INTRODUCTION
Genus Ichthyobodo comprise ectoparasitic flagellates that infect a wide range of fish species worldwide (Robertson, Reference Robertson, Muir and Roberts1985; Urawa et al. Reference Urawa, Ueki and Karlsbakk1998). Heavy Ichthyobodo spp. infections on skin or gills cause disease (ichthyobodosis) and mortalities and have represented a common problem in fish farms (Franke, Reference Franke1910; Schäperclaus, Reference Schäperclaus1929; Fish, Reference Fish1940; Bauer, Reference Bauer1959; Ellis and Wootten, Reference Ellis and Wootten1978; Urawa, Reference Urawa1996).
Ichthyobodo infections in salmonids in both fresh- and seawater have in the past been identified as Ichthyobodo necator, thereby implying that this species is euryhaline (Ellis and Wootten, Reference Ellis and Wootten1978; Wood, Reference Wood1979; Poppe and Håstein, Reference Poppe and Håstein1982; Urawa and Kusakari, Reference Urawa and Kusakari1990). Therefore, infections in seawater were assumed to be contracted in freshwater. Ichthyobodo spp. are attached to the host cell by an attachment disc through which a cytostome canal supported with microtubules penetrate to the host cell cytoplasm. Through this canal, cell contents are moved into the feeding parasite (see Robertson, Reference Robertson, Muir and Roberts1985; Lom and Dykova, Reference Lom and Dyková1992). Ultrastructural studies by Roubal and Bullock (Reference Roubal and Bullock1987) revealed that the attachments region differed between flagellates identified as I. necator infecting salmonids in fresh- and seawater, but suggested that the different environments influenced this structure. However, Bruno (Reference Bruno1992) found significant differences in the size of Ichthyobodo trophozoites from the gills of salmonids reared in freshwater and seawater, the latter being significantly smaller. He suggested that a marine Ichthyobodo species exists that is able to infect Atlantic salmon. Lamas and Bruno (Reference Lamas and Bruno1992) studied the ultrastructure of the attachment region of the smaller species infecting seawater reared salmon. They verified the findings of Roubal and Bullock (Reference Roubal and Bullock1987) but considered it a character distinguishing a separate species, Ichthyobodo sp. More recent molecular studies of the small subunit ribosomal RNA gene (SSU rDNA) have confirmed the existence of 2 distinct Ichthyobodo species infecting farmed Atlantic salmon (Salmo salar) in Norway; Ichthyobodo necator sensu stricto (s.s.) and Ichthyobodo sp. II (Todal et al. Reference Todal, Karlsbakk, Isaksen, Plarre, Urawa, Mouton, Hoel, Koren and Nylund2004). I. necator s.s., re-described by Isaksen et al. (Reference Isaksen, Karlsbakk and Nylund2007), appears to be restricted to freshwater fishes, while the undescribed Ichthyobodo sp. II infects salmon in both fresh- and seawater.
In the present study we used molecular methods to identify Ichthyobodo sp. II from salmon reared in fresh-, brackish- and seawater in Norway. Morphometric, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) studies were performed, in the search for distinguishing characters for the novel species Ichthyobodo salmonis sp. n. (syn. Ichthyobodo sp. II sensu Todal et al. Reference Todal, Karlsbakk, Isaksen, Plarre, Urawa, Mouton, Hoel, Koren and Nylund2004) when compared to I. necator s.s.
MATERIALS AND METHODS
Samples for identification and description of Ichthyobodo spp
Gill samples were collected from Ichthyobodo-infected Atlantic salmon (Salmo salar) from 3 farms in Western and Northern Norway (Table 1) and examined with molecular and morphological methods. All farms reported gill disease or increased mortality prior to the period of sampling.
Table 1. Samples from farmed Atlantic salmon (Salmo salar), representing 3 different fish farms in Norway

* Seawater acclimatization before smoltification, freshwater added salt/seawater to a salinity of 1·6–2·0%.
Samples from all farms included gills preserved in 90% ethanol (for PCR) and air-dried smears from the gills (for light microscopy). Gill samples fixed in a modified Karnovsky fixative (Nylund et al. Reference Nylund, Nylund, Watanabe, Arnesen and Karlsbakk2010) for electron microscopy were obtained from farms 2–3 (Table 1). In addition, material and measurements of I. necator s.s. from salmon parr (material of Isaksen et al. Reference Isaksen, Karlsbakk and Nylund2007), was used for interspecific comparisons.
