Premenstrual syndrome (PMS) is the most prevalent disorder in reproductive age women. According to the American College of Obstetricians and Gynecologists’ diagnostic criteria, PMS symptoms are classified as affective and somatic symptoms such as anger, anxiety, social withdrawal, depression, irritability, headache and swelling. Up to 85 % of menstruating women have one or more premenstrual symptoms, and 2–10 % of this population are severely affected. Although patients experience negative effects on quality of life, the aetiology of PMS is not entirely clear( 1 , Reference Steiner and Born 2 ).
Several studies report that women with PMS may be so physiologically hypersensitive to the normal cycling levels of oestrogen and progesterone that they experience more symptoms( Reference Schmidt, Nieman and Danaceau 3 ). Although twin studies suggest a heritable component, laboratory results from women with PMS demonstrate that an excess or deficiency of certain nutrients may result in hormonal and neurotransmitter imbalance( Reference Head 4 , Reference Kendler, Karkowski and Corey 5 ). A study that compared premenopausal women following a Western diet containing high fat and low fibre with vegetarians revealed that a low-fat vegetarian diet decreased plasma oestrogen levels and the duration of premenstrual symptoms( Reference Gorbach and Goldin 6 , Reference Barnard, Scialli and Hurlock 7 ). Changing dietary guidelines towards consuming more complex carbohydrates and vegetable proteins, less simple carbohydrates, animal proteins and SFA and increasing fibre intake for 3–6 months resulted in raised serum progesterone levels, whereas serum oestradiol 17-β fell in the middle of the luteal phase( Reference Abraham and Rumley 8 ). Results from food-intake studies show that excess consumption of sweet-tasting food items, fast food, deep-fried meals, coffee and alcohol and low intakes of vegetables and fruits are significantly related to PMS incidence( Reference Cheng, Shih and Yang 9 , Reference Seedhom, Mohammed and Mahfouz 10 ).
Nursing is one of the most stressful jobs with lots of responsibilities and mental pressure. Research on work stress revealed that PMS symptoms in women with high responsibilities can be more severe than that of in others( Reference Namavar Jahromi, Pakmehr and Hagh-Shenas 11 ). A study among Korean nurses revealed that 78·3 % were suffering from PMS symptoms( Reference Park, Jeong and Kim 12 ). No study has yet evaluated the relationship between dietary patterns and PMS. As nurses are at risk of more PMS morbidity, the aim of our study was to investigate the relationship between dietary patterns and PMS in nurses.
Methods
Participants
This case–control study was performed from February to October 2014 in nurses aged 20–45 years. Eight hospitals were randomly selected from seventeen hospitals affiliated to Tehran University of Medical Sciences, Iran. A list of all female nurses at these eight hospitals was prepared. Nurses were asked to fill in a premenstrual symptoms screening tool (PSST) questionnaire consecutively until 160 nurses with PMS and 160 nurses without PMS were selected for the study.
Exclusion criteria were as follows: presence of any diagnosed diseases, including diabetes, liver and kidney dysfunction, CVD, cancer, and polycystic ovary syndrome or premature menopause, BMI>39·9 kg/m2 or taking of contraceptive pills, anti-depressant and other psychotropic drugs, or thyroid and other endocrine drugs, or taking multivitamin and mineral supplements at least three times a week; women who were on a diet or those who smoked were also excluded. Furthermore, pregnant and lactating individuals were not included in the study. Ethical approval was obtained from the Medical Ethics Committee of Tehran University of Medical Sciences. A written informed consent was obtained from each participant after an explanation of the objectives and procedures of the study. We used four different instruments including: PSST questionnaire, socio-demographic questionnaire, semi-quantitative FFQ and International Physical Activity Questionnaire (IPAQ).
Participants completed the PSST questionnaire, and according to PMS criteria they were divided into two groups: PMS group (n 160) and healthy group (n 160) as controls.
Premenstrual symptoms
The PSST questionnaire consists of nineteen questions in two parts. The first fourteen questions are about the intensity of somatic and affective symptoms. The five remaining questions evaluate the effect of symptoms on social connections. Each item is rated on a scale of 0–3 points (not at all, mild, moderate and severe). According to the manual, the PMS group should have at least four moderate or severe symptoms from the first part (the first fourteen questions) with at least one being from the first four symptoms. Moreover, they must have one moderate or severe check mark from part two (the last five questions). The PSST is a simple user-friendly screening tool based on the fourth edition of Diagnostic and Statistical Manual of Mental Disorders’ criteria( Reference Steiner, Macdougall and Brown 13 ). The validity of the Iranian version of the PSST questionnaire has been demonstrated previously( Reference Hariri, Moghaddam-Banaem and Siah Bazi 14 ).
