INTRODUCTION
Diarrhoea, a common clinical presentation in bovine calves has a multifactorial aetiology [Reference Otter and Cranwell1], including Cryptosporidium and Eimeria spp., rotavirus, coronavirus, enteropathogenic Escherichia coli and Salmonella spp. [Reference Bangoura and Daugschies2]. The neonatal diarrhoeic syndrome caused by Cryptosporidium parvum is usually observed in 5–35-day-old claves with maximum incidence in the second week of life [Reference Gay and Kahn3], resulting in enormous direct and indirect economic loss to dairy industry [Reference Haschek4–Reference Cho and Yoon6]. In addition to bovine calves, it is known to infect humans, domestic and wild animals, and birds [Reference Chattopadhyay7–Reference Randhawa, Randhawa, Zahid, Singla and Juyal11]. The cryptosporidia, highly resistant to harsh environmental conditions, transmitted by faeco-oral route, have attained an increased zoonotic importance posing a great risk to dairy workers, milk handlers and general public through water contamination. The disease has particularly generated a great public health interest after a large human waterborne outbreak in Milwaukee in 1993 [Reference Randhawa, Randhawa, Zahid, Singla and Juyal11, Reference MacKenzie12].
Difficulties in the clinical diagnosis of infectious diarrhoea arise from frequent non-specific clinical signs and lesions, the presence of asymptomatic infections, the involvement of multiple agents, and the interplay of intrinsic and extrinsic factors that predispose the host to enteric dysfuction and pathology [Reference Athanassious13]. Traditionally, the infectious agents of diarrhoea have been identified using conventional techniques [Reference Ruest, Faubert and Couture14, Reference Nasir15]. However, these techniques are labour intensive, time consuming and less sensitive; therefore, advanced diagnostic methods may prove more efficacious in determining the precise aetiology of calf diarrhoea complex and thereby helpful in planning strategies for better herd management, disease surveillance and control programmes.
In India, extensive research work has been conducted on bovine calf diarrhoea with majority of infectious aetiological agents incriminated using conventional methods. Studies on Cryptosporidium in the country have mainly focused on the morphological identification of oocysts in faeces [Reference Singh5, Reference Chattopadhyay7, Reference Kumar, Sreekrishnan and Das8, Reference Randhawa, Randhawa, Zahid, Singla and Juyal11, Reference Nooruddin and Sarma16–Reference Randhawa20] with little emphasis on immunomolecular methods [Reference Roy21–Reference Bhat24]. These previous investigations were concentrated on isolated reports of cryptosporidia alone without an insight into multiple aetiologies. Even globally, very few studies have been conducted on Cryptosporidium-associated complex aetiology of bovine calf diarrhoea outbreaks [Reference Cho and Yoon6, Reference Cho25]. In fact very few comprehensive investigations ellucidating the most recognised agents, viz. protozoa (C. parvum, Eimeria spp.) viruses (rota and corona) and bacteria (E. coli, Salmonella spp., Clostridium perfringens) have been attempted [Reference Xiao, Herd and Rings26–Reference Fayer, Santin and Dumitru30]. Moreover, a comparative evaluation of multiple aetiological factors by traditional, immunological and molecular methods with a pursuit to determine their relative sensitivity using faecal samples is lacking. The present study deals with 17 periurban outbreaks of bovine calf diarrhoea investigated thoroughly comparing faecal examination, faecal ELISA and faecal PCR (most trusted methods) from 100 cases with 43% mortality.
MATERIALS AND METHODS
Study area and sample collection
The study was conducted in Ludhiana, located between north latitude 30°34′ and 31°01′ and east longitude 75°18′ and 76°20′. It is most populated and centrally located district of Punjab (India) with a huge periurban dairy animal population. The representative faecal samples were collected in sterile plastic bags directly from the rectum of 100 calves covering 17 outbreaks (four to six animals each) of diarrhoea from the organised dairy farms located in the periurban areas consisting of about 300 organised dairy farms in a radius of 25 km. To determine the exact age-wise distribution of aetiological factors of neonatal bovine diarrhoea, the animals were divided into five sub-groups, i.e. 1–7 days (group I), 8–14 days (group II), 15–21 days (group III), 22–30 days (group IV) and 31–60 days (group V) of age. Faecal samples were stored at −20 °C for ELISA and PCR.
The study was conducted after approval of the Institutional Animal Ethical Committee and consent of the dairy farmers was taken for collection of samples.
Detection of Cryptosporidium
Microscopic examination of faecal smears was conducted for bacteria and cryptosporidia by employing Leishman's and modified Zeihl–Neelsen's (mZN) stains [31].
Detection of infectious agents by ELISA
All the faecal samples collected from 100 neonatal bovine calves during scour outbreaks were subjected to faecal ELISA by using the protocol of commercial ELISA kits for C. parvum, rotavirus, coronavirus and E. coli (K99) (Bio-X Easy-Digest, Bio K 151; Bio-X Diagnostics, Belgique) and C. perfringens (type C) (BIO K 269; Bio-X Diagnostics, Belgique).
DNA extraction
DNA of target agents C. parvum, E. coli, Salmonella, Clostridium were extracted from faecal specimens using Nucleo-poreR Stool DNA Mini kit (HiMedia, Mumbai, India) as per the manufacturer's instructions.
RNA extraction
RNA from all the faecal samples for detection of coronaviruses was extracted using RNASure® Mini kit (Genetix, India; Table 1).
Table 1. Primers used for PCR