Small subunit ribosomal RNA gene sequences (SSU rDNA)
Total DNA from the ethanol-preserved tissue samples was isolated using QIAmp® DNA mini kit (Cat.no. 51304) and protocol for DNA purification from tissues (Qiagen). PCR and sequencing was performed using primers described by Isaksen et al. (Reference Isaksen, Karlsbakk, Sundnes and Nylund2010). PCR products were purified using E.Z.N.A.TM Cycle Pure Kit (Omega Bio-Tek) and then sequenced with BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystem). The sequence data were assembled with the software ContigExpress (VectorNTI Suite 9.0.0) and compared with sequences in the GenBank database with NCBI BLAST searching.
Morphological measurements
Samples for morphological analyses were tested by PCR using diagnostic primers specific for Ichthyobodo sp. II sensu Todal et al. (Reference Todal, Karlsbakk, Isaksen, Plarre, Urawa, Mouton, Hoel, Koren and Nylund2004) and for I. necator s.s. (CoEur and CoNec primers respectively; Isaksen et al. Reference Isaksen, Karlsbakk, Sundnes and Nylund2010). The studied samples (Table 1) were positive for Ichthyobodo sp. II and negative for I. necator s.s.
Air-dried smears were stained with Colorrapid-Set (Lucerna Chem AG) and the Ichthyobodo cells studied at 1000×magnification in a light microscope (ZEISS Axioskop 2 plus) equipped with a digital camera (Nikon Digital Sight DS-U1). The flagellates were measured on captured photos using the software ImageJ 1.42 (http://rsb.info.nih.gov/ij/). Morphometric data from Ichthyobodo cells were obtained as described by Isaksen et al. (Reference Isaksen, Karlsbakk and Nylund2007). Flagellates were only measured when principal characters (cytostomeal protrusion, nucleus, flagellar pocket) were visible. The cytostomeal protrusion represents an easily recognizable starting point for the measurements (Fig. 1). Flagellar length was measured as the free part from the edge of the cell outline, since the path of the flagella in the flagellar pocket often was difficult to ascertain. The number of visible cytoplasmic basophilic granules (kinetoplasts) was counted.

Fig. 1. Measurements of Ichthyobodo cells. L1, maximum cell length measured from a nose-like protrusion* (starting point); L2, maximum cell width measured right-angled to L1; L3 and L4, nucleus diameter measured paralleled to L1 and L2 respectively; L5, extents of the flagellar pocket; N1 and N2, nucleus position. Centre of nucleus measured parallel to L1 and L2.
Scanning electron microscopy (SEM)
Fixed gill tissues were washed in 0·2 m sodium phosphate buffer and post-fixed (1 h) in a 1% aqueous solution of osmium tetroxide (OsO4). The tissues were washed in distilled water and dehydrated with use of cold acetone in a 5×15 min series (60%; 90% and 3×100% acetone). Dehydrated tissues were further dried with the use of CO2 in a critical-point drier (Polaron), attached onto stubs, coated with gold-palladium (Polaron SC502 Sputter Coater, Fison Instruments) and examined in scanning electron microscopy (ZEISS Supra 55VP).
Transmission electron microscopy (TEM)
Fixed samples were washed in sodium phosphate buffer, post-fixed in OsO4 (2%) and dehydrated as described above for SEM. After acetone dehydration the tissues were embedded in EMbed 812 resin (Electron Microscopy Science, Hatfield, PA, USA). Ultrathin sections were cut and stained as described by Nylund et al. (Reference Nylund, Nylund, Watanabe, Arnesen and Karlsbakk2010).
Statistical methods
A dataset with measurements of I. necator s.s. referred to by Isaksen et al. (Reference Isaksen, Karlsbakk and Nylund2007) was used for statistical comparison with Ichthyobodo sp. n. (described below). The measurements of Ichthyobodo spp. were compared using Student's t-test, following normality testing in Statistica®. Since many measurements correlated with cell size, analysis of covariance (ANCOVA) was used to examine whether interspecific differences revealed by the Student's t-tests could be attributed to mere size differences of the cells in the two species. This was done to reveal good distinguishing characters. Probabilities (P)<0·05 were considered significant.
