Introduction
Olanzapine is an effective pharmacologic treatment for both the acute and maintenance treatment of schizophrenia and bipolar I disorder,Reference Huhn, Nikolakopoulou and Schneider-Thoma 1 -Reference Lindström, Lindström, Nilsson and Höistad 7 but its use in clinical practice is limited by the potential for substantial weight gain and other metabolic sequelae.Reference Lieberman, Stroup and McEvoy 4 , Reference Citrome, Holt, Walker and Hoffmann 8 -Reference Berkowitz, Patel, Ni, Parks and Docherty 12 In the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study in schizophrenia, olanzapine was the most effective agent, as measured by time to all-cause discontinuation; however, compared with the other antipsychotic treatment arms, olanzapine was also associated with a significantly greater proportion of patients who developed clinically significant (≥7%) weight gain from baseline and with a significantly greater percentage of patients who discontinued treatment because of weight gain and metabolic effects.Reference Lieberman, Stroup and McEvoy 4 In clinical studies with a duration of 48 weeks or longer conducted in adults, weight gain occurred in approximately 77–83% of patients treated with olanzapine. 13 Although several different patient demographic factors have been associated with an increased risk of olanzapine-associated weight gain,Reference Lipkovich, Jacobson, Caldwell, Hoffmann, Kryzhanovskaya and Beasley 14 the mechanisms underlying these effects remain unknown.Reference Dayabandara, Hanwella, Ratnatunga, Seneviratne, Suraweera and de Silva 15
A combination of olanzapine and the opioid receptor antagonist samidorphan (OLZ/SAM) was approved by the US Food and Drug Administration in May 2021 for the treatment of adults with schizophrenia or adults with bipolar I disorder, including indications for the acute treatment of mixed or manic episodes as monotherapy or adjunctively to lithium or valproate, and as a maintenance monotherapy. 16 As previously reported, treatment with OLZ/SAM in the ENLIGHTEN-2 study was associated with a significantly lower mean percent change in body weight and with a 50% reduction in the risk of developing clinically significant weight gain relative to olanzapine at both the 7% and 10% weight gain thresholds.Reference Correll, Newcomer and Silverman 17 Here, we report findings from a prespecified analysis exploring the consistency of the weight mitigation effect with OLZ/SAM vs olanzapine across demographic subgroups in ENLIGHTEN-2.
Methods
The phase 3, multicenter, randomized, double-blind, 24-week ENLIGHTEN-2 study (NCT02694328) enrolled adult outpatients aged 18–55 years with a Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) diagnosis of schizophrenia, stable body weight (ie, ≤5% change in the 3 months prior to study initiation, by self-report), and a body mass index (BMI) of 18–30 kg/m2. Patients were excluded if antipsychotic naive, treatment resistant, and/or if the onset of schizophrenia symptoms was within 1 year of study entry. Detailed eligibility criteria were reported previously.Reference Correll, Newcomer and Silverman 17 The study protocol and all amendments were approved by an institutional review board at each study site, and the study was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent before study participation.
The study design is provided in Supplementary Figure S1. Patients were randomized 1:1 to OLZ/SAM or olanzapine monotherapy for 24 weeks. Randomization was not stratified for baseline demographics. The initial olanzapine dose was 10 mg for both groups, given as OLZ/SAM (olanzapine 10 mg/samidorphan 10 mg [10/10 mg]) or olanzapine 10 mg. Between weeks 1 and 4, the olanzapine dose was titrated up to 20 mg (ie, administered as OLZ/SAM 20/10 mg or olanzapine 20 mg). If tolerability issues occurred, the olanzapine dose could be reduced at the investigator’s discretion in weeks 2 to 4; after week 4, olanzapine doses were fixed. Body weight (measured in triplicate) was assessed weekly through week 6 and then biweekly through week 24.
The analysis of covariance (ANCOVA) model included treatment group, race, and baseline age as factors, and baseline body weight as the covariate. Prespecified exploratory analyses of the ENLIGHTEN-2 co-primary endpoints (percent change in weight from baseline and the proportion of patients with ≥10% weight gain at week 24) were conducted in patient subgroups according to the following: sex (male, female), age (<30 years, ≥30 years), race (black, non-black), and baseline BMI (<27 kg/m2, ≥27 kg/m2). Percent change in body weight was determined by ANCOVA, with multiple imputation for handling missing values. The least squares mean difference in percent change in body weight and the associated 95% confidence intervals (CIs) were calculated for OLZ/SAM vs olanzapine by subgroup; if the 95% CIs crossed zero, differences between OLZ/SAM and olanzapine were considered nonsignificant. The proportion of patients with at least 10% weight gain was analyzed using a logistic regression model with multiple imputation for missing data, with the same covariates as for the ANCOVA analysis. The odds ratios (ORs) and 95% CIs for the OLZ/SAM vs olanzapine comparisons were calculated; an OR less than 1 with a 95% CI that did not cross 1 favored treatment with OLZ/SAM.