Confirmation of infectious agent by PCR
PCR was employed for Cryptosporidium, coronavirus, Salmonella, E coli and C. perfringens individually. The details of primers used and amplification conditions are given in Table 1. PCR reaction mixture 25 µl was constituted by adding 2·5 µl of 10× PCR buffer (HiMedia, Mumbai, India), 0·75 µl of 50 mM MgCl2, 2μl of template DNA, 1 µl of 20 pmol/μl of forward and reverse primer each, 0·2 µl of Taq DNA polymerase 5 U/μl and rest with nuclease-free water. Amplified PCR products were separated by gel electrophoresis using 1% agarose and visualisation of the product was carried out using UV transilluminator (Alpha Imager, San Jose, California, USA).
First strand cDNA synthesis
For the first strand cDNA synthesis from the RNA sample, the kit (Fermantas, Thermo Fisher Scientific, Waltham, MA, USA) was used, and the reaction was set up as per the manufacturer's instructions. In brief, the following reagents were added into a sterile nuclease-free tube on ice total RNA (0·1–5 µg), random hexamer primer (1 µl), nuclease-free water to make volume 11 µl. Subsequently, 5× reaction buffer (4 µl), Ribolock RNAase inhibitor (20 units/μl) 1 µl, 10 mM dNTP mix 2 µl, M-MuLV Reverse Transcriptase (20 units/μl), 2 µl was added and volume was made upto 20 µl with nuclease-free water. The above contents were mixed gently and centrifuged and incubated for 5 min at 25 °C, followed by 60 min at 37 °C, and finally the reaction was terminated by heating at 70 °C for 5 min. The reverse transcription reaction product was directly used in PCR.
Mortality analysis
During the investigation of 17 outbreaks in question, 43 claves died within 1 weak after sample collection, and the tissues from representative animals were collected for histopathology.
Statistical analysis
The data were statistically analysed by χ 2 test using SPSS 16.0 software.
Sensitivity of PCR in relation to ELISA, mZ–N stain and Leishman stain for detection of Cryptosporidium was done following the formulae of Perrin and Sureau [Reference Perrin and Sureau32].
RESULTS AND DISCUSSION
Detection of Cryptosporidium by conventional and immunological and molecular methods
Microscopic examination of the faecal smears stained with Leishman's stain showed cryptosporidial and eimerial oocysts, besides other faecal inclusions, viz. cocci, thick and thin bacilli, stumpy bacilli/coccobacilli, squamous epithelial cells, intestinal epithelial cells, cell debris, undigested plant fibres(s), red blood cells (RBCs), fungus/yeast, bile pigment, leucocytes and undigested (Table 3). The Leishman-stained smears proved highly useful in preliminary screening of faecal smears for the presence or absence of coocidia, particularly cryptosporidia. Morphometric analysis of oocysts of Cryptosporidium (n = 10; 10 field each sample) in Leishman (4·24 ± 0·28 µm) and mZN-stained (4·26 ± 0·29 µm) faecal smears revealed non-significant (0·16 NS) variation in the size. In the Romanowsky-stained faecal smears, the cryptosporidial oocysts were seen as hollow round bodies and with a size compatible with that recorded in this study and documented earlier by mZN staining [Reference Brar33] and the coocidian oocysts were larger hollows and oval (Fig.1a, b); but on mZN staining, cryptosporidial oocysts were seen as bright red, oval to spherical bodies in 25% cases (Fig. 1c; Table 2). Both the faecal antigen-ELISA and PCR showed an improved 35% prevalence of Cryptosporidium in diarrhoeic calves. In fact, direct visualisation by light microscopy of cryptosporidia in faeces or intestinal contents as well as the detection of its antigens (e.g. Ag-ELISA) or nucleic acids (e.g. PCR) in specimens have been widely accepted as alternative diagnostic methods [Reference Bhat, Juyal and Singla9, Reference Singla34].