RESULTS
SSU rDNA sequencing
Partial SSU rDNA sequences obtained from the different Ichthyobodo sp. samples from Atlantic salmon collected in fresh-, brackish- and seawater (Table 1) showed 99·9–100·0% identity with each other (GenBank Accession nos. JF290204, JF290203, JF290205, representing farms 1, 2 and 3 respectively) and with Ichthyobodo sp. II sensu Todal et al. Reference Todal, Karlsbakk, Isaksen, Plarre, Urawa, Mouton, Hoel, Koren and Nylund2004 (GenBank Accession nos. AY229972, AY229973, AY224685, AY224686).
Compared with Ichthyobodo sp. III of Todal et al. (Reference Todal, Karlsbakk, Isaksen, Plarre, Urawa, Mouton, Hoel, Koren and Nylund2004), from masu salmon (Oncorhynchus masou) in Japan (AY224689), the present Ichthyobodo sp. sequences differed by 2 deletions and 9–10 substitutions (99·3–99·4% identity). Ichthyobodo necator s.s. from freshwater reared salmon in Norway (AY224691, GQ184295, GQ184296) showed 92·3–92·5% similarity with the present Ichthyobodo sp. sequences. Based on 100% sequence similarity with the undescribed species designated Ichthyobodo sp. II by Todal et al. (Reference Todal, Karlsbakk, Isaksen, Plarre, Urawa, Mouton, Hoel, Koren and Nylund2004), the present material is identified with that species and described below as a novel species, Ichthyobodo salmonis sp. n.
Taxonomic summary
• Ichthyobodo salmonis sp. n.
• Synonym: Ichthyobodo sp. II sensu Todal et al. (Reference Todal, Karlsbakk, Isaksen, Plarre, Urawa, Mouton, Hoel, Koren and Nylund2004).
• Etymology: After the type host.
• Type host: Salmo salar L.
• Type locality: Grovfjord, Troms county, Northern Norway (68°39′N, 17°12′E).
• Additional locations: Etne and Eidfjord, Hordaland county, Western Norway (present study), Masfjorden and Ytre Sogn, Hordaland and Sogn and Fjordane counties, Western Norway (Todal et al. Reference Todal, Karlsbakk, Isaksen, Plarre, Urawa, Mouton, Hoel, Koren and Nylund2004).
• Site: Gills and skin.
• Museum material: Syntypes, air-dried stained slide deposited in Zoological Museum, University of Bergen, Norway, ZMBN 87075.
• SSU rDNA sequences: GenBank Accession numbers JF290203, JF290204, JF290205 (present study); AY229972, AY229973, AY224685, AY224686 (Todal et al. Reference Todal, Karlsbakk, Isaksen, Plarre, Urawa, Mouton, Hoel, Koren and Nylund2004) (partial sequences).
Novel species description
In live observations with the use of light microscopy, free forms of I. salmonis sp. n. move in a rapid, spinning and non-directional manner. No movement was apparent in the attached cells. The descriptions of the parasite refer to morphology of cells in stained smears (main characters shown in Fig. 2) and with the use of electron microscopy (SEM, TEM) of attached trophozoites.

Fig. 2. Characteristic structure of Ichthyobodo salmonis sp. n. (free form) from Atlantic salmon (Salmo salar). Kinetoplasts not drawn. Ventral view. A, axostyle; C, cytostomeal protrution (anterior part of the cell); F, flagella; Fp, flagellar pocket; Lg, longitudinal groove; N, nucleus; V, vacuoles.
Free form of I. salmonis sp. n. (Table 2)
The free form of the flagellates in stained smears is rounded or oval, with a length/width relationship (Ci) larger than 1.