Results
A total of 538 patients who received at least 1 dose of study medication and who had at least 1 postbaseline weight assessment were included (OLZ/SAM, n = 266; olanzapine, n = 272). Demographics and clinical characteristics for the subgroups were similar between treatment groups at baseline (Table 1).
Table 1. Demographics and Clinical Characteristics of Subgroups at Baseline
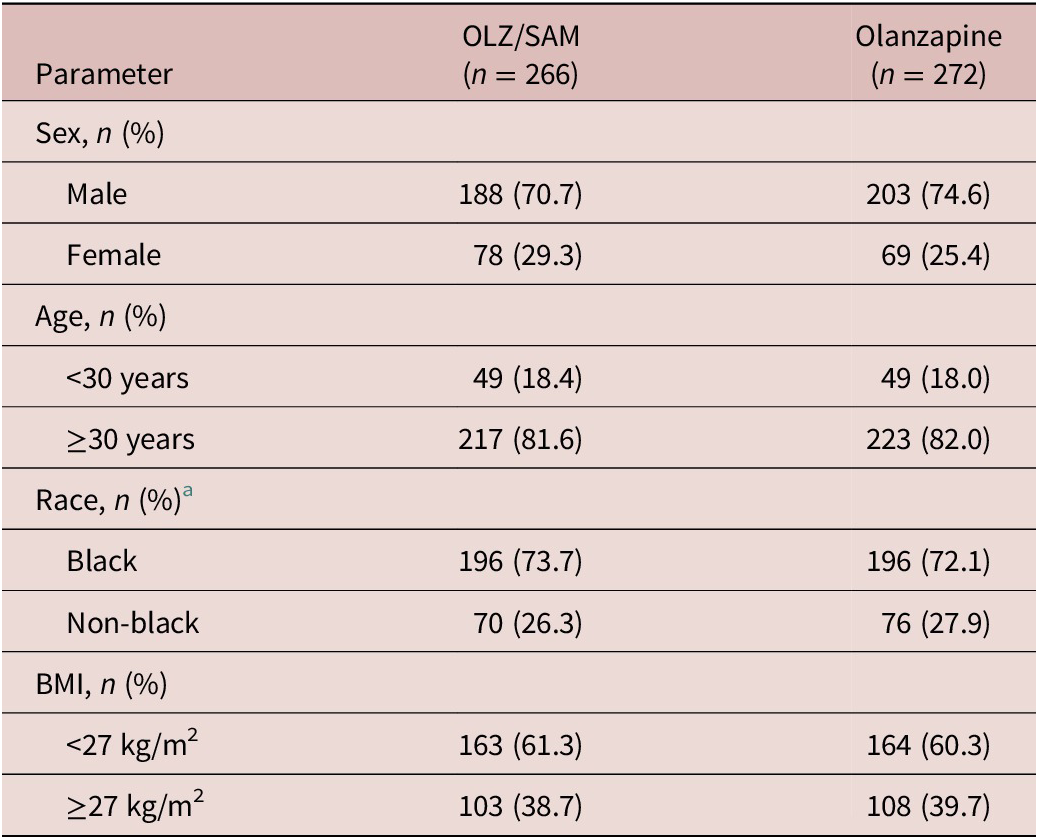
Abbreviations: BMI, body mass index; OLZ/SAM, combination of olanzapine and samidorphan.
a Self-identified.
The least squares mean differences in percent change from baseline body weight for OLZ/SAM vs olanzapine at 24 weeks are reported by subgroup in Figure 1. Across each of these subgroups, treatment with OLZ/SAM resulted in numerically less weight gain than olanzapine, with least squares mean differences ranging from −1.53% to −3.43% (Figure 1). For several subgroups (males, patients aged ≥30 years, black patients, and patients with a baseline BMI of either <27 kg/m2 or ≥ 27 kg/m2), the 95% CI of the least squares mean difference did not cross zero (Supplementary Figures S2–S5). For the subgroups of females, patients aged <30 years, and non-black patients, who each had smaller sample sizes, OLZ/SAM treatment resulted in numerically less percent weight gain, consistent with findings in the overall study population,Reference Correll, Newcomer and Silverman 17 but the 95% CIs crossed zero.

Figure 1. Percent change in weight from baseline in weight at week 24. Forest plot marker size is proportional to subgroup n. Analyses were conducted in the full-analysis set using analysis of covariance, with multiple imputation for missing postbaseline data. BMI, body mass index; CI, confidence interval; LS, least squares; OLZ/SAM, combination of olanzapine and samidorphan.
