Muscle fibre, as the basic unit of muscle composition, is one of the main factors affecting the meat quality of livestock. It has been reported that the amount of muscle fibres is invariable after livestock birth; however, the muscle fibre types transform continuously under the influence of the external environment( Reference Schiaffino and Reggiani 1 ). According to the polymorphism of myosin heavy chain (MyHC), muscle fibre is divided into four different types including type I with MyHC I (slow MyHC), type IIa with MyHC IIa, type IIx with MyHC IIx and type IIb with MyHC IIb( Reference Chang and Fernandes 2 , Reference Chang, Fernandes and Dauncey 3 ). MyHC IIa, MyHC IIx and MyHC IIb are collectively called fast MyHC. Under the influence of the external environment, muscle fibre types can transform from slow-twitch (slow MyHC) to fast-twitch (fast MyHC) or vice versa( Reference Pette and Staron 4 ). The transformation of muscle fibres can be regulated by myogenic factors, such as myogenin and myocyte enhancer factor-2 (MEF2)( Reference Sartorelli and Caretti 5 , Reference Sassoon 6 ) and also by a variety of signalling pathways. For example, it has been reported that serine phosphorylation and protein expression of protein kinase B (Akt), a serine/threonine protein kinase, are greater in oxidative muscle compared with glycolytic muscle( Reference Song, Ryder and Kawano 7 , Reference Turinsky and Damrau-Abney 8 ). Forkhead box 1 (FoxO1) is one of the downstream transcription factors of the phosphoinositide 3-kinase (PI3K)/Akt signalling pathway( Reference Stitt, Drujan and Clarke 9 ). Kamei et al. ( Reference Kamei, Miura and Suzuki 10 ) reported that over-expression of FoxO1 decreased the type I fibre-related gene expression and the number of type I fibres in mouse skeletal muscle.
Leucine is a branched-chain amino acid that mainly metabolises in skeletal muscle because the key enzymes of leucine metabolism are mainly distributed in skeletal muscle( Reference Shimomura, Honda and Shiraki 11 ). The balance of protein synthesis and degradation is crucial to the protein deposition of skeletal muscle. It is well known that leucine can stimulate muscle protein synthesis and suppress protein degradation( Reference Hernandez-Garcia, Columbus and Manjarin 12 , Reference Hong and Layman 13 ). Besides the regulation of protein turnover, leucine also plays a vital role in the process of myogenesis( Reference Averous, Gabillard and Seiliez 14 , Reference Dai, Yu and Shen 15 ). During the process of myogenesis, the formation of muscle fibre is an ultimate integral part, which is regulated by nutritive or non-nutritive factors. It has been reported that leucine increased the expression of oxidative muscle fibre marker genes, including sirtuin 1 (SIRT1), AMP-activated kinase (AMPK) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), in C2C12 myotubes and mice skeletal muscle( Reference Li, Xu and Lee 16 – Reference Sun and Zemel 18 ). Vaughan et al. ( Reference Vaughan, Garcia-Smith and Gannon 19 ) also found that treatment with leucine promoted oxidative preference and capacity of skeletal muscle cells. Although the role of leucine in oxidative fibre formation is well established( Reference Li, Xu and Lee 16 – Reference Vaughan, Garcia-Smith and Gannon 19 ), the effect of leucine on skeletal muscle fibre type transition is still unclear.
MicroRNA (miRNA) are highly conserved, non-coding RNA that have been regarded as important regulators in a variety of biological processes. Regulation of miRNA expression by nutrients has been recognised gradually( Reference Alvarez-Díaz, Valle and Ferrer-Mayorga 20 – Reference Drummond, Glynn and Fry 22 ). Our previous studies have provided evidence that microRNA-27a (miR-27a) promotes proliferation and differentiation of C2C12 cells( Reference Chen, Huang and Chen 23 , Reference Huang, Chen and Yu 24 ). However, the role of miR-27a in skeletal muscle fibre type transition and whether miR-27a contributes to leucine’s function in skeletal muscle fibre type transition are still unknown.
In this study, we investigated the role of leucine in porcine skeletal muscle fibre type transition in vitro. Its underlying mechanism was also investigated.
Methods
Ethics statement
All animal procedures were performed according to protocols approved by the Animal Care Advisory Committee of Sichuan Agricultural University.