Table 2. Measurements of the free form of Ichthyobodo salmonis sp. n. from the gills of Atlantic salmon (Salmo salar)
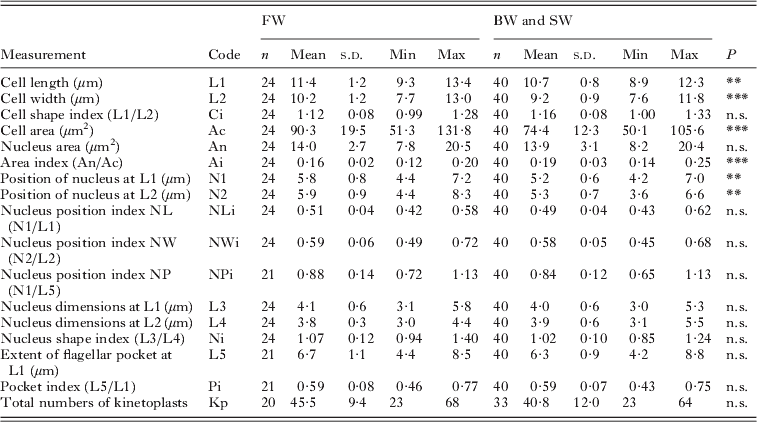
n.s., Not significant; *P<0·05;
** P<0·01;
*** P<0·001.
The flagellar pocket is seen as a bright area in the cell, to the right of the nucleus (e.g. Fig. 3 G, J, K). The pocket typically extends more than half the cell length, ending at the level of the posterior part of the nucleus. The pocket opens antero-ventrally into a longitudinal groove extending to the opposite side along the cell margin.

Fig. 3. Ichthyobodo spp. from skin and gills of Atlantic salmon (Salmo salar). Stained smears of Ichthyobodo salmonis sp. n. from gills of salmon in freshwater (A–D), brakish water (E–H) and seawater (I–L). Ichthyobodo necator from salmon parr shown for comparison (M–P). H and P, trophozoites; L, dividing form; C, cytostome; F, flagella; Fp, flagellar pocket; Kp, kinetoplasts; Lg, longitudinal groove; N, nucleus; V, vacuoles. Scale bars=5 μm.
Both bi- and quadri-flagellated cells were observed, with 1 short and 1 long flagellum or 2 pairs of unequal length. The free part of the shorter flagellum was generally half the length of the longer, in stained smears measuring 8·6±2·4 μm (mean±s.d.) (range 4–13 μm, n=22) and 15·0±3·4 (8–21 μm, n=32) respectively.
The nucleus is rounded and centrally located in the cell, constituting ca. 1/6th of the cell area.
A pale, bent, rod-like structure (axostyle) is often apparent along the right cell margin, originating in the cytostomeal protrusion and reaching to the posterior end.
One prominent apparent vacuole (V1) is commonly evident in an area close to the posterior part of the nucleus, on the left side. This vacuole typically takes a bluish colour in the stained preparations. Two additional, but colourless vacuoles may also be observed on the right side (V2 and V3), V3 is located close to the flagellar pocket (Figs 2 and 3).
Kinetoplasts (Kp, Fig. 3) are visible as intensely stained, regular-sized grains. No regular pattern of distribution in the cell was evident, but they rarely occurred in the pocket-groove region and on the nucleus.
Trophozoites of I. salmonis sp. n. (Table 3)
Trophozoites are pyriform in shape, pointed to the attachment area. The pointed end contains the anterior end of the axostyle. When apparent, the axostyle extends posterior along the right cell margin adjacent to the flagellar pocket. The longitudinal groove is more often easily visible in trophozoites than in free forms of the flagellate. In stained smears, the groove and the flagellar pocket form a characteristic Y-shaped pattern in the cell (e.g. Fig. 3H).
Table 3. Attached form (trophozoites) of Ichthyobodo salmonis sp. n. from gills of Atlantic salmon (Salmo salar)
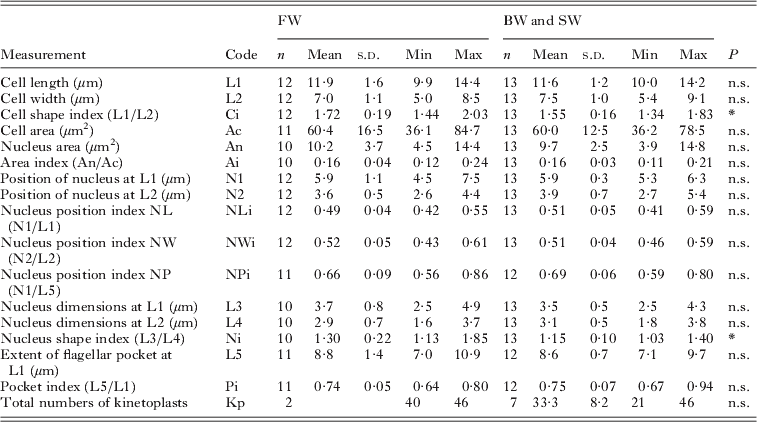
n.s., Not significant ;
* P<0·05; **P<0·01; ***P<0·001.