Fig. 1. Leishman-stained faecal smears showing large oval bodies resembling Eimeria spp. oocysts (left frame; a), small hollow round bodies resembling cryptosporidial oocysts (middle frame; b) and modified Zeihl–Neelson-stained faecal smear conforming the presence of cryptosporidial oocysts (right frame; c) besides several other faecal inclusions (original magnification × 1000×).
Table 2. Detection of Cryptosporidium parvum by conventional and modern techniques in different age groups of diarrhoeic bovine calf faeces (n = 100)

Table 3. Microscopic findings in Leishman's-stained faecal smears

The age-wise prevalence of Cryptosporidium by PCR and antigen detection ELISA showed non-significant variation with highest rate of 66·6% in 8–14 days group calves (Table 2). The maximal prevalence of Cryptosporidium was recoded in the 2-week-old diarrhoeic bovine calves. Similar prevalence of cryptosporidial infection in neonatal diarrhoeic calves with a gradual reduction with age has been reported previously from various regions of India [Reference Singh5, Reference Roy21, Reference Paul22, Reference Bhat, Juyal and Singla35] and across the globe [Reference de la Fuente27, Reference Castro-Hermida, González-Losada and Ares-Mazás36–Reference Fayer, Santin and Dargatz39]. In the present study, all calves positive by faecal ELISA were also positive for the Cryptosporidium by PCR, except one. The sensitivity of ELISA and mZN staining with respect to PCR was 97·14% and 72·72%, respectively (Table 4). Comparable results regarding sensitivity between ELISA and PCR, matching those of ours, were previously recorded by other workers [Reference Cho25, Reference Van den40] irrespective of the variations in the type of reagents and kits used. Settawy and Fathy [Reference Settawy MA and Fathy41] observed high detection rate by PCR (24·4%) and low by microscopy (18·6%), while it was 20·9% by ELISA. Their data showing higher sensitivity of PCR compared with ELISA are contradictory to our results showing equivalent positivity. In the differentiation of the aetiology of the diarrhoea in neonatal calves, PCR is being increasingly employed for the detection of Cryptosporidium during the last two decades mainly because of its accuracy, specificity and sensitivity and it has made significant contributions in understanding the epidemiology of Cryptosporidium infection [Reference Xiao, Ryan, Fayer and Xiao42–Reference Morgan46]. Moreover PCR has added advantages such as ease of use, ability to analyse large numbers of samples simultaneously, relatively low cost and ability to speciate (eliminating false positives and cross-reactions of antibodies to non-pathogenic species) [Reference Morgan and Thompson45]. Though nested PCR involving a second round of amplifications has been applied to Cryptosporidium to increase specificity and sensitivity [Reference Bhat, Juyal and Singla35, Reference Meyer and Palmer47, Reference Balatbat48], yet it has not been generally recommended for use in diagnostic laboratories owing to a major risk of contamination with PCR products [Reference Morgan and Thompson45, Reference Persing49].
Table 4. Sensitivity of ELISA, mZN and Leishman's staining of faecal smears with PCR as gold standard for determination of Cryptosporidium parvum in diarrhoeic bovine calf faeces (n = 100)