Culture and treatment of porcine myoblasts
Porcine myoblasts were isolated form 3-d-old male Duroc×Landrace×Yorkshire pigs. The cells were isolated as described previously( Reference Zhang, Chen and Huang 25 ). When the density reached about 80 % confluence, the cells were shifted to differentiation medium containing Dulbecco’s modified Eagle’s medium (DMEM)/F12 with 2 % horse serum (Invitrogen). After 72 h of differentiation, porcine myotubes cultured in differentiation medium were treated with different concentrations of leucine (0, 0·5, 2 and 4 mm) for 4 d.
Transfection of microRNA mimics and inhibitor
When porcine myoblasts reached about 80 % confluence, the cells were induced to differentiate in differentiation medium, which was replaced every day. In all, 3 d later, the cells were transfected with 100 nm miR-27a mimics, 100 nm miRNA mimics negative control, 200 nm miR-27a inhibitor or 200 nm miRNA inhibitor negative control (GenePharma). The process of transfection was conducted using the Lipofectamine 3000 (Invitrogen) according to the manufacturer’s instructions. In all, 24 h after transfection, the lipofectamine complex was removed, and the cells were continued to culture for 3 d.
Real-time quantitative PCR
Total RNA was extracted from cultured porcine myotubes using RNAiso Plus reagent (TaKaRa). RNA concentrations were determined by Nano-Drop ND 2000c Spectrophotometer (Thermo Scientific). In all, 10 ng of total RNA from each sample was reverse-transcribed using TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems). The expression of miRNA was determined using the TaqMan MicroRNA Assay kit (Applied Biosystems) in CFX96 Real-Time PCR Detection System (Bio-Rad). Relative expression of miRNA was quantified using
![]() $$2^{-} {^{\Delta \Delta C_{{\rm t}} }} $$
method and was normalised by U6 small nuclear RNA.
$$2^{-} {^{\Delta \Delta C_{{\rm t}} }} $$
method and was normalised by U6 small nuclear RNA.
Western blot
Protein samples were extracted from adhered porcine myotubes using radioimmunoprecipitation assay (RIPA) buffer (Pierce). Briefly, cells were washed once with PBS and then lysed in lysis buffer for 30 min at 4°C. The cell lysates were centrifuged at 14 000 g for 15 min at 4°C, and liquid supernatant was collected. Bicinchoninic acid (BCA) protein assay kit (Pierce) was used to determine the protein concentration by Nano-Drop ND 2000c Spectrophotometer (Thermo Scientific). In all, 20 µg of protein extractions were separated by 12 % SDS–polyacrylamide gel and then transferred to polyvinyldifluoride (PVDF) membrane (Millipore) using wet Trans-Blot System (Bio-Rad). After blocking with TRIS-buffered saline Tween 20 (TBS/T) containing 5 % bovine serum albumin for 2 h at room temperature, the membranes were incubated with primary antibodies at 4°C overnight against slow MyHC (1:400; Sigma, catalogue no. M8421), fast MyHC (1:400; Sigma, catalogue no. M4276), Akt (1:1000; Cell Signaling, catalogue no. 9272), phospho-Akt (P-Akt, 1:1000; Cell Signaling, catalogue no. 9271), FoxO1 (1:1000, Cell Signaling, catalogue no. 2880), phospho-forkhead box 1 (P-FoxO1, 1:1000, Cell Signaling, catalogue no. 9461) or β-actin (1:3000; Santa Cruz, catalogue no. sc-1616). The PVDF membranes were washed with TBS/T three times for 10 min each, incubated with second antibodies for 1 h at room temperature and then washed with TBS/T three times. Clarity Western enhanced chemiluminescence (ECL) Substrate (Bio-Rad) was used to visualise signals. The Gel-Pro Analyzer (Media Cybernetics) was used to quantify protein expression, and the ratio of target proteins expression was normalised to β-actin.
Statistical analysis
Data expressed as means with their standard errors were analysed by one-way ANOVA or Tukey’s tests using statistical software SPSS, version 11.5 (SPSS Inc.). In all analyses, P<0·05 was considered statistically significant.
Results
Effect of leucine on porcine myofibre type transformation
To determine whether leucine plays a role in porcine myofibre type transformation, we probed the protein levels of slow MyHC and fast MyHC in samples from cells treated with leucine. As shown in Fig. 1, leucine increased slow MyHC protein level and decreased fast MyHC protein level.