Two vacuoles may occur; one is commonly seen and shows the same characteristic staining as V1 in the free form (Figs 2 and 3). The second vacuole is occasionally seen near the flagellar pocket. The kinetoplasts are scattered as described for the free form.
SEM observations revealed 2 spine-like projections clearly visible on the ventral side of the cell (Fig. 4 D, E, G). One ‘spine’ appears close to the cell margin on the pocket side and the second one more posterior at the end of the longitudinal groove (Fig. 4). Both 2- and 4- flagellated trophozoites are seen with SEM, with 1 or 2 pairs of flagellae of unequal length. The long flagellae reach past the posterior tip of the trophozoite, while the short flagellae end inside the groove. TEM sections of the attachments disc and cytostome process show a smooth surface of this structure in I. salmonis sp. n. (Fig. 6).

Fig. 4. Scanning electron microscopy (SEM) of Ichthyobodo spp. trophozoites on gills of Atlantic salmon (Salmo salar). (A–G) Ichthyobodo salmonis sp. n. from brackishwater reared smolt. (A) Trophozoites attached to secondary gill lamellae; (B) trophozoites attached on the surface of a primary gill filament; (C) Single trophozoite, dorsal view; (D) trophozoite, ventral view; (E) quadri-flagellated (2 long, 2 short flagella) trophozoite, ventral view; (F) 2 trophozoites; (G) quadri-flagellated, ventral view (1short flagellum just visible in furrow); (H–K) Ichthyobodo necator s.s. from hatchery reared parr; (H) ventral view; (I) ventral view, flagella clearly visible; (J) same trophozoite shown in (I) close-up of the penetration area (scale bar=1 μm); (K) dorsal view; (D, E and G) arrows mark spine-like surface projections characteristic for I. salmonis sp.n. All scale bars except (J)=5 μm.
Intraspecific variations(Tables 2 and 3)
Measurements of the free forms of I. salmonis sp. n. from the different infections in fresh-, brackish- and seawater were compared. No significant differences in cell dimension between isolates from brackish- and seawater (n=19 and n=21 respectively) could be detected.
However, the flagellates in the freshwater infection were significantly larger compared to those from brackish and seawater (Ac: t=−4, d.f.=62, P<0·001). The cell shape (Ci) was similar, and there were no resultant relative changes in the position or extent of the nucleus and flagellar pocket, respectively, when cell size was accounted for (ANCOVA, P=0·93).
Vacuoles in positions V2 and V3 (Figs 2 and 3) are more frequently observed in flagellates from freshwater than those from brackish- and seawater.
The partial SSU sequences of I. salmonis sp. n. available (GenBank, present study) diverge by a maximum of 1 substitution (1801nt compared).
Comparison with I. necator (Tables 4 and 5)
Statistical comparison of measurements of I. salmonis sp. n. and I. necator s.s. (data of Isaksen et al. Reference Isaksen, Karlsbakk and Nylund2007) revealed significant differences in most dimensions and morphometric indices. I. salmonis sp. n. is significantly smaller than I. necator, being shorter and narrower with a clearly different shape (Ci) (Fig. 5 A). The flagellar pocket in I. salmonis sp. n. extends significantly more posterior than in I. necator s.s., and generally reaches posterior to the nucleus (NPi) (Fig. 5B).

Fig. 5. Morphometric comparison of the free forms of Ichthyobodo salmonis sp. n. (●) and Ichthyobodo necator (○) from Atlantic salmon (Salmo salar). (A) Scatterplot showing cell width (L2) against cell length (L1). Least squares trend lines and corresponding R2-values indicated. (B) Scatterplots (with 95% range ellipses) show the nucleus position index against the cell shape index. These indices represent the position of the nucleus related to extent of the flagellar pocket (N1:L5) and the length/width relationship (L1:L2); the measures which most clearly separate these species.
