Introduction
As an important biocontrol agent against aphids in greenhouses and open fields, Aphidius colemani Viereck has been used in biological control since the 1960s (Starý, Reference Starý1975) and commercially produced in Europe since 1992 (van Lenteren, Reference van Lenteren2007). However, a complicated taxonomical history has followed this parasitoid until recently (Starý, Reference Starý1975; Takada, Reference Takada1998; Kavallieratos and Lykouressis, Reference Kavallieratos and Lykouressis1999). A study by Tomanović et al. (Reference Tomanović, Petrović, Mitrović, Kavallieratos, Starý, Rakhshani, Rakhshanipour, Popović, Shukshuk and Ivanović2014) employing an integrated approach to taxonomy, recognised the Aphidius colemani species group, containing A. colemani, A. platensis Brethes and A. transcaspicus Telenga. The three species are easily distinguished from their congeners by the costate anterolateral area of the petiole, and from each other by a combination of morphological characters (number of palpomeres in labial palps, ratio between pterostigma and metacarpus (=R1 vein) length) (Tomanović et al., Reference Tomanović, Petrović, Mitrović, Kavallieratos, Starý, Rakhshani, Rakhshanipour, Popović, Shukshuk and Ivanović2014). Additionally, the costate anterolateral area of petiole can be found in A. avenae Haliday, A. bertrandi Benoit and A. pseudopicipes Starý.
Efforts to uncover aphidiin and braconid diversity in Africa go back to 1949 and the work on braconids of Madagascar by Granger (Reference Granger1949). Most of the subsequent studies also concentrated on a small area or specific countries. Starting in the 1970s, numerous studies were conducted concerning Sub-Saharan Africa (Starý and van Harten, Reference Starý and van Harten1972, Reference Starý and van Harten1992; Starý and Schmutterer, Reference Starý and Schmutterer1973; Starý et al., Reference Starý, Remaudiére and Autrique1985, Reference Starý, Remaudiére and Etienne1994; Starý, Reference Starý2005; Sæthre et al., Reference Sæthre, Godonou, Hofsvang, Tepa-Yotto and James2011; Muller et al., Reference Muller, Krüger and Kfir2014), while in recent years, more attention is given to North Africa (Ghelamallah et al., Reference Ghelamallah, Rakhshani, Bouhraoua, Michelena, Boualem, Ferrer-Suay and Pujade-Villar2018; Rakhshani et al., Reference Rakhshani, Barahoei, Ahmad, Starý, Ghafouri-Moghaddam, Mehrparvar, Kavallieratos, Čkrkić and Tomanović2019; Hemidi and Laamari, Reference Hemidi and Laamari2020). So far, only 22 species of Aphidiinae have been recorded from Sub-Saharan Africa (Petrović, Reference Petrović2022). In contrast, the latest checklist shows 108 species of Aphidiinae in North Africa and Middle East (Rakhshani et al., Reference Rakhshani, Barahoei, Ahmad, Starý, Ghafouri-Moghaddam, Mehrparvar, Kavallieratos, Čkrkić and Tomanović2019). Although research has been somewhat consistent during the years, there are still large areas of the continent without any data on Aphidiinae.
Based on specimens collected in Africa, we describe five new species of Aphidiinae belonging to the A. colemani species group. Four out of those are described from the Sub-Saharan part of the continent. We also discuss the implications of these findings for biocontrol purposes and the proposed origin of the group.
Material and methods
Specimens belonging to the A. colemani species group were collected in several African countries (Angola, Burundi, Cabo Verde, Egypt, Ethiopia, Kenya, Réunion Island, South Africa, and Tristan da Cunha) during the period 1964–2001, by sweeping or collecting plants infested with aphid colonies. Aphid colonies were kept under laboratory conditions for 3–4 weeks or until parasitoid emergence. Adult parasitoids were dissected and slide mounted. Photographs of relevant morphological characters were acquired using a Leica DM LS phase contrast microscope (Leica Microsystems GmbH, Wetzlar, Germany), while measurements were taken with ImageJ software (Schneider et al., Reference Schneider, Rasband and Eliceiri2012) (fig. 1). Morphological terminology follows Sharkey and Wharton (Reference Sharkey, Wharton, Wharton, Marsh and Sharkey1997).

Figure 1. Characters used in morphological analysis. a – longitudinal eye diameter; b – malar space; c – intertentorial line; d – tentoriocular line; e – maxillary palp; f – labial palp; g – F1 length; h – F1 width; i – F2 length; j – F2 width; k – oblique carina; l – postmedian carina; m – petiole length; n – petiole width; o – costae on anterolateral part of petiole; p – ovipositor sheath length; q – ovipositor sheath width; r – pterostigma length; s – metacarpus length; t – pterostigma width.
Results
In total, we examined 133 specimens belonging to Aphidius colemani species group collected in Africa. We uncovered five species clearly belonging to the A. colemani species group, but not fitting descriptions of currently known species.
Descriptions of new species
Aphidius angustastigmatus sp. n. Čkrkić & Tomanović
fig. 2
Material examined: 2♀, slide mounted, South Africa, Johannesburg, Olifantsvlei, 15. XI 1964, caught by sweeping. Holotype ♀ and paratype ♀ deposited in collection of Institute of Entomology, České Budějovice.