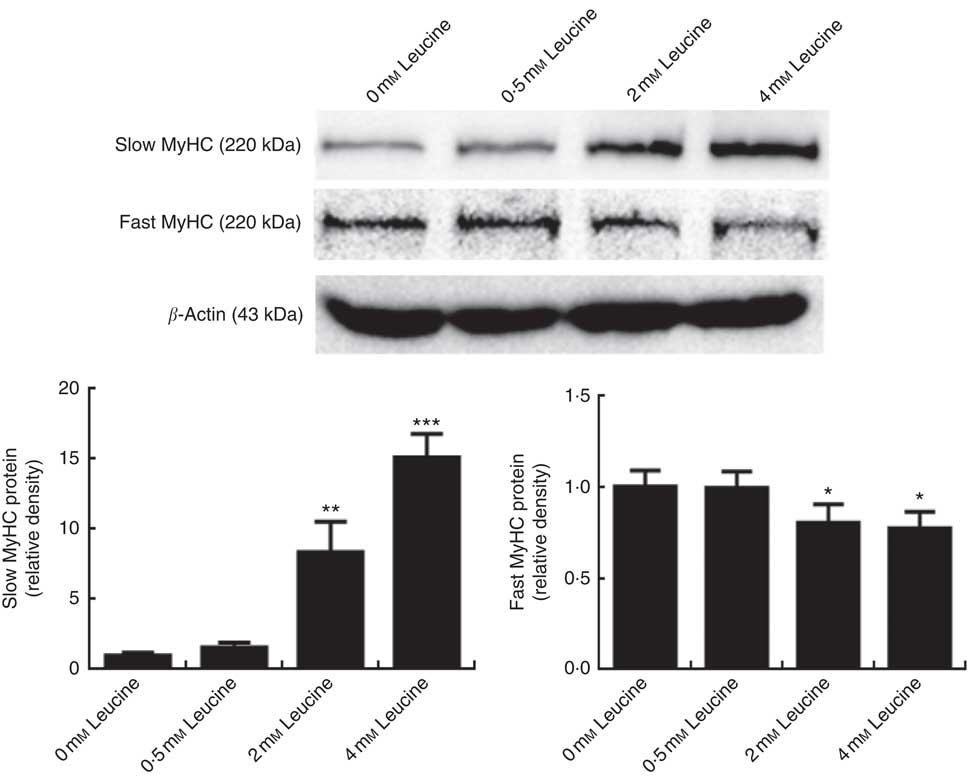
Fig. 1 Leucine promotes porcine myofibre type transformation from fast myosin heavy chain (MyHC) to slow MyHC. After 72 h of differentiation, porcine myotubes cultured in differentiation medium were treated with different concentrations of leucine (0, 0·5, 2 and 4 mm) for 4 d. Slow MyHC and fast MyHC protein levels were determined by Western blot analysis. Equal loading was monitored with anti-β-actin antibody. Values are means of the densitometry results from three independent experiments, with their standard errors represented by vertical bars. * P<0·05, ** P<0·01 and *** P<0·001 as compared with control.
Effect of leucine on the protein kinase B/forkhead box 1 signalling pathway
To determine whether the Akt/FoxO1 signalling pathway is activated in response to leucine treatment, we probed for P-Akt and P-FoxO1 levels after leucine treatment. As shown in Fig. 2, leucine increased the level of P-Akt/Akt and P-FoxO1/FoxO1, indicating that leucine activates the Akt/FoxO1 signalling pathway. Fig. 2 also showed that leucine decreased the protein level of FoxO1.