Detection of Cryptosporidium-associated enteropathogens
The progression of diarrhoea with multifactorial aetiology is rapid. Hence, a quick diagnosis is critical for not only rapidly confirming the causes but also helping clinicians and cattle producers to implement appropriate interventions in a timely manner. Antigen capture ELISAs are well known for rapid turnaround, high-throughput testing and portability [Reference Fenner, MacLachlan and Dubovi50]; whereas, PCR is especially useful for detecting viruses that are difficult to isolate in cell culture or bacteria that require a long time to grow [Reference Espy51]. Therefore, all the samples tested positive for other enteropathogens either/or by faecal antigen capture ELISA or PCR were considered positive as Cryptosporidium-associated enteropathogens.
Electrophoretic analysis of PCR products of Salmonella spp., C. perfringens and bovine coronavirus in association with C. parvum are shown in Figure 2. Majority of the cases, in this study, showed as many as 13 combinations of Cryptosporidium-associated infections (71·40%) with Cryptosporidium alone in 28·60% diarrhoeic calves only (Table 5). Moreover, the age-wise faecal prevalence of Cryptosporidium as a single agent and combination aetiologies with it, showed a decreasing and increasing trend (χ 2 13·28, P < 0·01), respectively (Table 5). This indicates that initial primary infection with Cryptosporidium led to significant enterocytic damage favouring flare up of other secondary infections with the passage of time. In addition, the findings of severe intestinal epithelial damage (Fig. 3), lymphoid depletion in mesenteric lymph nodes (Fig. 4) and demonstration of Cryptosporidium along with coccidiosis (eimeriosis) (Fig. 5) were amply augmented by histopathology of the representative tissue samples collected from calf mortality during the outbreaks. Histopathology is considered as a gold standard for demonstrating the intestinal involvement and cryptosporidia in one go in the animals dying of diarrhoeal disease [Reference Cho and Yoon6]. Furthermore, the haematobiochemical findings of significant neutrophilic leucocytosis, lymphopaenia and anaemia along with significant increase in serum albumin and blood urea nitrogen with corresponding significant decrease in total globulins hinted at secondary suppurative infection, dehydration and immunosuppression, respectively, as have been reported by the authors earlier [Reference Brar, Sood, Ahuja, Sandhu, Gupta and Singh52, Reference Brar, Ahuja, Sood, Sandhu and Gupta53]. Contrary to our findings, de la Fuente et al. [Reference de la Fuente27] reported a significant age-associated decrease in the detection rate of mixed infections. In fact, the epidemiological studies have proven that diarrhoea is more severe in mixed infection(s), especially in immunocompromised individuals [Reference Morin54]. In this study, the Cryptosporidium-associated pathogens responsible for bovine calf scour outbreaks and mortality were C. perfringens 45·71% (16/25), followed by Salmonella spp 40·0% (10/25), rotavirus 36·0% (9/25), coronavirus 16·0% (4/25), E. coli 12·0% (3/25) and Eimeria spp. 4·0% (1/4), respectively, which fell nearly in line with that for C. perfringens (54·0%) and in contrast to rotavirus (87%), coronavirus (11·1%), E. coli (27·8%) and Salmonella spp. (1·8%) in central Spain [Reference Bartels55]. Almost similar infection rate of Cryptosporidium (21·28%) with Blastocystis 19·15%, Giardia (51·06%) and Enterocytozoon 36·17%, although in a higher 3–5-month age group of diarrhoeic calves was reported [Reference Fayer, Santin and Dumitru30]. The severity of Cryptosporidium in combination with other agents, or whether other agents extend the risk period for clinical cryptosporidiosis remains to be proved further experimentally [Reference de la Fuente27]. The prevalence of each of pathogen and disease incidence can vary due to geographical location of the farms, farm managemental practices and herd size. A heavy mortality of 43% was recorded in the present study. A similar high mortality has been reported due to diarrhoea in the USA (57%) and Korea (53·4%) in unweaned dairy calves [56–Reference Hur57].