Table 4. Comparison of the free form of Ichthyobodo salmonis sp. n. and Ichthyobodo necator s.s. (data from Isaksen et al. Reference Isaksen, Karlsbakk and Nylund2007) from Atlantic salmon (Salmo salar)
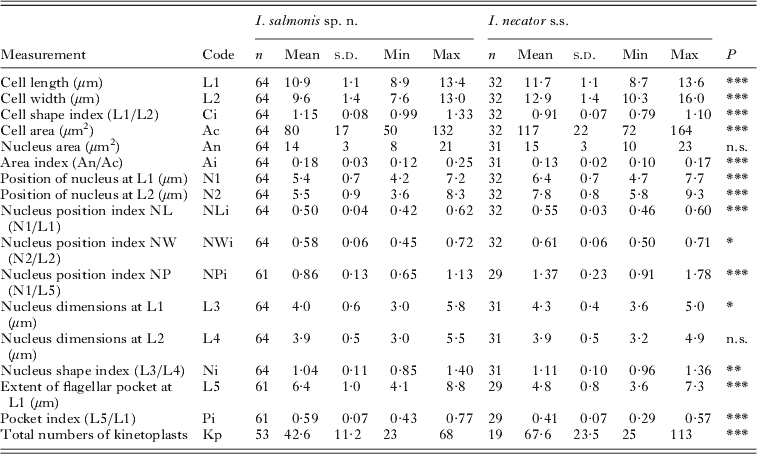
n.s., Not significant ;
* P<0·05;
** P<0·01;
*** P<0·001.
Table 5. Comparison of the attached form (trophozoites) of Ichthyobodo salmonis sp. n. and Ichthyobodo necator s.s. (data from Isaksen et al. Reference Isaksen, Karlsbakk and Nylund2007) from Atlantic salmon (Salmo salar). (n, Number of cells examined. Last column (P) gives the results of Student's t-tests, comparing measurements of I. salmonis and I. necator.)
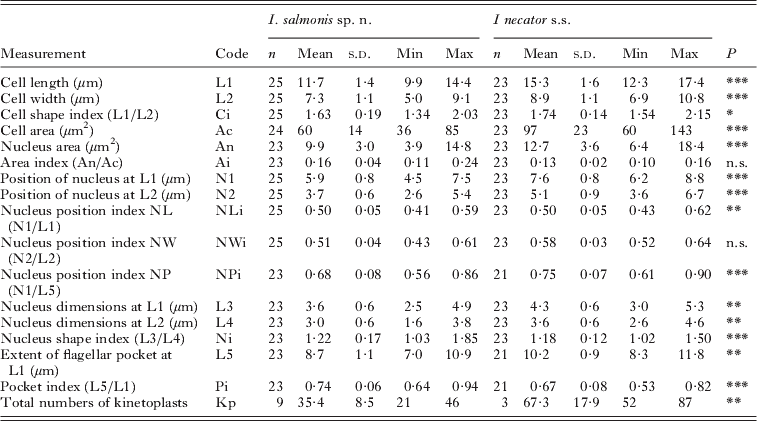
n.s., Not significant ;
* P<0·05;
** P<0·01;
*** P<0·001.
The larger I. necator s.s. contain more kinetoplasts than I. salmonis sp. n.; however, kinetoplast counts correlate positively with cell size in both species and counts did not differ significantly when cell size was accounted for (ANCOVA, P=0·28).
Trophozoites of I. salmonis sp. n. were clearly smaller compared to those of I. necator s.s. Significant differences in most other measures between the two species proved to be related to these size differences (ANCOVA, P>0·05). However, the shape of the cells and the size of the nucleus differed when cell size was accounted for (ANCOVA, P<0·01).
The SEM observations of the trophozoites of I. salmonis sp. n. revealed a characteristic cell shape in dorsal view (Fig. 4B, C), and 2 spine-like projections clearly visible on the ventral side of the cell (Fig. 4D, E, G). No such structures can be detected in our SEM images of I. necator (e.g. Fig. 4H, I).
Longitudinal TEM sections revealed a smooth surface of the attachment disc and cytostome process in the trophozoites of I. salmonis sp.n. from different macrohabitats (Fig. 6). However, in I. necator the interface between the attachment disc and the host cell is conspicuously jagged (Fig. 7).

