Introduction
Autism spectrum disorders (ASDs) are characterized by impairments in communication, social reciprocity, and imagination, accompanied by limited, repetitive interests and behaviours. There is strong evidence from different lines of research that ASD is influenced by prenatal factors (Lyall et al. Reference Lyall, Schmidt and Hertz-Picciotto2014); and there is evidence through neuroanatomic, epidemiologic, and animal studies (Courchesne et al. Reference Courchesne, Yeung-Courchesne, Press, Hesselink and Jernigan1988; Rodier et al. Reference Rodier, Ingram, Tisdale, Nelson and Romano1996; Hultman et al. Reference Hultman, Sparen and Cnattingius2002; Shi et al. Reference Shi, Fatemi, Sidwell and Patterson2003; Herbert et al. Reference Herbert, Ziegler, Deutsch, O'Brien, Kennedy, Filipek, Bakardjiev, Hodgson, Takeoka, Makris and Caviness2005; Larsson et al. Reference Larsson, Eaton, Madsen, Vestergaard, Olesen, Agerbo, Schendel, Thorsen and Mortensen2005) that specific patterns of maternal diet represent a biologically plausible potential risk factor for ASD.
Recent studies (Schmidt et al. Reference Schmidt, Hansen, Hartiala, Allayee, Schmidt, Tancredi, Tassone and Hertz-Picciotto2011, Reference Schmidt, Tancredi, Ozonoff, Hansen, Hartiala, Allayee, Schmidt, Tassone and Hertz-Picciotto2012; de Steenweg et al. Reference de Steenweg, Ghassabian, Jaddoe, Tiemeier and Roza2015), including results from the large Norwegian MoBa Cohort, suggest that prenatal folic acid supplement use may protect the child against developing autism (Suren et al. Reference Suren, Roth, Bresnahan, Haugen, Hornig, Hirtz, Lie, Lipkin, Magnus, Reichborn-Kjennerud, Schjolberg, Davey, Oyen, Susser and Stoltenberg2013). Folate is necessary for normal fetal development (Morse, Reference Morse2012), and plays a key role in DNA methylation (Reynolds, Reference Reynolds2006) and could therefore impact risk of ASD. Previous studies have for the most part been case-control studies (Schmidt et al. Reference Schmidt, Hansen, Hartiala, Allayee, Schmidt, Tancredi, Tassone and Hertz-Picciotto2011, Reference Schmidt, Tancredi, Ozonoff, Hansen, Hartiala, Allayee, Schmidt, Tassone and Hertz-Picciotto2012), or have had incomplete ascertainment of ASD due to a relatively short follow-up period (Suren et al. Reference Suren, Roth, Bresnahan, Haugen, Hornig, Hirtz, Lie, Lipkin, Magnus, Reichborn-Kjennerud, Schjolberg, Davey, Oyen, Susser and Stoltenberg2013).
A previous report based on a subsample of the large prospective Danish National Birth Cohort (DNBC) was not able to substantiate a protective effect of prenatal folic acid supplement use against autism (Virk et al. Reference Virk, Liew, Olsen, Nohr, Catov and Ritz2016); therefore we aimed to test such a hypothesis using data from the entire DNBC.
Methods
The DNBC included more than 100 000 pregnancies, and details of the cohort have been reported previously (Olsen et al. Reference Olsen, Melbye, Olsen, Sorensen, Aaby, Andersen, Taxbol, Hansen, Juhl, Schow, Sorensen, Andresen, Mortensen, Olesen and Sondergaard2001, Reference Olsen, Mikkelsen, Knudsen, Orozova-Bekkevold, Halldorsson, Strøm and Osterdal2007); briefly, recruitment for the DNBC took place through the General Practitioners (GPs) when women consulted them for the first antenatal visit which, in Denmark, usually takes place during gestation weeks 6–10. The GP gave oral and written information about the DNBC and if the woman decided to participate, she was asked to send the completed recruitment form by post to the research centre in a pre-stamped envelope.