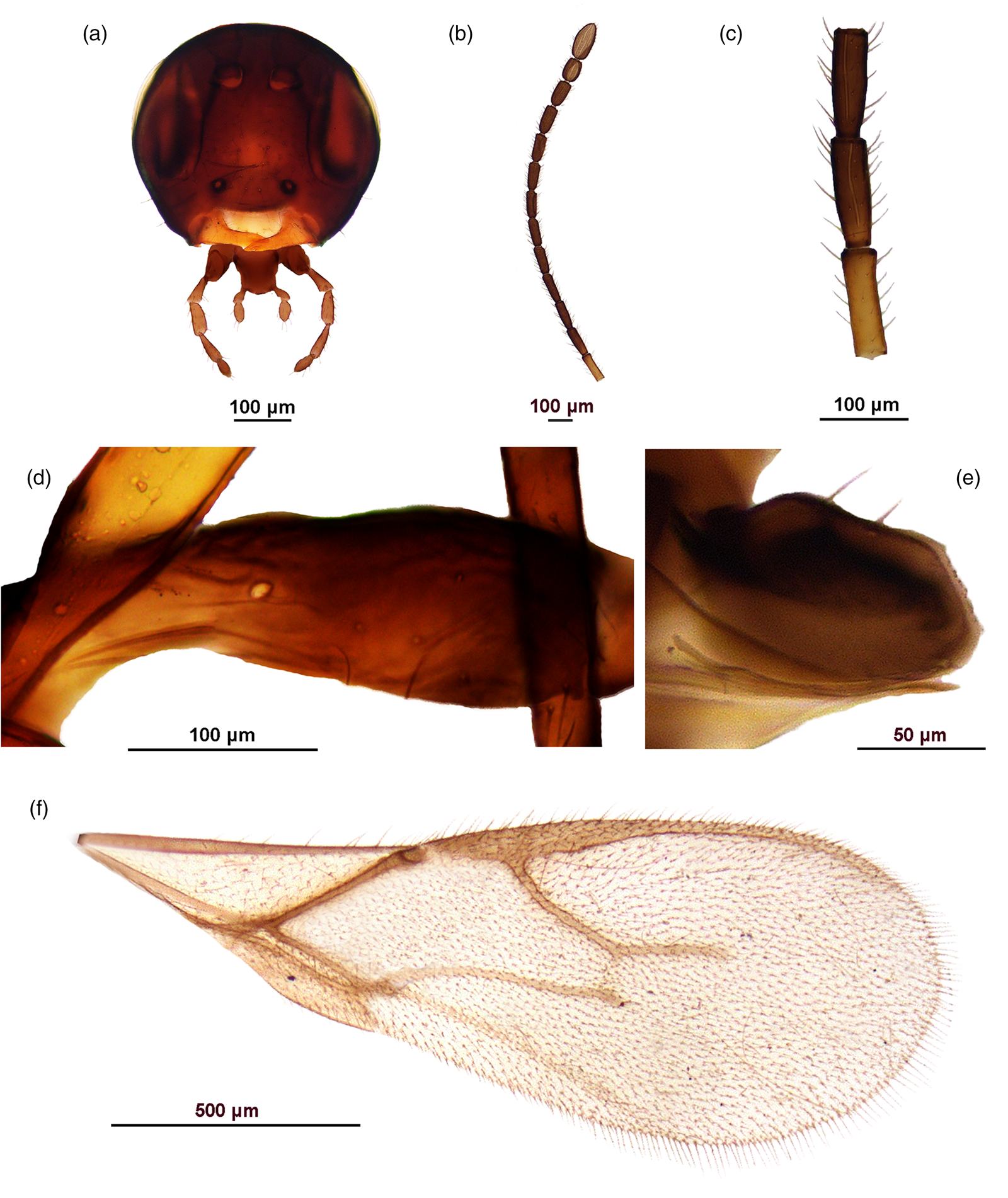
Figure 2. Aphidius angustastigmatus sp. n., female. A. Head. B. Antenna. C. Flagellomeres 1–3. D. Petiole, lateral view. E. Ovipositor sheaths. F. Fore wing.
Diagnosis: This species is distinguished from other members of the A. colemani group and any congeners by a very elongate fore wing pterostigma, which is approx. 7.5 times as long as wide (3.5–4.0 times in A. colemani and A. platensis) and by irregular pattern of costae on the anterolateral part of the petiole. Additionally, it can easily be differentiated from A. transcaspicus by 2-segmented labial palps (3-segmented in A. transcaspicus).
Description.
Female. Head (fig. 2a). Moderately setose with long setae. Tentorial index (tentoriocular line/intertentorial line) approx. 0.5, malar space approx. 0.3 of longitudinal eye diameter. Clypeus with 1–2 very long setae. Antenna 15-segmented, moderately thickened at apex, with setae subequal to segment diameter (fig. 2b). Flagellar segment 1 (=F1) 3.5–3.6 times as long as wide, without longitudinal placodes. Flagellar segment 2 (=F2) 2.8–3.0 times as long as wide, with 1–2 longitudinal placodes. F1 subequal to F2 (fig. 2c). Maxillary palp with four palpomeres, labial palp with two palpomeres.
Mesosoma. Mesoscutum damaged. Fore wing pterostigma unusually elongated, approx. 7.5 times as long as wide and subequal to metacarpus (pterostigma length/metacarpus length ratio approx. 0.9) (fig. 2f). Propodeum damaged, with indication of existing central areola.
Metasoma. Petiole slender, with 2–3 irregular costae in the anterolateral area. (fig. 2d) Ovipositor sheath slightly concave at the dorsal margin and short, length/width ratio 1.3–1.4 (fig. 2e).
Coloration. Head brown, mouthparts light brown. Scapus, pedicel and F1 yellow, remainder of antenna brown. Mesoscutum and propodeum brown, legs light brown. Wings hyaline with brown venation. Metasoma brown.
Male. Unknown.
Host: unknown.
Etymology: The name of the new species is derived from its unique diagnostic feature, the very long and narrow forewing pterostigma.
Aphidius brevicarpus sp. n. Čkrkić & Tomanović
fig. 3
Material examined: 1♀, slide mounted, Ethiopia, Fiche, 2850 m.a.s.l., reared from Diuraphis noxia (Kurdjumov) on Hordeum vulgare. Holotype ♀ deposited in collection of Institute of Entomology, České Budějovice.