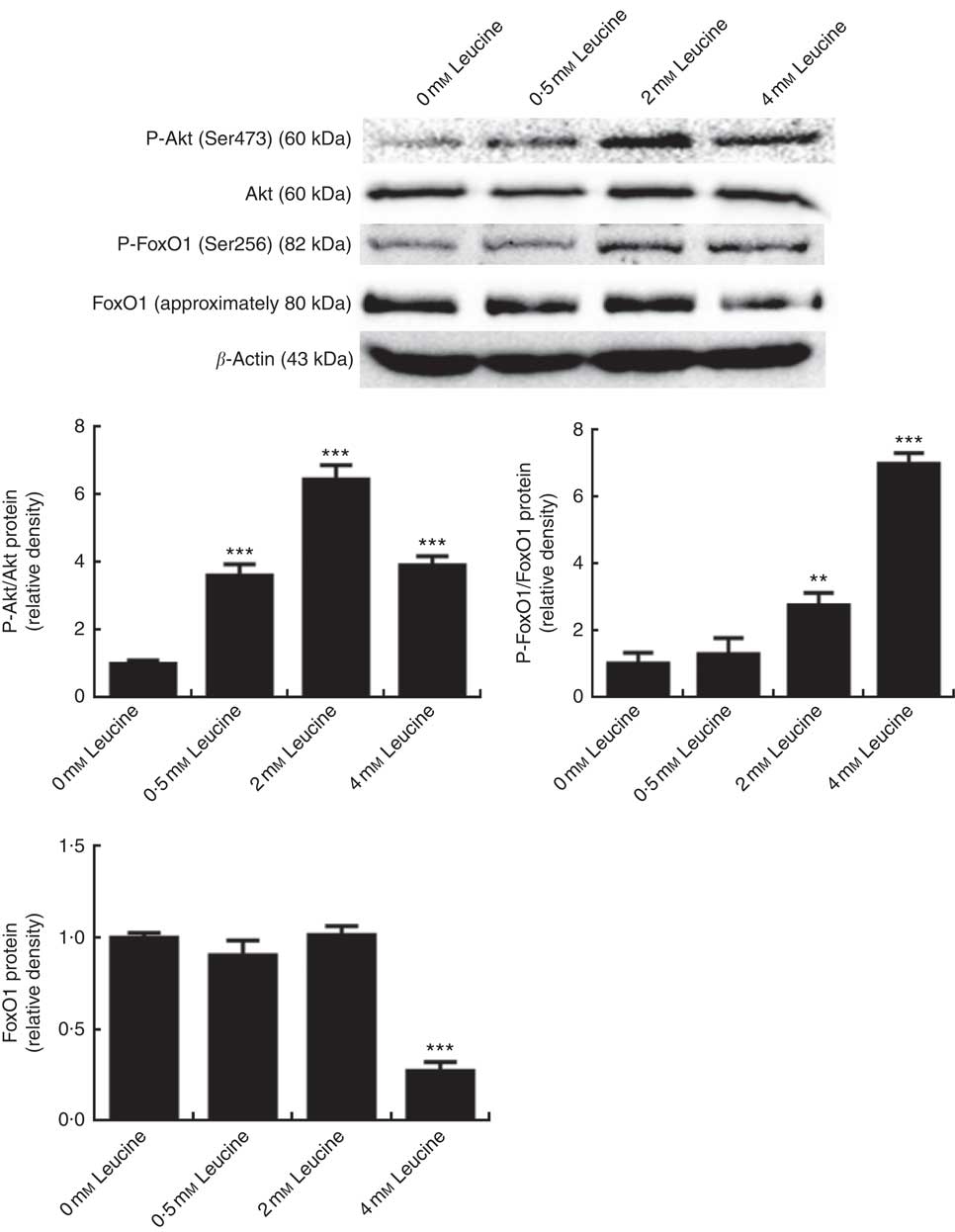
Fig. 2 Effect of leucine on the protein kinase B (Akt)/forkhead box 1 (FoxO1) signalling pathway. Samples were prepared as described in Fig. 1. Akt, phospho-Akt (P-Akt), phospho-forkhead box 1 (P-FoxO1) and FoxO1 protein levels were determined by Western blot analysis. Equal loading was monitored with anti-β-actin antibody. Values are means of the densitometry results from three independent experiments, with their standard errors represented by vertical bars. ** P<0·01 and *** P<0·001 as compared with control.
Leucine regulates porcine myofibre type transformation through the protein kinase B/forkhead box 1 signalling pathway
To explore whether leucine regulates porcine myofibre type transformation through the Akt/FoxO1 signalling pathway, leucine and wortmannin (a specific PI3K/Akt inhibitor) were added to the differentiation medium simultaneously. As shown in Fig. 3, leucine increased slow MyHC protein level and decreased fast MyHC protein level, whereas wortmannin attenuated this effect, indicating that leucine regulates porcine skeletal myofibre type transformation via the Akt/FoxO1 signalling pathway.