Mothers provided written informed consent on behalf of their children. The Regional Scientific Ethics Committee for the municipalities of Copenhagen and Frederiksberg approved all study protocols, and all procedures were in accordance with the Declaration of Helsinki.
Data collection in the DNBC included a recruitment form, two telephone interviews (gestation weeks 12 and 30, approximately); a semiquantitative food frequency questionnaire (FFQ) that was mailed to women in gestation week 25 asking about food consumption and supplement use in the previous 4 weeks (Olsen et al. Reference Olsen, Mikkelsen, Knudsen, Orozova-Bekkevold, Halldorsson, Strøm and Osterdal2007); and two postpartum telephone interviews (6 and 18 months postpartum, approximately). The main data source for our study was the recruitment form, which among other components had a section asking women to report on supplements and medication used in the periconceptional period. The format of this component was changed halfway through the recruitment period. In both versions, the woman was first asked if she had taken any drug or supplement prior to and/or during pregnancy. If so, she was asked to complete questions regarding a maximum of eight different drugs and supplements and two examples were provided to illustrate how to complete the form. In the first version of the recruitment form women were asked to write the brand names of all supplements that they had used during the preceding 3 months, and to write in their own words the amount and period that they had taken the supplement. The aim was to have the information computerized continuously as the recruitment forms were received at the research centre containing name of supplement product, dates of start and end of use and daily dosage. However, the manual process implied a considerable amount of interpretation of information, and in some cases (14%) the information was never computerized. To reduce the amount of manual processing a second version of the recruitment form was launched halfway through the recruitment. The new version included a table where the women ticked off which weeks (from gestation week −4 to 14) they had taken the supplement and asked her to write the average number of units (e.g. tablets) taken per week. Recently, the task of making the data from the first version of the recruitment form electronically available was taken up, which implied interpretation and coding of electronical text variables. For a smaller proportion of pregnancies, for which the first version of the recruitment form had not been computerized, the original questionnaires had to be manually processed.
Study sample
Our analyses included all singleton, liveborn children (n = 92 676). We excluded children with birthweights <2500 g or gestational age <32 weeks (n = 89 293), or missing information on supplement use, leaving us with 87 210 mother–children pairs in our analyses.
Exposure
For our analysis we defined ‘users’ as women who reported taking a supplement containing folic acid during week −4 to −1, 1–4 or 5–8. In sensitivity analysis we defined ‘consistent users’ as those who had taken supplements with folic acid during the whole period −4th to 8th week of gestation. Also, in sensitivity analyses we tested an association of periconceptional vitamin B12 with ASD, as well as an interaction between periconceptional B12 and folic acid supplementation in association with ASD.
In supplementary analyses using data from the midpregnancy FFQ, we compared users v. non-users of folic acid containing supplements (0, <400, ⩾400 µg/day), as well as groups of women categorized into quintiles of estimated dietary folate intake; a trend test was performed by entering the median in each quintile as a continuous variable in the model (Olsen et al. Reference Olsen, Mikkelsen, Knudsen, Orozova-Bekkevold, Halldorsson, Strøm and Osterdal2007).
Outcome
We used diagnoses of ASD from two mandatory national registers: the Danish Central Psychiatric Research Registry (Mors et al. Reference Mors, Perto and Mortensen2011), and the Danish National Patients Registry (Lynge et al. Reference Lynge, Sandegaard and Rebolj2011). Children with ASD were identified by International Classification of Diseases (ICD)-10 diagnosis codes F840, F841, F845, F848, and F849; ‘childhood autism’ by diagnosis code F840.
In sensitivity analyses we examined ASD subtypes: excluding the ‘atypical syndrome’ and ‘other pervasive developmental disorder’ groups, as these may include a broader range of developmental delays; looking separately at ‘Asperger syndrome’ (F845), and ‘pervasive developmental disorder, not otherwise specified’ (F841, F848, F849). We furthermore restricted cases to ASD and childhood autism with intellectual disability (F70–79).