Figure 3. Aphidius brevicarpus sp. n., female. A. Head. B. Antenna. C. Flagellomeres 1–3. D. Petiole, lateral view. E. Ovipositor sheaths. F. Fore wing.
Diagnosis. This species closely resembles A. colemani, from which it can be distinguished by a very short metacarpus; pterostigma length/metacarpus length is 1.8–2 in Aphidius brevicarpus sp. n., while it is 1.1–1.2 in A. colemani and 1.5 in the morphologically similar A. platensis. It is easily distinguished from A. transcaspicus by 2-segmented labial palps (3-segmented in A. transcaspicus).
Description.
Female. Head (fig. 3a). Sparsely setose. Antenna 15-segmented, moderately thickened at apex, with setae subequal to half of segment diameter (fig. 3b). Terminal segment long and undivided, indicating a possibility of individuals with 16-segmented antennae within this species. F1 2.6–2.7 times as long as wide, without longitudinal placodes. F2 2.1 times as long as wide, with 2–3 longitudinal placodes. F1 subequal to F2 (fig. 3c). Maxillary palp with four palpomeres, labial palp with two palpomeres.
Mesosoma. Mesoscutum damaged. Fore wing pterostigma elongated, 3.7–4.0 times as long as wide and almost double the length of metacarpus (pterostigma length/metacarpus length ratio 1.8–2.0) (fig. 3f). Propodeum damaged.
Metasoma. Petiole slender, with 4–5 costae in the anterolateral area (fig. 3d). Ovipositor sheath slightly concave at the dorsal margin, length/width ratio 1.5 (fig. 3e).
Coloration. Head brown, mouthparts light brown. Antenna brown, except for a yellow annellus at the base of F1. Mesoscutum and propodeum brown, legs light brown. Wings hyaline with brown venation. Metasoma light brown to brown.
Male. Unknown.
Host: Diuraphis noxia.
Etymology: The name of the new species is derived from its diagnostic feature, the very short fore wing metacarpus.
Aphidius egypti sp. n. Čkrkić & Tomanović
fig. 4
Material examined: 1♀, slide mounted, Egypt, reared from ‘aphids on tomatoes’. Holotype ♀ deposited in collection of Institute of Entomology, České Budějovice.

Figure 4. Aphidius egypti sp. n., female. A. Head. B. Mesoscutum. C. Propodeum. D. Petiole, lateral view. E. Ovipositor sheaths. F. Detail of fore wing – pterostigma and metacarpus.
Diagnosis. This species is distinguished from other members of the A. colemani group by fore wing pterostigma shape, which is elongated and approx. 5 times as long as wide (3.5–4.0 times in A. colemani and A. platensis, 7.5 times in Aphidius angustastigmatus sp. n.). Additionally, it can easily be differentiated from A. transcaspicus by 2-segmented labial palps (3-segmented in A. transcaspicus).
Description.
Female. Head (fig. 4a). Moderately setose. Tentorial index approx. 0.4, malar space approx. 0.2 of longitudinal eye diameter. Clypeus oval with 4–5 setae. Antennae missing. Maxillary palp with four palpomeres, labial palp with two palpomeres.
Mesosoma. Mesoscutum with notaulices only in anterior part, dorsal surface sparsely setose (fig. 4B). Fore wing pterostigma elongated, about 5 times as long as wide; pterostigma length/metacarpus length ratio 1.5 (fig. 4F). Propodeum with a central pentagonal areola (fig. 4C).
Metasoma. Petiole slender, with 4 costae in the anterolateral area (fig. 4D). Ovipositor sheath slightly concave at the dorsal margin, length/width ratio 1.67 (fig. 4E).
Coloration. Head light brown, mouthparts yellow. Mesoscutum light brown, propodeum yellow. Wings hyaline with light brown venation. Metasoma yellow.
Male. Unknown.
Host: unknown aphids on tomatoes.
Etymology: The name of the new species is derived from its known country distribution.
Aphidius remaudierei sp. n. Čkrkić & Tomanović
fig. 5
Material examined: 1♀, slide mounted, Burundi, reared from Sitobion autriquei Remaudière. Holotype ♀ deposited in collection of Institute of Entomology, České Budějovice.