Fig. 2. Agarose gel electrophoretic analysis showing PCR amplified products of multiple agents of diarrhoea. Lane 1: Salmonella sp(s) positive (575 bp), lane 2: Salmonella sp negative, lane 3: Clostridium perfringens (Cl) positive (279 bp), lane 4: C. perfringens (Cl) negative, lane M: DNA marker (100 bp plus, SRL), lane 5: bovine coronavirus (Co) positive (406 bp), lane 6: bovine coronavirus (Co) negative, lane 7: Cryptosporidium parvum (Cr) positive (194 bp), lane 8: C. parvum (Cr) negative control.
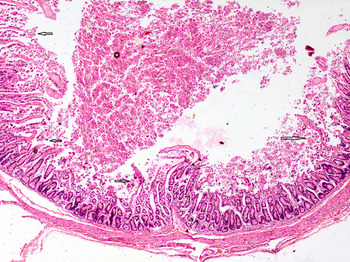
Fig. 3. Severe intestinal damage due to multiple aetiological agents characterised by massive superficial and deep necrosis extending into crypts with formation of marked debris in the lumen. H&E × 4× original magnification.

Fig. 4. Section of a mesenteric lymph node of the small intestine segment affected with severe diarrhoea showing massive lymphoid cell depletion. H&E × 10× original magnification.

Fig. 5. Section of small intestine showing various developmental stages of coccidia and an oocyst (arrow) of Cryptosporidium present superficially. H&E × 100× original magnification.
Table 5. Detection of Cryptosporidium and its combination with other enteropathogens in different age groups of diarrhoeic calves

Figures in parentheses indicate percentage.
All the agents shown in the table were detected by both ELISA and PCR except Salmonella spp., for which only PCR was performed and for rotavirus only ELISA was performed.
Among the various risk factors analysed during the study, poor hygiene, overcrowding, bad calf nutrition, including deprivation of colostrum and inadequate milk feeding, and sudden weather changes were found to be the major epidemiological factors. Poor managemental practices, including the method of cleaning, the type of flooring and the frequency of cleaning and deprivation of colostrum in suckling calves were also found to be the main risk factors associated with cryptosporium infections [Reference Mohammed, Wade and Schaaf58, Reference José59].
From this first comprehensive report of 17 cryptosporidial outbreaks from Punjab State of North India, diagnosed based on a combination of techniques, we conclude that when C. parvun occurs in association with other concurrent enteropathogens, it may result into severe infection leading to outbreaks of neonatal bovine calf diarrhoea, resulting in huge mortality perpetuated by poor managemental practices and immunosuppression as augmented by histopathology of lymph nodes. In addition to C. parvum, the other aetiological agents, viz. C. perfringens, Salmonella spp and E. coli detected in the present study also carry marked zoonotic potential in children and immunocompromised people.
ACKNOWLEDGEMENTS
Thanks are due to the Director of Research, Guru Angad Dev Veterinary and Animal Sciences University for providing financial support to carry out the research work under RKVY project entitled, ‘Rapid and precise diagnostics of important gastrointestinal and respiratory diseases of bovines’.