Analytical strategy
We investigated associations between folic acid supplementation and dietary folate intake on the one hand and ASD/childhood autism on the other using Cox regression models with age of the child as the underlying time scale and stratifying by birthyear. Children were followed in the analyses from birth until date of diagnosis of ASD/childhood autism, death, emigration or end of follow up (31 December 2013), whichever came first. We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) while adjusting for the following covariates, selected a priori based on previous literature: maternal age, paternal age, parity, maternal smoking during pregnancy, maternal primary and secondary education, family socioeconomic status (based on occupation and education), whether the pregnancy was planned, maternal pre-pregnancy body mass index (BMI) and sex of the child. Missing data for covariates (range 0 for gender and maternal age to 8% for maternal pre-pregnancy BMI) were replaced using the mean/mode method. In sensitivity analysis complete case analysis was run.
Results
We identified 1234 cases of ASD during follow up in our study sample. Maternal folic acid supplementation was significantly associated with all the selected covariates, except for offspring sex (Table 1). For maternal and paternal age there was an inverse u-shaped association with maternal folic acid supplementation, whereas rate of supplementation decreased with increasing parity, maternal BMI, smoking, family socioeconomic group, and pregnancies that were not planned. There was no detectable association between maternal folic acid supplementation in the periconceptional period and offspring ASD, adjusted HR for use v. non-use was 1.06 (95% CI 0.94–1.19) (Table 2). The same was the case when we looked at childhood autism as an outcome, and when we examined supplementation during weeks −4 to −1, 1–4 and 5–8, separately. Results from the analyses using midpregnancy exposure data were similar: there was no association with ASD/childhood autism neither for folic acid supplementation nor for dietary folate intake. Sensitivity analyses using alternative definitions of periconceptional folic acid users, investigating the association between vitamin B12 and ASD, and omitting restrictions on birthweight or gestational age did not change our results. The same was the case when we looked at total maternal intake of folate, either periconceptionally or in midpregnancy, and when we restricted analyses of folic acid supplementation to those with low dietary folate intake, and vice versa, restricting analyses of dietary folate to those who did not take supplements containing folic acid.
Table 1. Mother-child pairs in study sample (n = 87 210), according to maternal, family and child characteristics, and distribution according to maternal folic acid supplementation during gestation weeks −4 to 8
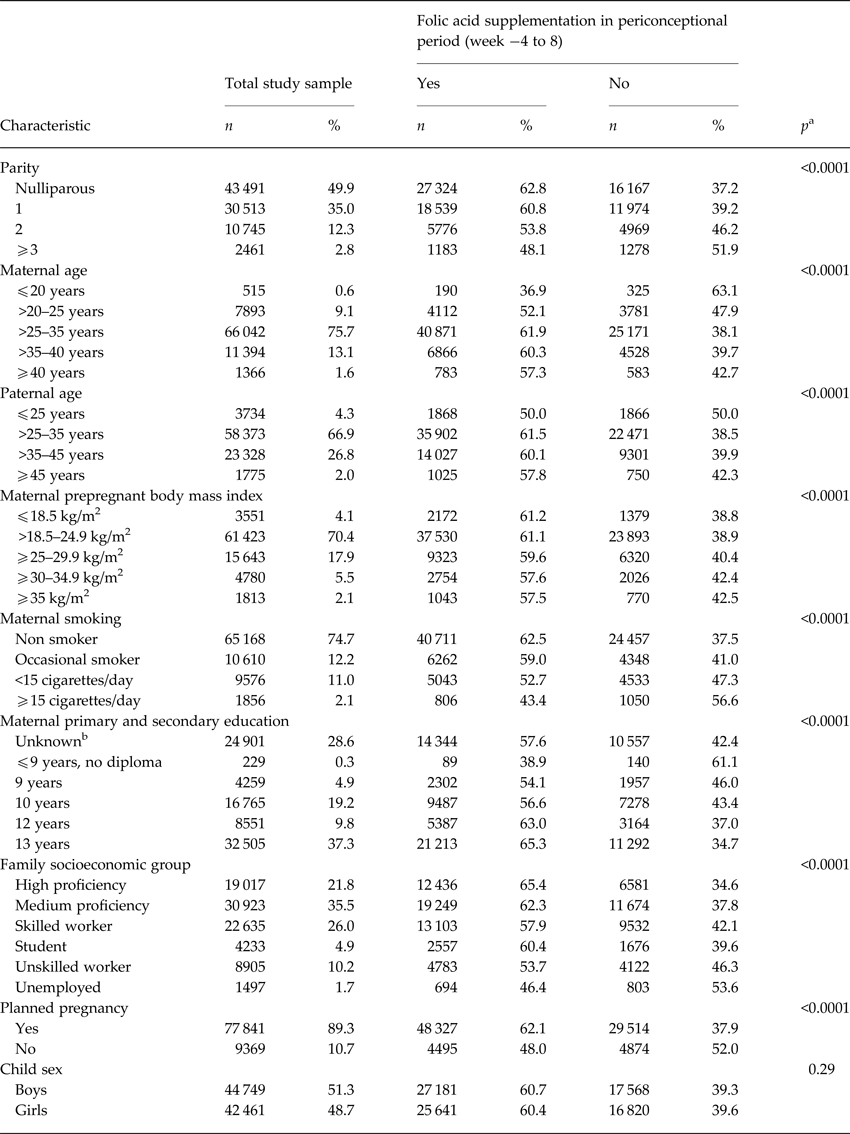
a p value from categorical χ2 test.
b We included ‘unknown’ as a category, since information on education was missing for 28%.
Table 2. Hazard Ratios (HR) for ASD and Childhood autism according to maternal folic acid use during gestation weeks −4 to 8 among 87 210 mother-child pairs in the Danish National Birth Cohort
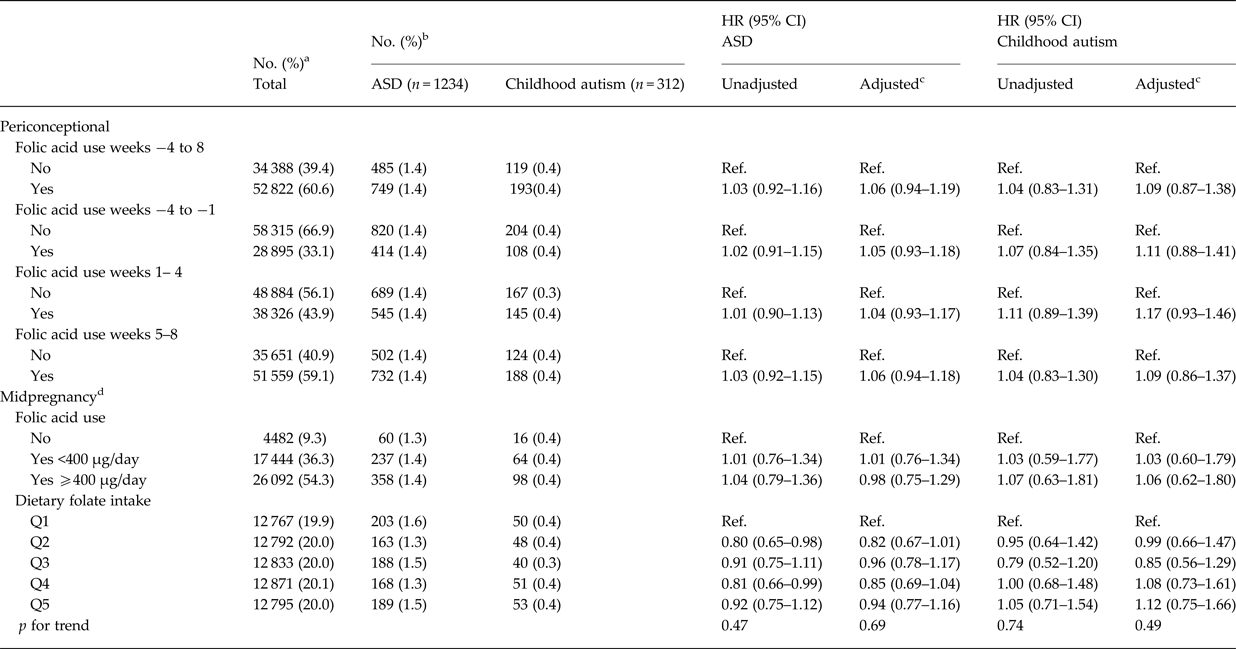
a Column percentages.