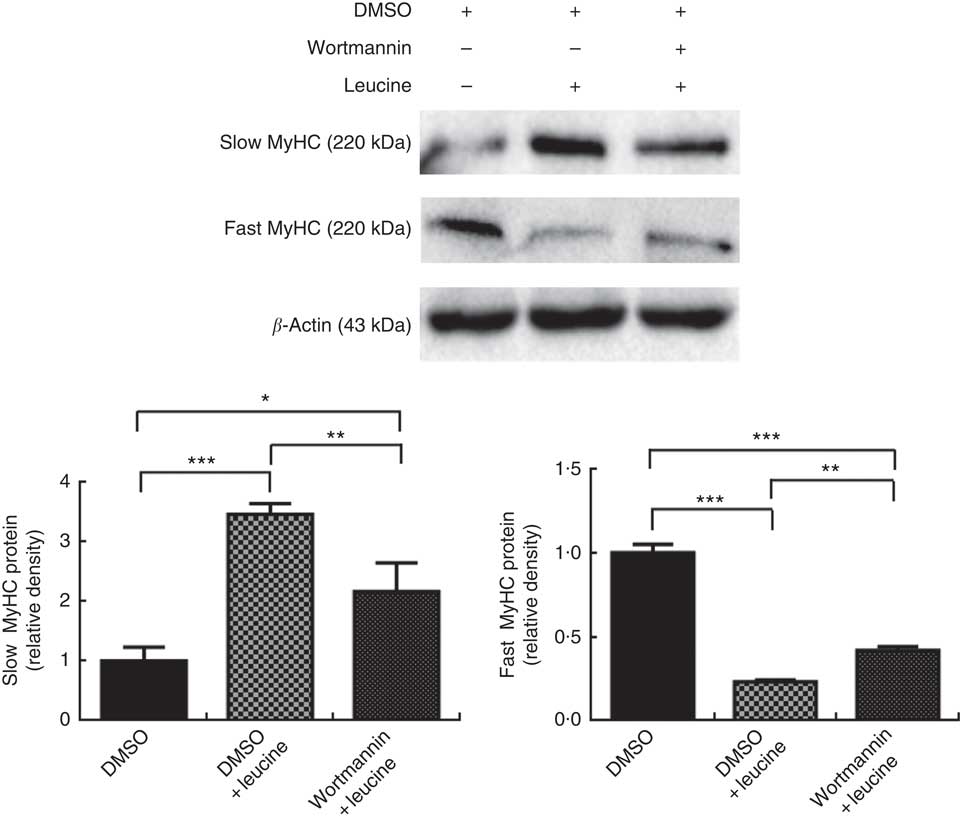
Fig. 3 Leucine regulates porcine myofibre type transformation through the protein kinase B/forkhead box 1 signalling pathway. After 72 h of differentiation, porcine myotubes cultured in differentiation medium were treated with 4 mm leucine and 1 µm wortmannin for 4 d. Dimethyl sulfoxide (DMSO) was used to dissolve wortmannin. Slow myosin heavy chain (MyHC) and fast MyHC protein levels were determined by Western blot analysis. Equal loading was monitored with anti-β-actin antibody. Values are means of the densitometry results from three independent experiments, with their standard errors represented by vertical bars. * P<0·05, ** P<0·01 and *** P<0·001 as compared with control.
Effect of microRNA-27a on porcine myofibre type transformation
To determine whether miR-27a plays a role in porcine myofibre type transformation, we transfected miR-27a mimics, miRNA mimics negative control, miR-27a inhibitor or miRNA inhibitor negative control into porcine myotubes. As shown in Fig. 4(a) and (b), the level of miR-27a was significantly increased in cells transfected with miR-27a mimics, compared with miRNA mimics negative control, whereas miR-27a inhibitor decreased the level of miR-27a, indicating that the transfection was efficient. Under these conditions, over-expression of miR-27a decreased slow MyHC protein level and increased fast MyHC protein level. In contrast, inhibition of miRNA-27a increased slow MyHC protein level and decreased fast MyHC protein level (Fig. 4(c)).