The subgroup analysis of the proportion of patients with at least a 10% weight gain is shown in Figure 2. As previously reported, the odds of gaining at least 10% of baseline body weight were reduced by half with OLZ/SAM treatment vs treatment with olanzapine (OR, 0.50; 95% CI: 0.31, 0.80; P = 0.003).Reference Correll, Newcomer and Silverman 17 In this subgroup analysis, OLZ/SAM treatment was associated with lower odds of having 10% or greater body weight gain from baseline vs olanzapine in each subgroup tested. The reduced OR favored OLZ/SAM vs olanzapine for the following subgroups: male patients (OR, 0.41; 95% CI: 0.23, 0.73), patients aged 30 years or older (OR, 0.46; 95% CI: 0.27, 0.78), black patients (OR, 0.47; 95% CI: 0.27, 0.82), and patients with a baseline BMI of at least 27 kg/m2 (OR, 0.38; 95% CI: 0.16, 0.87; Supplementary Figures S2–S5). Although females, patients younger than 30 years, non-black patients, and those with a BMI of less than 27 kg/m2 were numerically less likely to gain at least 10% of their baseline body weight with OLZ/SAM vs olanzapine treatment, the 95% CIs for those comparisons crossed 1, indicating no statistically significant differences between treatment groups.
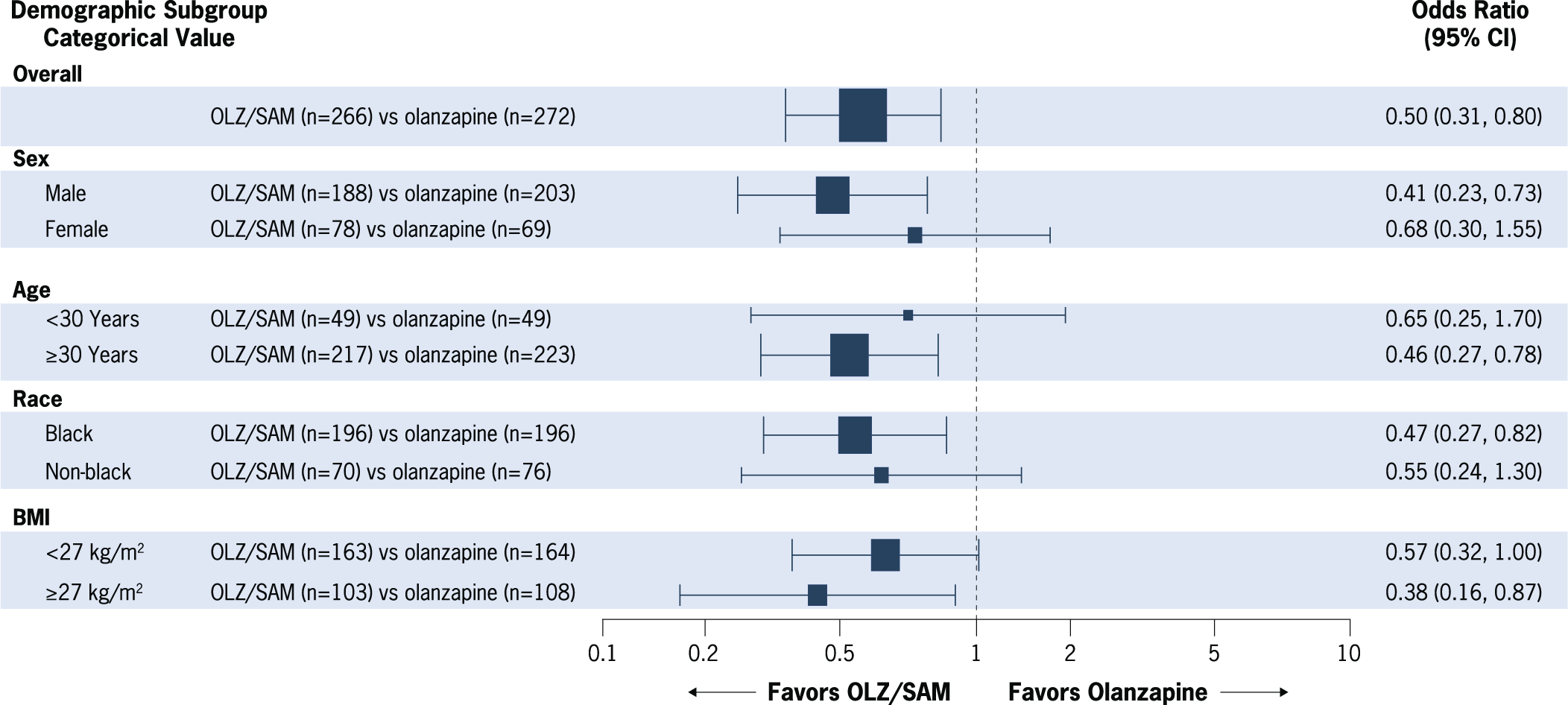
Figure 2. Proportion of patients with at least 10% weight gain from baseline at week 24. Forest plot marker size is proportional to subgroup n. Analyses were conducted in the full-analysis set using logistic regression, with multiple imputation for missing postbaseline data. BMI, body mass index; CI, confidence interval; OLZ/SAM, combination of olanzapine and samidorphan.