b Row percentages.
c Adjusted for maternal age, paternal age, parity, maternal smoking during pregnancy, maternal education, family socioeconomic status, whether the pregnancy was planned, maternal pre-pregnancy body mass index (BMI) and sex of the child
d Due to a smaller proportion of DNBC participants filling in the food frequency questionnaire in midpregnancy, study sample was restricted for the analyses using these measures: n (folic acid use in midpregnancy) = 48 018, n (dietary folate intake in midpregnancy) = 64 058.
There was no indication of sex specific effects, and adjusting for birth weight and gestational age did not change our results. Likewise, our results were not altered when we excluded atypical autism and pervasive developmental disorder, or when we looked at Asperger syndrome and pervasive developmental disorder separately. When we restricted cases to ASD with intellectual disability we saw similar results; for childhood autism with intellectual disability adjusted HR (95% CI) was 0.88 (0.52–1.48).
Discussion
In the largest study to date, we found no association between maternal folic acid supplementation and offspring ASD. While in accordance with a previous report from a subsample of the DNBC (Virk et al. Reference Virk, Liew, Olsen, Nohr, Catov and Ritz2016), this finding stands in contrast to results from two US case-control studies (Schmidt et al. Reference Schmidt, Hansen, Hartiala, Allayee, Schmidt, Tancredi, Tassone and Hertz-Picciotto2011, Reference Schmidt, Tancredi, Ozonoff, Hansen, Hartiala, Allayee, Schmidt, Tassone and Hertz-Picciotto2012), and the large (n = 85 176) prospective Norwegian MoBa Cohort (Suren et al. Reference Suren, Roth, Bresnahan, Haugen, Hornig, Hirtz, Lie, Lipkin, Magnus, Reichborn-Kjennerud, Schjolberg, Davey, Oyen, Susser and Stoltenberg2013). Results from the Dutch Generation R Study (n = 3893) were not able to substantiate an association when they investigated biomarkers for folate concentration in maternal serum from gestation week 13, but found an inverse association between selfreported folic acid supplementation and parent-reported autistic traits (de Steenweg et al. Reference de Steenweg, Ghassabian, Jaddoe, Tiemeier and Roza2015).
At present we are not able to present any viable explanation for these discrepant results. Rather than relying on self-reported measures of intake, biomarker studies may be an approach that permits an investigation into the mechanisms by which folate exerts its neurodevelopmental effects. In a study relating maternal folic acid supplementation to child language delay, inspired by animal data, the authors suggest as a potential explanation for their findings that folic acid supplements may facilitate reversal or compensation of the epigenetic effects of other early prenatal exposures that disrupt neurodevelopment (Roth et al. Reference Roth, Magnus, Schjolberg, Stoltenberg, Suren, McKeague, Davey, Reichborn-Kjennerud and Susser2011).
Interestingly, the US case-control studies investigated genetic influences and found that an association between folic acid supplementation and ASD was stronger for those genetically susceptible through polymorphisms related to inefficient folate metabolism (Schmidt et al. Reference Schmidt, Tancredi, Ozonoff, Hansen, Hartiala, Allayee, Schmidt, Tassone and Hertz-Picciotto2012). Differences in genetic background might thus explain discrepant findings for the USA and European studies, since associations for the MTHFR polymorphism with diseases such as dementia and schizophrenia have been shown to vary between ethnicities and populations (Liew & Gupta, Reference Liew and Gupta2015). However, this is unlikely to explain the inconsistent results from the closely related populations of Norwegians (the MoBa cohort) and Danes (the DNBC).