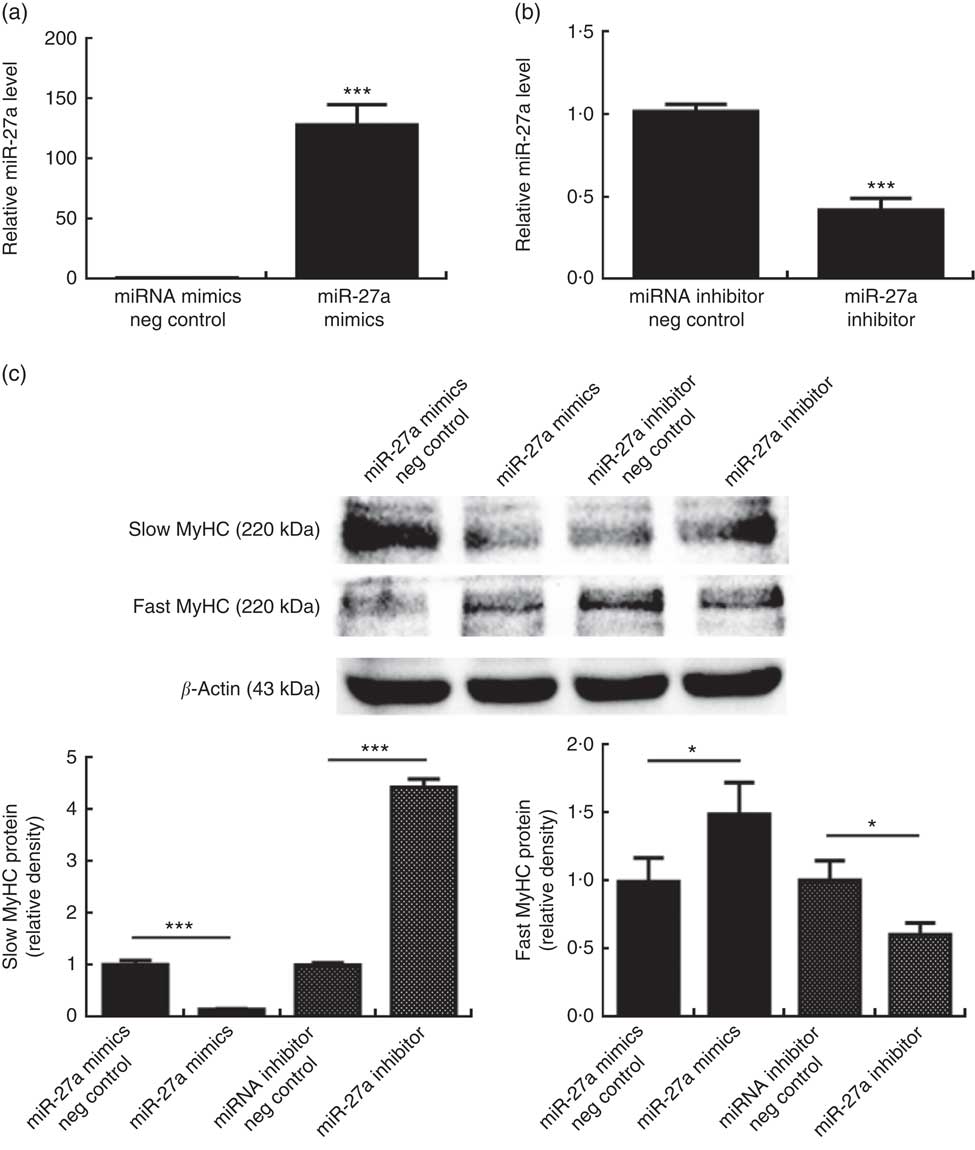
Fig. 4 Effect of microRNA-27a (miR-27a) on porcine myofibre type transformation. After 72 h of differentiation, porcine myotubes cultured in differentiation medium were transfected with 100 nm microRNA (miRNA) mimics negative (neg) control, 100 nm miR-27a mimics, 200 nm miRNA inhibitor negative control or 200 nm miR-27a inhibitor for 3 d. MiR-27a expression (a, b) was determined by real-time quantitative PCR normalised to the amount of U6 small nuclear RNA. Slow myosin heavy chain (MyHC) and fast MyHC protein levels (c) were determined by Western blot analysis. Equal loading was monitored with anti-β-actin antibody. Values are means of the densitometry results from three independent experiments, with their standard errors represented by vertical bars. * P<0·05 and *** P<0·001 as compared with negative control.
Effect of leucine on microRNA-27a expression in porcine myotubes
Expression of miR-27a in leucine-treated porcine myotubes was determined by real-time quantitative PCR. As shown in Fig. 5, expression of miR-27a in porcine myotubes was decreased following leucine treatment.
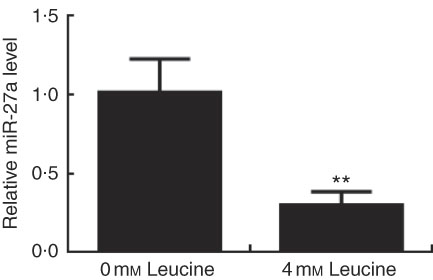
Fig. 5 Effect of leucine on microRNA-27a (miR-27a) expression. After 72 h of differentiation, porcine myotubes cultured in differentiation medium were treated with 4 mm leucine for 4 d. miR-27a level was determined using real-time quantitative PCR normalised to the amount of U6 small nuclear RNA. Values are means from three independent experiments, with their standard errors represented by vertical bars. ** P<0·01 as compared with control.