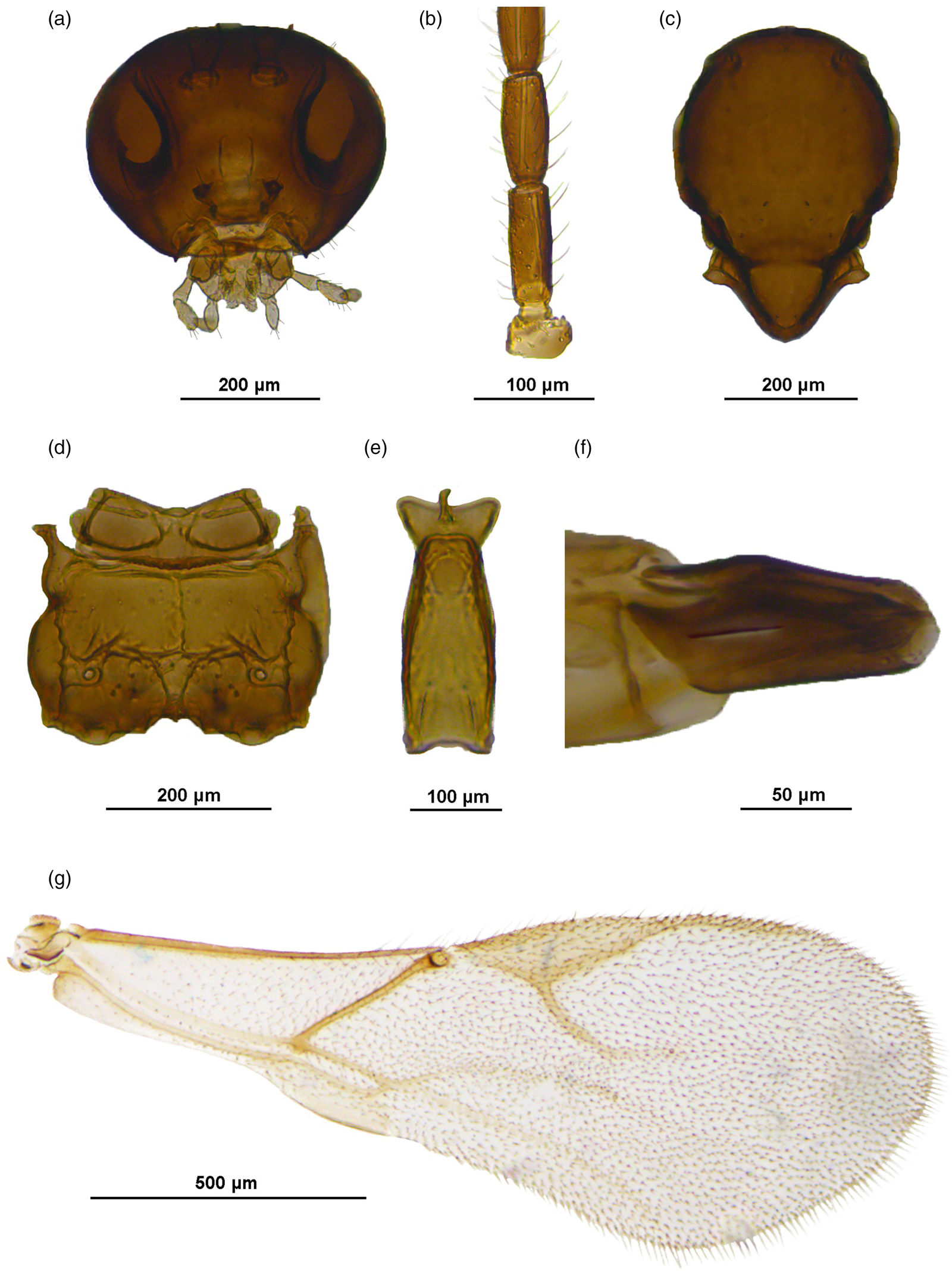
Figure 5. Aphidius remaudierei sp. n., female. A. Head. B. Flagellomeres 1–2. C. Mesoscutum. D. Propodeum. E. Petiole, dorsal view. F. Ovipositor sheaths. G. Fore wing.
Diagnosis. This species is distinguished from other members of the A. colemani group by 3-segmented maxillary palps (4-segmented in all other species). Additionally, it differs from A. colemani in having a relatively shorter metacarpus (pterostigma length/metacarpus length ratio is 1.4, while 1.1–1.2 in A. colemani).
Description.
Female. Head (fig. 5a). Moderately setose. Tentorial index 0.4, malar space 0.3 of longitudinal eye diameter. Clypeus with 4–5 setae. Antennae damaged, first 9 flagellomeres of one antenna and last 9 flagellomeres of the other antenna remaining. F1 2.5 times as long as wide, with 2 longitudinal placodes. F2 2.2 times as long as wide, with 3 longitudinal placodes. F1 equal to F2 (fig. 5b). Maxillary palp with three palpomeres, labial palp with two palpomeres.
Mesosoma. Mesoscutum with incomplete notaulices visible in anterior part, dorsal surface sparsely setose (fig. 5c). Fore wing pterostigma elongated, 3.8–4.0 times as long as wide; pterostigma length/metacarpus length ratio 1.4 (fig. 5g). Propodeum with a central pentagonal areola, oblique carinae almost horizontal (fig. 5d).
Metasoma. Petiole slender (fig. 5e). Ovipositor sheath slender and slightly concave at the dorsal margin (fig. 5f).
Coloration. Head light brown, mouthparts yellow. Mesoscutum light brown, propodeum yellow. Wings hyaline with light brown venation. Petiole and metasoma light brown, ovipositor sheath brown.
Male. Unknown.
Host: Sitobion autriquei.
Etymology: The name of the new species is derived in honour of the famous French aphidologist Georges Remaudière who described many aphid species from Africa.
Aphidius petrstaryi sp. n. Čkrkić & Tomanović
fig. 6
Material examined: Holotype: 1♀, slide mounted, Angola, Chianga, 12. II 1971, reared from Hyperomyzus carduelinus (Theob.) on Sonchus sp.; Paratypes: 3♀, slide mounted, Ethiopia, Fiche, 2850 m.a.s.l., reared from D. noxia on Hordeum vulgare. Holotype and paratypes deposited in collection of Institute of Entomology, České Budějovice.

Figure 6. Aphidius petrstaryi sp. n., female. A. Head. B. Antenna. C. Flagellomeres 1–2. D. Mesoscutum. E. Propodeum. F. Petiole, dorsal view. G. Petiole, lateral view. H. Ovipositor sheaths. I. Fore wing.
Diagnosis. This species differs from A. transcaspicus in F1 length/width ratio (2.6–3.0 in A. petrstaryi sp. n., while 3.5–4.0 in A. transcaspicus) and narrower pterostigma (pterostigma length/width is 3.7–4.0 in A. petrstaryi sp. n., while 3.6 in A. transcaspicus). Its host range is also different from that of A. transcaspicus – while A. transcaspicus has been reliably recorded on Hyalopterus spp. and Melanaphis donacis (Passer.), A. petrstaryi sp. n. is found on D. noxia and H. carduelinus.
Description.