Socio-demographic characteristics
The socio-demographic questionnaire consisted of general information such as age, marital status, educational level, position group, position ward, work shift, menstrual cycle status and bleeding period. In this study, regular menstrual cycle was defined as a normal period lasting 3–7 d in a 21–35 d cycle( 15 ).
Anthropometric measurements
Participants were required to stand on digital scales (Omron BF508 0·1–150 kg; OMRON HEALTHCARE Co., Ltd) to measure their weight to the nearest 100 g while wearing light clothes without shoes. Standing height was measured without shoes to the nearest 0·5 cm using a tape measure while shoulders were in a relaxed position. To calculate the BMI, we divided the weight (kg) by square of height (m2). Waist circumference was measured at the mid-point between the last rib and the iliac crest to the nearest 0·5 cm at the end of normal expiration using a non-elastic tape and without exerting any pressure on the body.
Assessment of food intakes
Intakes of macro- and micronutrients during the previous year were measured using a semi-quantitative FFQ. Participants were asked how frequently they consumed a specific portion size of 147 food items. We then noted down and multiplied the portion size and frequency of eating each item in order to obtain the total intake for the previous year. To save the nurses’ time, we divided the FFQ into three parts, and each time we completed forty-nine items. The validity and reliability of the FFQ has been evaluated previously( Reference Esfahani, Asghari and Mirmiran 16 ).
Physical activity measurement
The short form of IPAQ was used to assess physical activity of participants during the previous 7 d( Reference Moghaddam, Aghdam and Jafarabadi 17 ). The questionnaire includes seven items evaluating vigorous and moderate intensity of activities and walking for at least 10 min/d during the previous week. To compute activities, we multiplied the duration and number of activity days by the metabolic equivalent task (MET) value of the activity and summed all activities to gain an overall estimate of total physical activity.
Statistical analysis
The Statistical Package for Social Sciences, version 15 (SPSS Inc.), was used for analysis. To compare the socio-demographic characteristics between the two groups, first we assessed the normality of variables via the Shapiro–Wilk’s test. Variables with P>0·05 were considered as normal. We employed independent t tests and Mann–Whitney’s U tests for continuous variables and χ 2 tests for categorical variables. To ascertain the dietary patterns, factor analysis was performed. According to the similarity of food items, twenty-five food groups were created (Table 1). To explain sampling adequacy and inter-correlation of variables, Kaiser–Meyer–Olkin value and Bartlett’s test of sphericity were marked. The principal components method, scree plot and eigenvalues over 1·4 were used to select the number of factors and extract the main dietary patterns. Varimax rotation was carried out to create a simple and definitive component matrix. Factor loading weighted the consumption of each food group to produce the factor score for each pattern, and each subject got a particular factor score for each of the three patterns. Then, based on quintile, factor scores were classified into five groups. Logistic binary regression was used to assess the association of quintile of dietary pattern scores with incidence of PMS among groups. Two models were formed: model 1 was crude, and model 2 was controlled for age, menstrual cycle status, BMI, energy intake and physical activity score.
Table 1 Food groupings used in factor analysis

Results
Characteristics of cases and controls are defined in Table 2. There were no significant differences in quantitative variables between the two groups (P>0·05), but the number of cases who had irregular cycle status was significantly higher than that was in controls (P=0·04). Alcohol consumption was ignored as all nurses marked a negative answer for this except for four persons. Three main dietary patterns came to light from factor analysis, and these were named in terms of the highest loading factor groups in each pattern (Table 3). Food groups with factor loadings over 0·20 were considered as making a significant contribution to the pattern. These three dietary patterns accounted for 29·97 % of the total variance in food intake. The healthy dietary pattern was high in vegetables, fruits, natural juice, olive, tea and coffee, fish, low-fat dairy products, legumes and nuts. The Western dietary pattern was high in red and visceral meats, fast foods, vegetable oil and mayonnaise, sweets and desserts, salty snacks, refined grains, sugar and soft drinks, high-fat dairy products, spices and fried potato. The traditional dietary pattern was high in eggs, cooked potatoes, legumes and nuts, poultry, hydrogenated oil, cabbage, sweets and desserts. The OR of PMS incidence across quintiles of dietary pattern scores is shown in Table 4. We found a significant association between the Western dietary pattern and PMS in both models. Participants in the second (OR 2·29; 95 % CI 1·12, 4·66) and third (OR 3·65; 95 % CI 1·75, 7·57) quintiles were more likely to experience PMS compared with those in the first quintile in the crude model. The association between PMS and the Western dietary pattern remained significant after further adjustment for age, BMI, menstrual cycle status, physical activity and energy intake (OR 2·53; 95 % CI 1·18, 5·43 and OR 4·39; 95 % CI 1·97, 9·81, respectively). A dose–response relationship was not evident after testing PMS across the quintiles of the Western dietary pattern. No significant relationship was found between either the healthy or traditional dietary patterns and PMS even after adjusting for potential confounders (P trend>0·05).