Fig. 6. Transmission electron microscopy (TEM) of Ichthyobodo salmonis sp.n. on the gills of Atlantic salmon (Salmo salar). (A–B) Trophozoites on salmon from brackish water; (C–D) trophozoites on salmon from seawater. Ad, Attachment disc; Cp, cytostome process; F, flagella; Kp, kinetoplasts; N, nucleus. All scale bars=1 μm.

Fig. 7. Transmission electron microscopy (TEM) of Ichthyobodo necator on gills (A–B) and skin (C–D) of Atlantic salmon parr (Salmo salar) from freshwater. Ad, Attacment disc; Cp, cytostome process; F, flagella; Kp, kinetoplast; N, nucleus. All scale bars=1 μm.
DISCUSSION
The genus Ichthyobodo (syn. Costia Leclerq, 1890) contains 3 named species, I. necator Henneguy, 1883, I. nitschei (syn. Tetramitus nitschei Weltner, 1894) and I. hippoglossi Isaksen et al. Reference Isaksen, Karlsbakk and Nylund2007. A fourth species, Costia pyriformis Davis, 1943 likely represents a member of a different genus (see Isaksen et al. Reference Isaksen, Karlsbakk and Nylund2007). The inadequately described I. nitschei from goldfish (Carassius auratus) may represent one of the several undescribed Ichthyobodo species detected in infected goldfish and other cyprinids through SSU rDNA sequencing (Ichthyobodo spp. VII and VIII, see Isaksen et al. Reference Isaksen, Karlsbakk and Nylund2007). Ichthyobodo hippoglossi, a parasite of the Atlantic halibut (Hippoglossus hippoglossus), is the only named marine species in the genus. It was distinguished from I. necator sensu stricto (s.s.) by Isaksen et al. (Reference Isaksen, Karlsbakk and Nylund2007) on the basis of SSU rDNA sequences, cell shape, kinetoplast number and size and by the extent of the flagellar pocket.
Ichthyobodo salmonis sp. n. is clearly distinguished from I. necator and I. hippoglossi (DQ414520) by SSU sequence similarity (92 and 94%, respectively). Morphologically, I. salmonis can be distinguished from I. hippoglossi by its larger number of evenly sized small kinetoplasts, its characteristic stained vacuole-like structure (V1) and by the presence of spine-like surface structures on the ventral surface. The latter structures have never previously been detected in Ichthyobodo spp.
The most relevant morphological comparison is between I. necator and I. salmonis, since these may infect the same salmonid hosts. The very wide conception of I. necator sensu lato (s.l.) is partly due to the past confusion of these species in connection with ichthyobodosis in reared salmonids. When Isaksen et al. (Reference Isaksen, Karlsbakk and Nylund2007) re-described I. necator from juvenile salmon farmed in freshwater, they provided morphometric characters that may be used to separate Ichthyobodo spp. recognized by sequence information (e.g. SSU rDNA). In the present study, we show that free forms and trophozoites of I. salmonis sp. n. from both fresh- and seawater were smaller than those of I. necator s.s. Bruno (Reference Bruno1992) performed morphometric comparisons of Ichthyobodo spp. trophozoites in gill sections from farmed salmon in seawater and salmon and other salmonids in freshwater. He found that the trophozoites from seawater-infections were significant smaller than those in freshwater. He considered it likely that 2 different Ichthyobodo spp. were involved. Since the trophozoites of I. salmonis sp. n. are some 60–80% the size of those of I. necator s.s., it now appears likely that Bruno (Reference Bruno1992) studied I. salmonis sp. n. infections (seawater) and infections dominated by I. necator s.s. (freshwater). It has been observed that Ichthyobodo sp. infections (usually identified as I. necator s.l.) acquired by salmonids in freshwater apparently survive sea-transfer of the smolt, often with proliferation of the parasite in the seawater phase leading to ichthyobodosis (gill disease) (Ellis and Wootten, Reference Ellis and Wootten1978; Wood, Reference Wood1979; Urawa and Kusakari, Reference Urawa and Kusakari1990). However, sodium chloride (NaCl) or seawater addition (salinity 1%) has been used to treat ichthyobodosis in freshwater salmonid hatcheries (see Todal et al. Reference Todal, Karlsbakk, Isaksen, Plarre, Urawa, Mouton, Hoel, Koren and Nylund2004), including