the roots in all cases. However, there were no clear differences that would help to explain differential sensitivity between the rosette and upright growth types with known glyphosate resistance (Table 3). There was minimal translocation to the roots (0.77% to 3.8%), but the S upright type translocated less [14C]glyphosate to the roots compared with all other growth type by biotype combinations. Previous research found lower glyphosate levels in the treated leaf of susceptible biotypes compared with resistant biotypes (Feng et al. Reference Feng, Tran, Chiu, Sammons and Heck2004; Koger and Reddy Reference Koger and Reddy2005). However, we only observed this when examining the S rosette type compared with the R rosette type. No differences were observed between the upright type from the R and S biotypes. In addition, prior studies have observed reduced [14C]glyphosate translocation to the crown and other leaves in resistant biotypes (Feng et al. Reference Feng, Tran, Chiu, Sammons and Heck2004; Koger and Reddy Reference Koger and Reddy2005); however, when comparing growth types, we observed that this was not always the case. Conversely, González-Torralva et al. (Reference González-Torralva, Rojano-Delgado, Luque de Castro, Mülleder and De Prado2012) reported no differences in translocation to the leaves between resistant and susceptible C. canadensis biotypes. Dinelli et al. (Reference Dinelli, Marotti, Bonetti, Minelli, Catizone and Barnes2006) reported that more [14C]glyphosate was translocated to the leaves compared with the roots in rosette C. canadensis, supporting what we found across all growth-type biotype combinations. In contrast, prior research has found roots to be the strongest sink when applying [14C]glyphosate to rosette C. canadensis (Feng et al. Reference Feng, Tran, Chiu, Sammons and Heck2004; González-Torralva et al. Reference González-Torralva, Rojano-Delgado, Luque de Castro, Mülleder and De Prado2012; Koger and Reddy Reference Koger and Reddy2005). This may have been due to different growing conditions before and after [14C]glyphosate application.

Figure 3. [14C]glyphosate translocation over time out of the treated leaf in rosette and upright glyphosate-resistant (MSU-18) and glyphosate-susceptible (S-117) Conyza canadensis biotypes.
Table 3. [14C]glyphosate translocation and distribution (±SE) in rosette and upright glyphosate-resistant and glyphosate-susceptible Conyza canadensis biotypes at 168 h after treatment. a

a Plants were grown in the greenhouse before [14C]glyphosate application at 25 ± 5 C with a 16 h photoperiod. After application, plants were maintained in a growth chamber at 25/20 C day/night temperature with a 16-h photoperiod. Means followed by the same letter within a column are not statistically different at α ≤ 0.05.
b Abbreviations: MSU-18, glyphosate resistant; S-117, glyphosate susceptible.
c [14C]glyphosate outside of treated leaf (above treated leaf, below treated leaf, and roots) is considered translocation.
d [14C]glyphosate distribution throughout the plant is based on percent of [14C]glyphosate absorbed.
Generally, non–target site resistance mechanisms such as impaired translocation due to rapid vacuolar sequestration have been identified as the most common mechanism of glyphosate resistance in C. canadensis (Dinelli et al. Reference Dinelli, Marotti, Bonetti, Minelli, Catizone and Barnes2006; Feng et al. Reference Feng, Tran, Chiu, Sammons and Heck2004; Ge et al. Reference Ge, d’Avignon, Ackerman and Sammons2010; González-Torralva et al. Reference González-Torralva, Rojano-Delgado, Luque de Castro, Mülleder and De Prado2012; Koger and Reddy Reference Koger and Reddy2005; Moretti and Hanson Reference Moretti and Hanson2016). This is likely due to prior research primarily investigating non–target site resistance mechanisms and only in rosette growth types. Our research supports these findings, as translocation was impeded in the R rosette type compared with the S rosette type at 168 HAT. Interestingly, our research differs when considering upright plants. Translocation differences were not evident at 168 HAT between the S upright growth type and the R upright and rosette growth types despite R upright plants still being very glyphosate resistant; therefore, it is likely that a yet to be discovered resistance mechanism is at least partially responsible for glyphosate resistance in the MSU-18 biotype. We predict that this mechanism is either differential expression of a metabolism gene or increased expression of EPSPS itself, which has been found in other plant species (Pan et al. Reference Pan, Yu, Han, Mao, Nyporko, Fan, Bai and Powles2019; Patterson et al. Reference Patterson, Pettinga, Ravet, Neve and Gaines2018). We have ruled out translocation and absorption as possibilities, and theoretically, a target-site polymorphism should be unaffected by growth type and should present a fairly consistent resistance response.