Table 2 Characteristics of premenstrual syndrome cases and controls (Mean values and standard deviations; numbers and percentages)
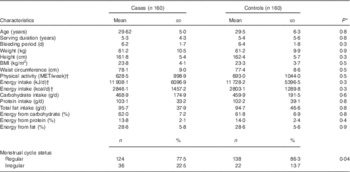
MET, metabolic equivalent tasks.
* The t test was used for parametric variables and the Mann–Whitney’s U test was used for non-parametric variables.
† For non-parametric variables, instead of mean and standard deviation, median and interquartile range are reported.
Table 3 Factor loading matrix for dietary pattern identified by factor analysis (n 320)Footnote *
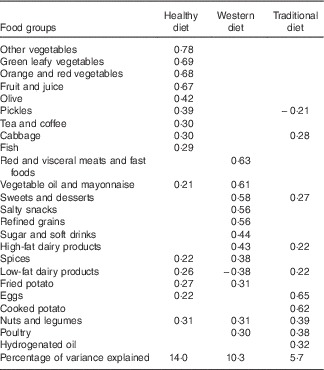
* Values<0·20 were excluded; Bartlett’s test of sphericity<0·0001; Kaiser–Meyer–Olkin=0·73; total variance=29·9 %.
Table 4 Premenstrual syndrome across quintiles (Q) of dietary pattern scores (Odds ratios and 95 % confidence intervals)
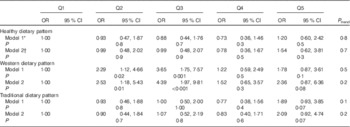
* Model 1: crude.
† Model 2: adjusted for age, BMI, menstrual cycle status, physical activity and energy intake.
Discussion
For the first time, we have investigated the association between dietary patterns and PMS morbidity. The results showed that the Western dietary pattern might be associated with PMS, although a distinct dose–response relationship was not found. The healthy and traditional dietary patterns did not show any significant relationship with this disorder.
Although many studies have been conducted on PMS worldwide, few researchers have focused on the relationship between food intake and PMS morbidity. In the present study, the Western dietary pattern has the greatest score for red meat and fast foods. Previous results revealed that higher consumption of red meat( Reference Sánchez-Villegas, Delgado-Rodríguez and Alonso 18 ) and fast foods and lower consumption of fruits and vegetables, fish and olive oil( Reference Sanchez-Villegas, Toledo and de Irala 19 ) was related to higher depression risk. Depression is the most outstanding feature of severe PMS( Reference Ko, Long and Chen 20 ). Following consumption of protein or protein plus fat-rich meals, plasma concentrations of tryptophan (the essential amino acid precursor of serotonin) show a greater decrease compared with that of large neutral amino acids (LNAA). Tryptophan and LNAA compete with each other for blood–brain barrier transport( Reference Wurtman, Wurtman and Regan 21 ). Individuals with lower levels of brain serotonin are considered to be vulnerable to affective disorders such as depression( Reference Markus, Firk and Gerhardt 22 ).
We observed that subjects with PMS consumed more refined grains and fewer vegetables and fruits. Previous studies revealed that fruit and vegetable diets with whole grains can treat mild forms of PMS and decrease the menstrual pain( Reference Balbi, Musone and Menditto 23 , Reference Tekač and Kotnik 24 ). Dietary fibre intake has shown a significantly inverse relationship to menstrual pain scale( Reference Nagata, Hirokawa and Shimizu 25 ).
In this study, the Western dietary pattern was high in Na from salty meals and snacks. Studies have suggested that excess salt intake, by decreasing Mg levels, may worsen premenstrual symptoms( Reference Johnson 26 ). In addition, Western diets with low vegetable intakes and high intakes of refined grains and meats are poor sources of Mg( Reference Beyer 27 ).