MicroRNA-27a contributes to leucine-induced porcine myofibre type transformation
Next, we examined whether the inhibition of miR-27a could be relevant for the myofibre type transformation effect of leucine on porcine myotubes. To accomplish this aim, porcine myotubes were transfected with miR-27a mimics followed by leucine treatment. As shown in Fig. 6, leucine increased slow MyHC protein level and decreased fast MyHC protein level, whereas miR-27a mimics attenuated the effect of leucine on slow MyHC and fast MyHC expressions.
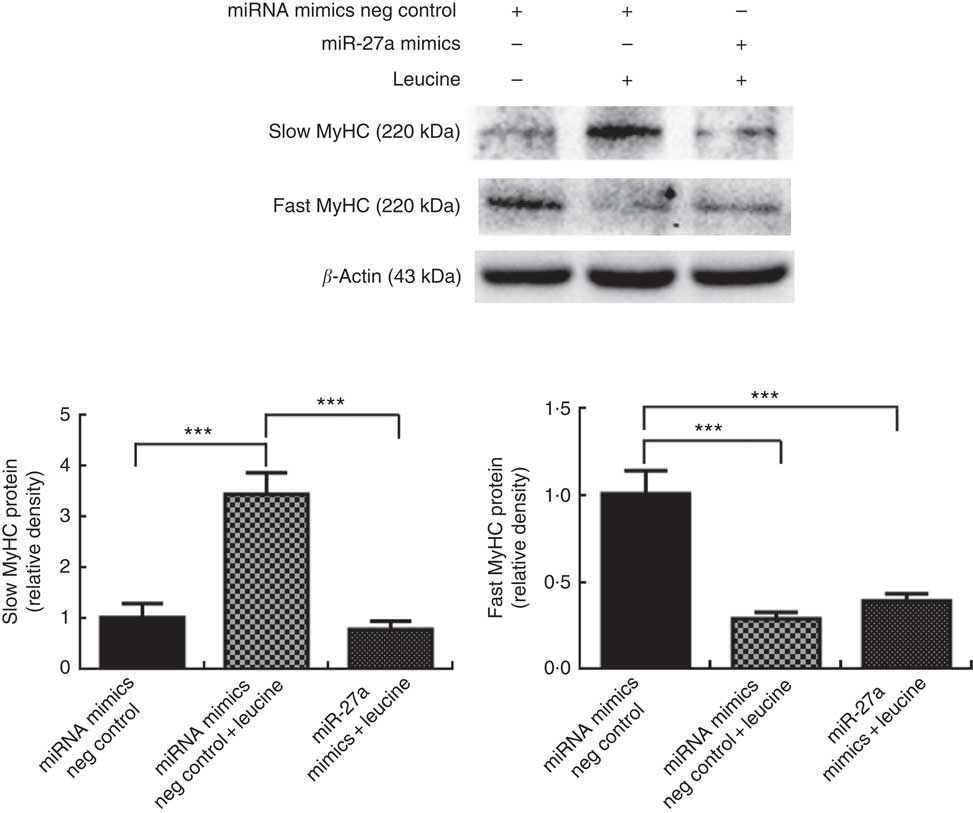
Fig. 6 MicroRNA-27a (miR-27a) contributes to leucine-induced porcine myofibre type transformation. After 72 h of differentiation, porcine myotubes cultured in differentiation medium were transfected with 100 nm microRNA (miRNA) mimics negative (neg) control or 100 nm miR-27a mimics. After 24 h of transfection, 4 mm leucine was added to differentiation medium simultaneously for another 72 h. Slow myosin heavy chain (MyHC) and fast MyHC protein levels were determined by Western blot analysis. Equal loading was monitored with anti-β-actin antibody. Values are means of the densitometry results from three independent experiments, with their standard errors represented by vertical bars. *** P<0·001 as compared to control.