Female. Head (fig. 6a). Moderately setose. Tentorial index 0.3–0.5, malar space 0.3 of longitudinal eye diameter. Clypeus with 2–5 setae. Antenna 16-segmented, slightly thickened at apex, with setae subequal to half of segment diameter (fig. 6b). F1 2.6–3.2 times as long as wide, without longitudinal placodes. F2 2.4–3.2 times as long as wide, with 1–2 longitudinal placodes. F1 equal to F2 (fig. 6c). Maxillary palp with four palpomeres, labial palp with three palpomeres.
Mesosoma. Mesoscutum with incomplete notaulices visible in anterior part, dorsal surface sparsely setose (fig. 6d). Fore wing pterostigma elongated, 3.7–4.0 times as long as wide; proportion between pterostigma length and metacarpus length 1.3–1.7 (fig. 6i). Propodeum with a central pentagonal areola (fig. 6e).
Metasoma. Petiole slender, 3.8 times as long as wide at spiracles, with 4–5 costae in the anterolateral area (fig. 6f, g). Ovipositor sheath slender and concave at the dorsal margin, length/width ratio 1.5 (fig. 6h).
Coloration. Head brown, mouthparts light brown. Scapus and pedicel light brown, F1 with a yellow ring at the base, remainder of antenna brown. Mesoscutum brown, propodeum light brown. Wings hyaline with brown venation. Petiole and metasoma light brown, ovipositor sheath brown.
Male: Unknown.
Etymology: The name of the new species is derived in honour of the late Petr Starý, Czech braconologist and his extraordinary contributions to aphidiinae taxonomy.
Uncertain taxa
In addition to the specimens clearly different from currently known species of the A. colemani species group and described here as new species, there were some individuals with somewhat unclear morphological characters and their combinations. These specimens do not warrant new species designations yet, most of them showing slight deviations from ranges of variation currently established for known species. Some examples are described below.
Aphidius sp. 1
Material examined: 1♀, slide mounted, Egypt, Beni Suef, I 1975, reared from Rhopalosiphum padi on Triticum sp. Deposited in collection of Institute of Entomology, České Budějovice.
Female. Head. Moderately setose. Tentorial index 0.4, malar space 0.3 of longitudinal eye diameter. Clypeus with 3–4 setae. Antenna 16-segmented, with setae subequal to half of segment diameter. Flagellar segment 1 (=F1) 3.3 times as long as wide, without longitudinal placodes. F2 2.8 times as long as wide, with 0–1 longitudinal placodes. F1 equal to F2 (fig. 7a). Maxillary palp with four palpomeres, labial palp with three palpomeres.
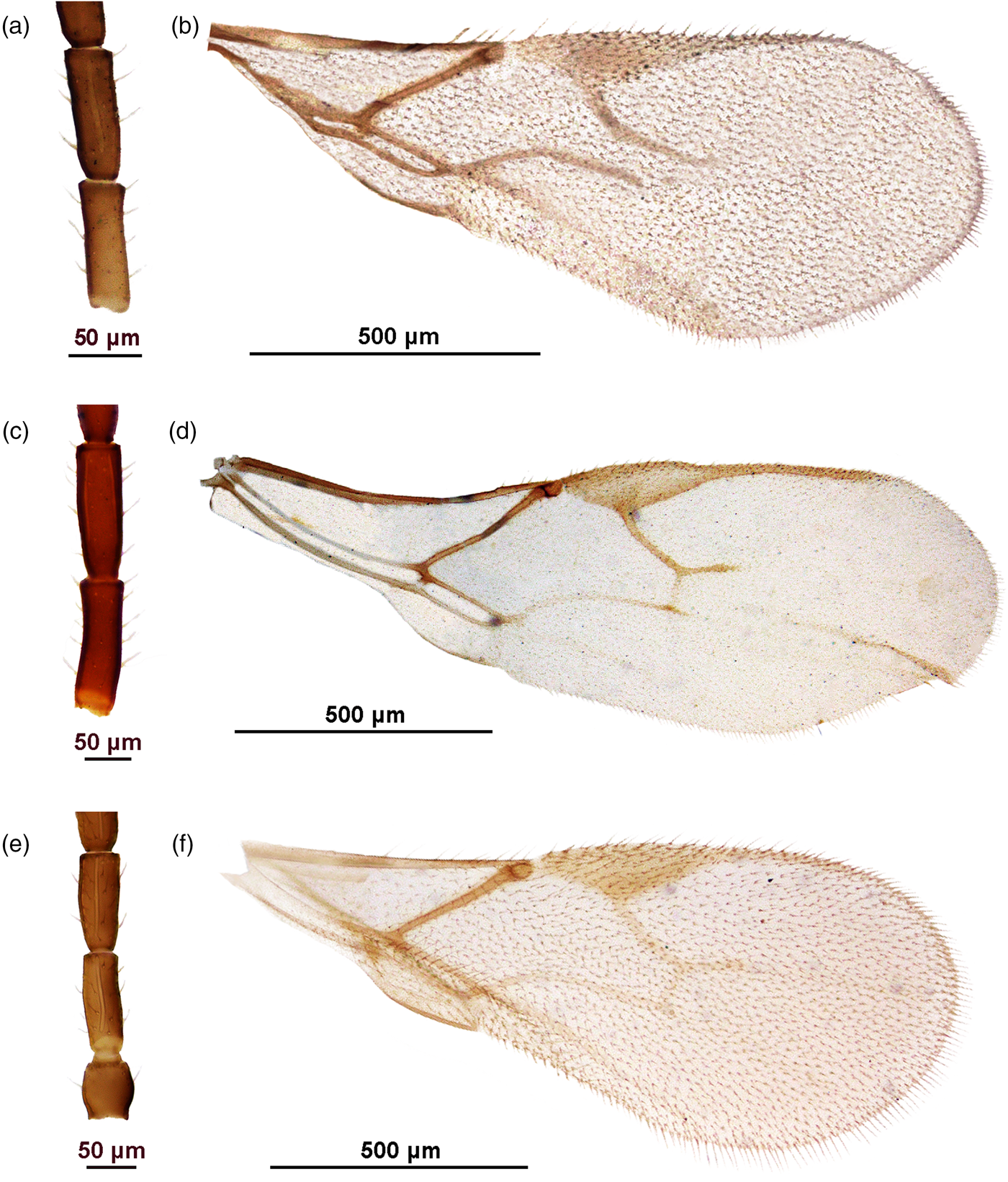
Figure 7. Uncertain taxa. Aphidius sp. 1: A. F1 and F2; B. Fore wing. Aphidius sp. 2: C. F1 and F2; D. Fore wing. Aphidius sp. 3: E. F1 and F2; F. Fore wing.