Discussion
Based on these prespecified exploratory analyses from the ENLIGHTEN-2 study, there was consistent weight mitigation by treatment with OLZ/SAM vs olanzapine across the subgroups examined. For each subgroup, treatment with OLZ/SAM was associated with a lower percent change in body weight and with fewer patients experiencing at least 10% weight gain compared with olanzapine. Overall, the cumulative findings are consistent with the primary analysis of ENLIGHTEN-2, in which OLZ/SAM treatment mitigated olanzapine-associated weight gain in the overall patient population.Reference Correll, Newcomer and Silverman 17
Although many antipsychotics are associated with some degree of weight gain, olanzapine is considered a medication with high risk for this effect.Reference Lieberman, Stroup and McEvoy 4 , Reference De Hert, Detraux, van Winkel, Yu and Correll 18 , Reference McEvoy, Lieberman and Perkins 19 Indeed, in clinical studies lasting 48 weeks or longer, approximately 77–83% of all individuals treated with olanzapine experienced weight gain, 13 with certain subgroups having more vulnerability to this effect than others.Reference Lipkovich, Jacobson, Caldwell, Hoffmann, Kryzhanovskaya and Beasley 14 Importantly, the weight gain mitigation benefits observed with OLZ/SAM are not limited to a specific subset of patients but are consistently seen across subgroups. The decision to use any medication should rest on the risk–benefit analysis for that individual patient.Reference Hasan, Falkai and Wobrock 20 -Reference Remington, Addington, Honer, Ismail, Raedler and Teehan 22 The results from these data analyses indicate that, across the patient subgroups evaluated, including those subgroups previously identified as having high risk for olanzapine-associated weight gain, all benefited from OLZ/SAM, as did the overall population, in terms of weight gain mitigation. Thus, OLZ/SAM may provide a treatment option for a broader population of patients in need of the efficacy of olanzapine with less risk of weight gain.
Several study limitations must be considered in the interpretation of these analyses. First, sample sizes for some patient subgroups were small, and this can contribute to wide CIs. Additionally, no data in these subgroup analyses pertain to obese patients, as enrollment in the study was limited to those with a BMI of 18–30 kg/m2. Finally, these analyses were exploratory in nature, as subgroups were not stratified at randomization and no adjustments were made to the analysis to address potential confounding factors at baseline.
Conclusion
In these prespecified exploratory analyses of data from the phase 3 ENLIGHTEN-2 study, consistency in the weight-mitigating effects of OLZ/SAM vs olanzapine was observed across subgroups. While no treatment differences were observed in some smaller groups, numerically, OLZ/SAM resulted in a lower percent change in body weight and in fewer patients with clinically significant weight gain (≥10% from baseline) across all subgroups examined. These data indicate that OLZ/SAM has the potential to mitigate olanzapine-associated weight gain across a broad range of patient subgroups.
Acknowledgments
The authors thank the ALK3831-A303 Study Group, as well as Mark S. Todtenkopf, PhD, of Alkermes, Inc., who assisted in the preparation and proofreading of the manuscript. Medical writing and editorial support were provided by Judith Bammert Adams, PharmD, and John H. Simmons, MD, of Peloton Advantage, LLC, an OPEN Health company, and funded by Alkermes, Inc.
Financial support
This study was sponsored by Alkermes, Inc.
Author contributions
All authors contributed to data interpretation, collaborated in the preparation of the manuscript, and participated in the critical review and revision of the manuscript. All authors granted approval of the manuscript for submission.
Disclosures
The data collected in this study are proprietary to Alkermes, Inc. Alkermes, Inc. is committed to public sharing of data in accordance with applicable regulations and laws, and requests may be submitted to the corresponding author. Jonathan M. Meyer has served as a speaker or advisor for Acadia Pharmaceuticals, Alkermes, Inc., Intra-Cellular Therapies, Karuna, Neurocrine, Noven, Otsuka America, Inc., and Sunovion Pharmaceuticals. Adam Simmons, Ying Jiang, Christine Graham, and Sergey Yagoda were employees of Alkermes, Inc., at the time of this analysis and may own stock/options in the company. David McDonnell is an employee of Alkermes Pharma Ireland Ltd. and may own stock/options in the company.
Supplementary materials
To view supplementary material for this article, please visit https://doi.org/10.1017/S1092852922000967.