Our results indicate that low-fat dairy products were inversely related to the Western dietary pattern, whereas high-fat dairy products had a positive score. It has been shown that consuming whole milk rather than skimmed or low-fat milk slightly increased the risk for PMS( Reference Bertone-Johnson, Hankinson and Bendich 28 ).
Barnard et al.( Reference Barnard, Scialli and Hurlock 7 ) investigated the effects of a low-fat, vegetarian diet on premenopausal women. Study findings showed decreased premenstrual symptoms in women, with moderate-to-intense pain in menstruation. Furthermore, a low-fat, high-fibre diet significantly decreased serum estrone sulphate levels( Reference Woods, Gorbach and Longcope 29 ).
Vitamin D food sources such as fish and eggs did not feature in the Western dietary pattern. Previous studies found an inverse relationship between intake of fish and eggs and dysmenorrhoea( Reference Balbi, Musone and Menditto 23 , Reference Di Cintio, Parazzini and Tozzi 30 ).
We observed that the Western dietary pattern included high scores for sweets, desserts, sugar and soft drinks. Results of a previous study showed that subjects with premenstrual tension consumed more refined sugar and carbohydrates than did the normal people( Reference Goei, Ralston and Abraham 31 ). Other studies on PMS patients showed increased intake of cakes and desserts during the late luteal phase( Reference Wurtman, Brzezinski and Wurtman 32 , Reference Cross, Marley and Miles 33 ). Women with severe PMS have higher cravings for high-sweet-fat foods( Reference Yen, Chang and Ko 34 ). This may be due to the effects of premenstrual low serotonin activity, which often coexists with food cravings and depression in PMS patients( Reference Dye and Blundell 35 ). By eating high-glycaemic index carbohydrates, the ratio of plasma tryptophan:LNAA increases, which helps allay some of the psychological symptoms( Reference Murakami, Sasaki and Takahashi 36 ).
In the present study, we observed that participants in the fourth quintile of the healthy dietary pattern had an OR of 0·73 for PMS morbidity. However, it is possible that the total intake of healthy food items by our population is insufficient to yield substantially beneficial effects.
Our findings may not be directly comparable to previous research because of differences in study design and methods and our specific population. However, in terms of foods and food groups, we arrived at the same conclusion that a Western dietary pattern with meals and snacks high in fat, sugar and salt might be related to PMS morbidity.
This study does have some limitations that may affect interpretation of our findings. First, we could not include nurses from all parts of the hospitals, because we were not allowed to enter some areas such as emergency rooms, neonatal intensive-care units and surgical, infection and cancer wards either because personnel were too busy to find time to cooperate with us or because these wards were not safe for non-medical personnel to stay in for a long time. Therefore, we missed the opportunity to recruit certain nurses who were possibly under greater work stress. Second, in trying to document food consumption among nurses over the previous year, both the quantification of portion sizes and the frequency of consumption may have been subject to recall bias. However, in spite of these biases that can occur with the use of FFQ, they are still considered the best method for gathering dietary data in large population studies – such as the Nurses’ Health Study 2 cohort( Reference Colditz and Hankinson 37 ). Third, like all case–control studies, we could not determine the temporal relationship between dietary patterns and PMS. It is possible that premenstrual symptom experience influences diet choices (specifically preferences for fatty, salty or sweet foods) rather than the reverse. Fourth, the findings of our study might not be generalisable to other populations. One of the limitations of data-driven diet pattern analysis is that the derived patterns tend to be specific to the population under evaluation and may not easily be translated into broader recommendations for other populations with different diets and among whom correlations between foods are different. Despite these limitations, this is the first study to examine the relationship of major dietary patterns and PMS.
In conclusion, we have investigated the relationship between dietary patterns and PMS morbidity in a nurse population that has high work pressures and responsibilities. Among this population, we found that a Western dietary pattern might be associated with increased PMS morbidity. However, this result should be interpreted with caution because of the lack of a dose–response relationship. Further studies are needed to confirm this finding.
Acknowledgements
The authors would like to thank the hospital staff and all nurses for their participation, cooperation and collaboration in this study.
This research has been supported by Tehran University of Medical Sciences and Health Services grant no. 24367-161-03-92.
The authors’ contributions are as follows: F. S. and G. S. contributed to the study design and revised the manuscript. N. F. and K. A. contributed to data collection and wrote the manuscript. M. Q. and F. K. contributed to data analysis and interpretation of the data. All authors listed approved the content of the submitted manuscript.
There are no conflicts of interests to declare.