Mesosoma. Mesoscutum damaged. Fore wing pterostigma elongated, 4 times as long as wide; proportion between pterostigma length and metacarpus length 1.6 (fig. 7b). Propodeum with a central pentagonal areola.
Metasoma. Petiole slender, with 4 costae in the anterolateral area. Ovipositor sheath slender and slightly concave at the dorsal margin, length/width ratio 1.9.
Coloration. Head light brown, mouthparts yellow. Scapus and pedicel light brown, F1 yellow, remainder of antenna brown. Mesoscutum brown, propodeum light brown. Wings hyaline with brown venation. Petiole, metasoma and ovipositor sheath light brown.
Note: this specimen differs slightly from A. transcaspicus in having a narrower pterostigma (length/width ratio 4, while 3.6 in A. transcaspicus), and from A. colemani and A. platensis in having 3-segmented labial palps. It was designated as ‘A. mediterraneus’ (= A. petrstaryi sp. n.) by Dr Petr Starý, although it can be differentiated from those specimens by a slightly more elongated F1, a larger portion of F1 being yellow (almost the entire segment, while only a yellow ring at the base of F1 is present in A. petrstaryi sp. n.) and a general lighter/yellow coloration.
Aphidius sp. 2
Material examined: 1♀, slide mounted, Ethiopia, Alidoro/Selale, 2820 m.a.s.l., 24. X 2001, reared from Diuraphis noxia on Hordeum vulgare. Deposited in collection of Institute of Entomology, České Budějovice.
Female. Head. Moderately setose. Tentorial index 0.7, malar space 0.3 of longitudinal eye diameter. Clypeus with 4–5 setae. Antenna 17-segmented, with setae subequal to half of segment diameter. Flagellar segment 1 (=F1) 3.2–3.3 times as long as wide, without longitudinal placodes. F2 2.8–3.3 times as long as wide, with 2–4 longitudinal placodes. F1 equal to F2 (fig. 7c). Maxillary palp with four palpomeres, labial palp with three palpomeres.
Mesosoma. Mesoscutum with incomplete notaulices visible in anterior part, dorsal surface sparsely setose. Fore wing pterostigma elongated, 3.3–3.6 times as long as wide; metacarpus slightly longer than pterostigma (pterostigma length/metacarpus length ratio 0.9) (fig. 7d). Propodeum missing.
Metasoma. Petiole missing. Ovipositor sheath slender and slightly concave at the dorsal margin, length/width ratio 1.8.
Coloration. Head light brown, mouthparts yellow. F1 with a yellow ring at the base, remainder of antenna light brown. Mesoscutum light brown. Wings hyaline with brown venation. Metasoma and ovipositor sheath light brown.
Note: this specimen was designated as ‘A. mediterraneus’ (= A. petrstaryi sp. n.) by Dr Petr Starý, but it can be differentiated clearly from other ‘A. mediterraneus’ specimens by its very long metacarpus (pterostigma length/metacarpus length ratio is 0.9, while 1.6 in A. petrstaryi), 17-segmented antenna (16-segmented in ‘A. mediterraneus’) and a general yellow/light brown coloration. F1 length/width ratio of 3.2 differentiates it from A. transcaspicus (3.5–4.0), while the 3-segmented labial palps separate it from A. colemani and A. platensis.
Aphidius sp. 3
Material examined: 1♀, slide mounted, Burundi, reared from Aphis fabae on Phaseolus sp.
Deposited in collection of Institute of Entomology, České Budějovice.
Description.
Female. Head. Densely setose with short setae. Tentorial index 0.75, malar space approx. 0.3 of longitudinal eye diameter. Clypeus with 3–4 setae. Antenna 15-segmented, slightly thickened at apex, with setae subequal to half of segment diameter. Flagellar segment 1 (=F1) 2.5–2.7 times as long as wide, with 1–2 longitudinal placodes. F2 2.2–2.4 times as long as wide, with 3–4 longitudinal placodes. F2 equal to F1 (fig. 7e). Maxillary palp with four palpomeres, labial palp with three palpomeres.
Mesosoma. Mesoscutum damaged. Fore wing pterostigma elongated, 3.4 times as long as wide; pterostigma length/R1 length ratio 1.4 (fig. 7f). Propodeum damaged.
Metasoma. Petiole slender, with 4 costae in the anterolateral area. Ovipositor sheath concave at the dorsal margin, length/width ratio 1.4.
Coloration. Head brown, mouthparts light brown, antenna light brown. Mesonotum and propodeum brown, legs light brown. Wings hyaline with brown venation. Metasoma light brown.
Host: Aphis fabae.
Note: this specimen was identified as A. colemani. It differs from it in having 3-segmented labial palp and a shorter metacarpus. The 3-segmented labial palp are a shared trait with A. transcaspicus, but there is some slight variation in this specimen – F1 and F2 are less slender in Aphidius sp. 3 (F1 length/width ratio 2.5–2.7, while 3.5–4 in A. transcaspicus; F2 length/width ratio 2.2–2.4, while 2.9 in A. transcaspicus), and the metacarpus is just slightly longer than what has been recorded for A. transcaspicus so far (pterostigma length/metacarpus length 1.4 in Aphidius sp. 3, 1.6–2.0 in A. transcaspicus).