Residual confounding by socioeconomic status or other factors influencing ASD diagnosis and health related factors could underlie previously reported associations, since periconceptional folic acid supplement use, as clearly shown by our data, is strongly associated with health consciousness and cognitive skills. In contrast to the later Norwegian MoBa cohort, recruitment to the DNBC was ongoing when recommendations of folic acid supplementation for women who planned to become pregnant was first introduced and data from the DNBC has indicated that compliance with the recommendations was strongly associated with sociodemographic and lifestyle factors (Knudsen et al. Reference Knudsen, Orozova-Bekkevold, Rasmussen, Mikkelsen, Michaelsen and Olsen2004; Olsen & Knudsen, Reference Olsen and Knudsen2008). Since the folic acid awareness initiatives preceded the recruitment for the Norwegian MoBa Cohort, the covariate structure may have been even stronger in those analyses, compared with the analyses in the DNBC. But whereas confounding and differential uptake in the recommendations for folic acid supplementation may have affected the level of folic acid supplementation, it is less likely to have affected the internal association between maternal folic acid supplementation and child ASD; a point that is supported by the little effect confounder adjustment had in our analyses.
Previously, differences in intake of folic acid and folate have been mentioned as a potential explanation for discrepant findings. Folate deficiency rate has been reported to be higher in Norwegian compared with Danish pregnant women (Virk et al. Reference Virk, Liew, Olsen, Nohr, Catov and Ritz2016), perhaps reflecting a higher habitual dietary folate intake in the DNBC (Olsen et al. Reference Olsen, Birgisdottir, Halldorsson, Brantsaeter, Haugen, Torjusen, Petersen, Strom and Meltzer2014) and suggested by Virk et al. (Reference Virk, Liew, Olsen, Nohr, Catov and Ritz2016) to mask any beneficial effect that folic acid would have in more deficient populations. However, we looked at the association of folic acid supplementation in the lowest quintile of dietary folate intake (n = 12 767) (and vice versa), and still were not able to substantiate any beneficial effect of folic acid or folate with regard to ASD in the DNBC.
The strengths of our study include the prospective study design and large sample size with complete follow up of all children by our use of registry data. Furthermore, data on folic acid supplementation, available at two different occasions during pregnancy, was concurrently assessed so we were able to effectively investigate two different time windows of exposure. Limitations of our study include selfreported exposure measures rather than biomarkers and that we used diagnoses from registries for our outcome assessment, which may have been prone to misclassification. However, previous work has suggested high validity of ASD diagnoses in the Danish registries that we used (Lauritsen et al. Reference Lauritsen, Jorgensen, Madsen, Lemcke, Toft, Grove, Schendel and Thorsen2010), making this explanation unlikely. In supplementary analyses we looked at ASD/childhood autism with intellectual disability. For childhood autism with intellectual disability the risk estimate was in the direction of a beneficial effect of folic acid supplementation, but this was not statistically significant (adjusted HR (95% CI) 0.88 (0.52–1.48)), perhaps because of a relatively low number of cases in the analysis (n = 60).
In conclusion, we were not able to substantiate a hypothesized beneficial effect on child risk of ASD by maternal folic acid supplementation in the periconceptional period. Continued study of maternal folate and child ASD, using biomarkers for exposure measurement and taking careful consideration of genetic and other potentially confounding factors, is warranted.
Acknowledgements
Dr Strøm had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design was done by all the authors. All the authors were involved in acquisition, analysis, or interpretation of data. Strøm drafted the manuscript. Critical revision of the manuscript for important intellectual content was done by all the authors. Strøm performed statistical analysis. Olsen, Strøm obtained funding. This study was funded by the Danish Council for Independent Research (4092-00499). The study is furthermore part of the programme of the Centre for Fetal Programming, which is funded by the Danish Council for Strategic Research (09-067124). The funding bodies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Declaration of Interest
All authors report no conflicts of interest.