infections now verified as being due to I. necator s.s. (Isaksen et al. Reference Isaksen, Karlsbakk and Nylund2007, Reference Isaksen, Karlsbakk, Sundnes and Nylund2010). Therefore, it appears that I. necator s.s. is a freshwater parasite, unable to survive in the marine environment. Hence the observations by Ellis and Wootten (Reference Ellis and Wootten1978) from Scotland likely represent I. salmonis sp. n. infections, as do other European reports of salmonid gill inflammation due to I. necator s.l. in the seawater phase (e.g. Poppe and Håstein, Reference Poppe and Håstein1982). The euryhaline Ichthyobodo sp. infecting Pacific salmonids (Wood, Reference Wood1979; Urawa and Kusakari, Reference Urawa and Kusakari1990) may represent I. salmonis sp. n. or other species. Todal et al. (Reference Todal, Karlsbakk, Isaksen, Plarre, Urawa, Mouton, Hoel, Koren and Nylund2004) found a species closely related to I. salmonis sp. n. infecting Japanese masu salmon (Oncorhynchus masou). The masu parasite (Ichthyobodo sp. III) may prove conspecific with the euryhaline Ichthyobodo species studied and described (as I. necator s.l.) by Urawa and Kusakari (Reference Urawa and Kusakari1990) from chum salmon (Oncorhynchus keta) in Japan. Measurements of the rounded free form of that parasite (10·1×9·6 μm) compares well with I. salmonis sp. n., but SEM studies of the flagellates does not contain observations of the characteristic spine-like surface structures seen in I. salmonis sp.n. (Urawa and Kusakari, Reference Urawa and Kusakari1990).
Applying transmission electron microscopy (TEM), Roubal and Bullock (Reference Roubal and Bullock1987) noted ultrastructural differences in the attachment disc of I. necator s.l. from the skin and gills of Scottish salmonids in freshwater and from the gills of salmon in seawater. These authors concluded that the smooth disc in seawater and ridged disc seen in freshwater was associated with the macrohabitat, but did not exclude the possibility that 2 different Ichthyobodo spp. were involved. In the present study we confirm the observations by Roubal and Bullock (Reference Roubal and Bullock1987), and show that smooth attachment discs occur in I. salmonis sp. n., while the jagged or ridged attachment disc is a (so far) a unique character of I. necator s.s.
I. salmonis sp. n., isolated from hosts reared in different environments (macrohabitat; fresh-, brackish- and seawater) were similar, but some differences were apparent. Notably, the free forms of the parasite from salmon in freshwater were larger than those in brackish and seawater, without this difference affecting cell shape. Also, in the free forms of I. salmonis sp. n. from the freshwater infection, a characteristic large vacuole (V3) close to the flagellar pocket was more commonly observed than in brackish- and seawater. The same observation was made by Urawa and Kusakari (Reference Urawa and Kusakari1990) on Ichthyobodo sp. infecting O. keta. This vacuole is most likely a contractile vacuole needed for osmoregulation (see Joyon and Lom, Reference Joyon and Lom1969; Kivic and Walne, Reference Kivic and Walne1984), which is probably much less conspicuous in the marine environment. The prominence of these vacuoles in freshwater may contribute to the larger cell size of I. salmonis sp. n. from freshwater as observed in our study.
A bluish stained vacuole-like structure (V1) characteristic for I. salmonis sp. n. shows a rather fixed position in the cell. Large vacuole-like structures with vesicular content were often seen in TEM images in this position, perhaps representing V1. The other vacuoles observed (V2) may represent food vacuoles (see Joyon and Lom, Reference Joyon and Lom1969; Isaksen et al. Reference Isaksen, Karlsbakk and Nylund2007).
ACKNOWLEDGEMENTS
We would like to thank the managers of the salmon farms in Troms and Hordaland for collaboration and for supplying fish material used in this study. In particular, we would like to thank Bjørn Hugo Erntsen (Troms), Glenn Arve Sundnes (Hordaland) and Randi Haldorsen (Hordaland).
FINANCIAL SUPPORT
The present study was funded by the Norwegian Research Council (Project no. 190448).