Additionally, there are a number of specimens that could be placed between A. colemani and A. platensis based on metacarpus length. Some specimens have one 2-segmented and one 3-segmented labial palp (fig. 8a). Usually, this would be a consequence of the palp segment not splitting fully, and the specimens in question would normally have 3-segmented labial palps. However, a few specimens with 2- and 3-segmented labial palps were collected in the same sample as specimens with 2-segmented labial palps. A detailed comparison of these individuals does not show clear separation based on relevant morphological characters. As another peculiarity, wing shape in a couple of specimens is distinctly different from the general wing shape of Aphidiinae. The wings are clearly much wider, with a constriction at the distal margin (fig. 8b).
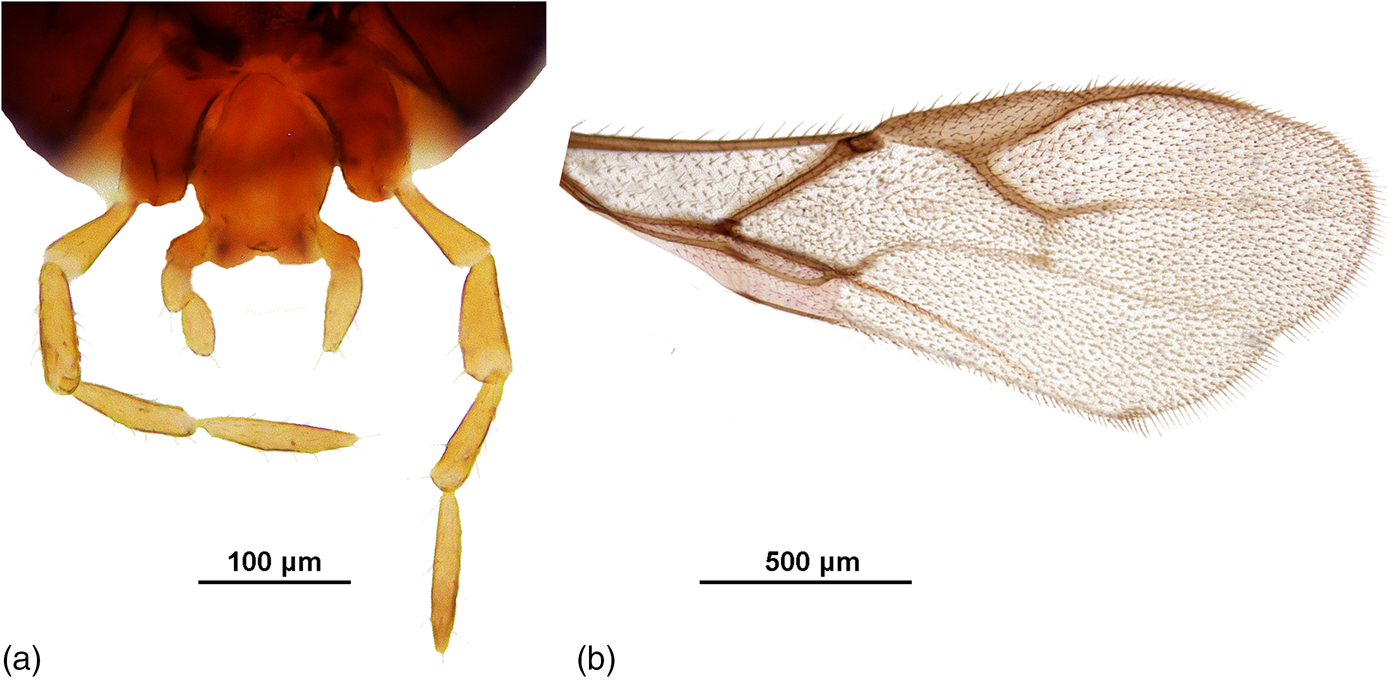
Figure 8. A. Specimen with one 2-segmented and one 3-segmented labial palp. Collected in Burundi, reared from A. fabae; B. Unusual fore wing shape. Specimen collected in Burundi, reared from A. gossypii on Solanum nigrum.
Additional examined material
Aphidius colemani
reared from Aphis craccivora: 2 females, Cabo Verde, 1 female, Egypt, Giza, 1 female, Angola, San Jorge; reared from A. gossypii on Solanum nigrum: 7 females, Burundi; on Phaseolus sp.: 1 male, Burundi; on unknown plant: 6 females, 2 males, Burundi; 13 females, Reunion Island; reared from A. fabae: 12 females, Burundi; reared from Brachycaudus helichrysi: 3 females, 1 male, Burundi; reared from B. schwartzi: 2 females, Burundi; reared from Diuraphis noxia: 1 female, Ethiopia; reared from Melanaphis sacchari on Sorghum sp.: 3 females, Burundi; reared from Rhopalosiphum maidis: 2 females, 1 male, Angola, 1 female, Reunion Island; reared from R. padi: 2 females, Angola, 6 females, 2 males, Burundi; reared from Schizaphis rotundiventris: 3 males, Angola, 1 female, Burundi; reared from unknown aphid on Cucurbita sp.: 1 female, Egypt, Oman, 1995; collected by Malaise trap: 1 female, Tristan da Cunha, Gough Island.
Aphidius platensis
reared from B. helichrysi: 2 females, Burundi; reared from ‘cereal aphids’ on ‘spring barley’: 19 females, Ethiopia, Fiche, 2850 m.a.s.l.; on unknown plant: reared from Hyalopterus pruni on Phragmites sp.: 1 female, Burundi, 1983; reared from unknown aphid on Solanum lycopersicum: 2 females, Egypt, Oman, 1995; on Zea mays: 3 females, Egypt, Oman, 1995.