The first documented case of target site–mediated glyphosate resistance in C. canadensis in the United States was recently observed in highly resistant biotypes, at 20X to 40X the field rate (1X = 840 g ae ha−1), from Ohio and Iowa (Beres et al. Reference Beres, Giese, Mackey, Owen, Page and Snow2020). A proline to serine mutation at position 106 of EPSPS2 was detected, which is the same target-site mutation that was identified in 21 glyphosate-resistant C. canadensis accessions from Canada (Page et al. Reference Page, Grainger, Laforest, Nurse, Rajcan, Bae and Tardif2018). The recent discovery of an EPSPS target-site mutation in glyphosate-resistant C. canadensis biotypes coupled with what we found regarding translocation indicates that the primary mechanism of resistance in C. canadensis biotypes should not be assumed to be reduced translocation. This is especially true when only examining rosette growth types. However, it is also possible that vacuolar glyphosate sequestration or another, yet to be described resistance mechanism is working at a greater capacity on the R and S upright biotypes, thus making differences in translocation negligible.
In the future, researchers should consider including both growth forms when studying glyphosate resistance in C. canadensis (Dinelli et al. Reference Dinelli, Marotti, Bonetti, Minelli, Catizone and Barnes2006; Feng et al. Reference Feng, Tran, Chiu, Sammons and Heck2004; Ge et al. Reference Ge, d’Avignon, Ackerman and Sammons2010; González-Torralva et al. Reference González-Torralva, Rojano-Delgado, Luque de Castro, Mülleder and De Prado2012; Koger and Reddy Reference Koger and Reddy2005; Moretti and Hanson Reference Moretti and Hanson2016). It is also possible that the biotypes used in this study, including MSU-18, possess a target-site mutation that works synergistically with non-target or other unknown mechanisms. Stacked target-site and non–target site resistance mechanisms have been observed in waterhemp [Amaranthus tuberculatus (Moq.) Sauer], rigid ryegrass (Lolium rigidum Gaudin), and annual bluegrass (Poa annua L.) (Bostamam et al. Reference Bostamam, Malone, Dolman, Boutsalis and Preston2012; Kaundun et al. Reference Kaundun, Dale, Zelaya, Dinelli, Marotti, McIndoe and Cairns2011; Laforest et al. Reference Laforest, Brahim, Patterson, Vargas, Boggess, Houston, Trigiano and Brosnan2021; Nandula et al. Reference Nandula, Ray, Ribeiro, Pan and Reddy2013).
These results suggest that differences in glyphosate sensitivity among the rosette and upright growth types with known glyphosate resistance were not due to higher glyphosate absorption, translocation, or the total amount of glyphosate intercepted and retained on the C. canadensis leaf surface. As expected, we did not observe these differences in the glyphosate-susceptible biotype either. However, the upright growth type intercepted and retained 21% and 18% less glyphosate on a per-weight and per-area bases. Thus the concentration of glyphosate may be diluted, resulting in slightly higher glyphosate tolerance in the upright growth type in the R biotype, although this difference is not likely fully responsible for the 3- to 4-fold difference in glyphosate sensitivity between the rosette and upright growth types in the glyphosate-resistant biotype.
Recently, Laforest et al. (Reference Laforest, Martin, Bisaillon, Soufiane, Meloche and Page2020) reported the first chromosome-scale genome sequence of C. canadensis, which revealed at least 4 EPSPS-like genes (three of which seem to be pseudogenized). Because of this, care should be taken when amplifying and sequencing EPSPS from C. canadensis so as not to accidentally sequence one of these nonfunctional copies of EPSPS. This genome will greatly assist a genome-wide association study to look for genetic variations among S and R biotypes to identify the mechanism of glyphosate resistance. Once the mechanism of resistance has been established, additional studies should examine whether there are differences within the resistance mechanism between the rosette and upright types with known glyphosate resistance. There may be other factors contributing to the differential sensitivity among the rosette and upright growth types studied here, such as differences in EPSPS gene expression, which would be supported if a target-site mutation is discovered similar to ones recently found in other C. canadensis biotypes (Beres et al. Reference Beres, Giese, Mackey, Owen, Page and Snow2020; Page et al. Reference Page, Grainger, Laforest, Nurse, Rajcan, Bae and Tardif2018).
Acknowledgments
We would like to thank the Michigan Soybean Committee for their support, Jinye Chen, Gary Powell, and Brian Stiles for their technical assistance, and Erin Burns for her statistical assistance. No conflicts of interest have been declared.