Discussion
Leucine, a well-known anabolic factor that regulates skeletal muscle protein turnover, plays a vital role in muscle protein synthesis and degradation( Reference Hernandez-Garcia, Columbus and Manjarin 12 , Reference Hong and Layman 13 ). Leucine also plays an important role in the formation of skeletal muscle fibres( Reference Li, Xu and Lee 16 – Reference Vaughan, Garcia-Smith and Gannon 19 ). In this study, we showed that leucine promoted slow MyHC expression and inhibited fast MyHC expression, indicating that leucine promotes porcine myofibre type transformation from fast-twitch to slow-twitch.
It has been reported that the expression of Akt was higher in oxidative muscle( Reference Song, Ryder and Kawano 7 , Reference Turinsky and Damrau-Abney 8 ). FoxO1, a downstream transcription factor of the PI3K/Akt pathway, has a closer relation with muscle myofibre type distribution under controlled conditions; moreover, the expression level of FoxO1 in fast-twitch muscle is higher than that in slow-twitch muscle( Reference Azad, Khaledi and Hedayati 26 – Reference Yuan, Shi and Liu 29 ). Swimming endurance training promoted fast- to slow-twitch transformation in mouse muscle, accompanied by reduction in FoxO1 expression; in addition, over-expression of FoxO1 increased the formation of fast-twitch fibres in C2C12 cells( Reference Yuan, Shi and Liu 29 ). Wortmannin or LY294002, the specific blocker of the PI3K/Akt pathway, can suppress the activity of Akt, increase the expression of FoxO1 and then result in the accumulation of oxidative fibre( Reference Sakamoto, Aschenbach and Hirshman 30 – Reference Sequea, Sharma and Arias 32 ). In the present study, we showed that leucine could affect the Akt/FoxO1 signalling pathway, and wortmannin attenuated the effects of leucine on porcine fast MyHC and slow MyHC expressions, indicating that the Akt/FoxO1 signalling pathway contributes to leucine-induced porcine skeletal myofibre type transformation.
miRNA are a class of endogenous non-coding RNA that play critical roles in a variety of biological processes( Reference Bartel 33 , Reference Ambros 34 ). Studies have shown that the expression of majority of miRNA was different in slow- and fast-twitch muscles, indicating that miRNA might play a crucial role in muscle fibre type transition( Reference Liu, Li and Ma 35 , Reference Muroya, Taniguchi and Shibata 36 ). Allen & Loh( Reference Allen and Loh 37 ) reported that the expression of miR-27a and b was abundant in the adult mouse muscle, and expression of miR-27a and b in slow-twitch muscle was higher than that in fast-twitch muscle. It has been reported that over-expression of miR-27b might decrease slow muscle fibre composition in C2C12 cells( Reference Shen, Chen and Zhang 38 ). In the present study, we found that transfection of miR-27a mimics in porcine myotubes decreased slow MyHC protein expression and increased fast MyHC protein expression, whereas transfection of miR-27a inhibitor gave an opposite result, indicating that miR-27a promotes porcine myofibre type transformation from slow-twitch to fast-twitch. Recently, growing studies have reported that nutrients could regulate the expression of miRNA( Reference Alvarez-Díaz, Valle and Ferrer-Mayorga 20 – Reference Drummond, Glynn and Fry 22 ). In this study, we observed that treatment with leucine decreased the expression of miR-27a in porcine myotubes. We also observed that over-expression of miR-27a repressed transformation from fast MyHC to slow MyHC caused by leucine. These results indicated that leucine promotes porcine skeletal myofibre type transformation from fast-twitch to slow-twitch by inhibiting the expression and function of miR-27a. miRNA negatively regulate gene expression at the post-transcriptional level via binding to the 3′-untranslated region of target mRNA. Although human FoxO1 and mouse FoxO1 are targeted by miR-27a( Reference Guttilla and White 39 , Reference Sun, Li and Shao 40 ), there is no evidence that porcine FoxO1 is a target of miR-27a, as leucine (4 mm) down-regulated both the expression of miR-27a and the protein expression of FoxO1 in this study.
In conclusion, we provided the first evidence that leucine promotes porcine myofibre type transformation from fast MyHC to slow MyHC through the Akt/FoxO1 signalling pathway and miR-27a.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (no. 31672432 and no. 31272459) and the National Key R&D Program of China (no. 2018YFD0500403).
X. C. and Z. H. conceived the study and designed the experiments. S. Z. carried out the experiments, analysed the data and wrote the manuscript. X. C., Z. H., D. C., B. Y., H. C., J. H., J. L., P. Z., J. Y. and Y. L. contributed reagents/materials/analysis tools. Z. H. revised the manuscript. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.