Aphidius transcaspicus
reared from B. helichrysi: 1 female, Burundi; reared from H. pruni on Phragmites sp.: 1 female, Burundi, 1983; on Prunus persica: 2 females, Egypt; on unknown plant: 3 females, Burundi; reared from Hyperomyzus carduelinus: 2 females, Burundi; reared from Macrosiphum euphorbiae: 2 females, Burundi; reared from R. maidis on Z. mays: 1 male, Kenya, Chiromo; collected by sweeping: 3 females, South Africa, Mondeor, Johannesburg, 26 XI 1964; 2 females, South Africa.
Discussion
Aphidius colemani group has long been puzzling researchers, despite including a small number of species. The three species included in it were considered valid species, then synonymised (Starý, Reference Starý1975), then restored as separate species again (Tomanović et al., Reference Tomanović, Petrović, Mitrović, Kavallieratos, Starý, Rakhshani, Rakhshanipour, Popović, Shukshuk and Ivanović2014). As a consequence, earlier literature data about their distribution and hosts can lead to erroneous conclusions if not examined thoroughly. This problem is compounded with the shared host range between A. colemani and A. platensis. One such example is the Biological Control Program of Wheat Aphids (BCPWA) undertaken in Brazil in the 1970s and 1980s, where A. colemani was introduced from France and Israel. Santos et al. (Reference Santos, Sampaio, Lau, Redaelli, Jahnke, Pivato and Carvalho2019) re-examined specimens collected during the introduction and the monitoring period, thought at the time to be A. colemani, and found that they, in fact, all belong to A. platensis. A similar problem probably still exists in the light of commercially produced A. colemani, and it is likely that what is distributed as A. colemani actually contains a combination of two or more species from this group (Tomanović et al., Reference Tomanović, Petrović, Mitrović, Kavallieratos, Starý, Rakhshani, Rakhshanipour, Popović, Shukshuk and Ivanović2014).
As well as identification, finding a potential origin area and actual distribution of species from the A. colemani species group is complicated by its taxonomic history. So far, different origin areas have been proposed by different authors and with different taxonomic treatments of species. Starý (Reference Starý1972) proposed India as area of origin for A. platensis, as opposed to it being a member of the Neotropical faunistic complex, since it was described from South America. After synonymising the three species under A. colemani, Starý (Reference Starý1975) merged their distribution data and again postulated the Oriental region as the place of origin of this species. Takada (Reference Takada1998) confirmed Oriental origin for A. colemani, but assigned Palaearctic origin to A. magdae (=A. transcaspicus). Finally, Tomanović et al. (Reference Tomanović, Petrović, Mitrović, Kavallieratos, Starý, Rakhshani, Rakhshanipour, Popović, Shukshuk and Ivanović2014) postulated a Mediterranean origin for all three species.
Regarding the species described in this study, we can assume Afrotropical origin based on the current data. These findings may imply an area of origin for A. colemani species complex different from what has been considered in literature so far. With nine species, Africa currently holds the greatest diversity of the group. This might suggest that the group originated there and subsequently spread mainly through the tropical and subtropical regions. The findings of Woolley et al. (Reference Woolley, Tembo, Ndakidemi, Obanyi, Arnold, Belmain, Ndakidemi, Ogendo and Stevenson2021) support this hypothesis with a high haplotype diversity found in A. colemani species group in East Africa. Unfortunately, specimens from that study were destroyed during DNA extraction and no photographs or morphological analyses are given in the study, so a comparison with our specimens is impossible. Interestingly, none of the specimens examined in our study belong to A. bertrandi, a species from the A. colemani group reported to be the most common aphidiid in East Africa. Aphidius bertrandi is easily distinguished from other members of the colemani group by 18–19-segmented antenna (Starý et al., Reference Starý, Remaudiére and Autrique1985).
In contrast to the diversity of A. colemani group in Africa, reported Aphidiinae diversity in this region is very low. With occasional studies conducted since the 1970s, the current number of Aphidiinae in Sub-Saharan Africa (until this study) stands at only 22 (Starý and van Harten, Reference Starý and van Harten1972, Reference Starý and van Harten1992; Starý and Schmutterer, Reference Starý and Schmutterer1973; Starý et al., Reference Starý, Remaudiére and Autrique1985, Reference Starý, Remaudiére and Etienne1994; Sæthre et al., Reference Sæthre, Godonou, Hofsvang, Tepa-Yotto and James2011; Muller et al., Reference Muller, Krüger and Kfir2014). The question arises whether this is a consequence of fewer research efforts, or the actual state of aphidiine diversity. On one hand, the diversity of aphids is greater in the Northern hemisphere, so it would make sense to expect a greater diversity of their parasitoids. On the other hand, there is an apparent shortage of Aphidiinae taxonomists in the Southern hemisphere, as shown in a recent review by Petrović (Reference Petrović2022), although this could actually be a shortage of funding opportunities for researchers in the global south.
The five newly described species from A. colemani species group have great potential for the biocontrol market. Since these species share the same host range pattern as the nominal species, and probably similar biological and ecological traits, we can assume that existing production technologies for A. colemani could work for them too. The increasing pressure of aphid pests affected by climate changes necessitates use of a greater diversity of Aphidiinae biocontrol agents in open fields and glasshouses. Further research of A. colemani species group from Sub-Saharan Africa could lead to their taxonomical clarification and confirmation of the high diversity. Hopefully this could lead to a more diverse Aphidiinae presence in the biocontrol market.
Acknowledgements
We thank the late Dr Petr Starý (Laboratory of Aphidology, Institute of Entomology, Academy of Sciences of the Czech Republic) for providing the specimens used in this study. This study was partially funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant numbers 451-03-65/2024-03/ 200178 and 451-03-66/2024-03/ 200178) and the Serbian Academy of Sciences and Arts (grant no. F131).
Competing interests
None.











