Psychological distress, such as depressive and anxiety symptoms, has been associated with higher risk of coronary heart disease (CHD)-, stroke-, and cardiovascular disease (CVD)-related mortality (Batelaan et al., Reference Batelaan, Seldenrijk, Bot, van Balkom and Penninx2016; de Miranda Azevedo et al., Reference de Miranda Azevedo, Roest, Hoen and de Jonge2014; Moise et al., Reference Moise, Khodneva, Richman, Shimbo, Kronish and Safford2016; Rutledge et al., Reference Rutledge, Linke, Krantz, Johnson, Bittner, Eastwood and Merz2009; Salaycik et al., Reference Salaycik, Kelly-Hayes, Beiser, Nguyen, Brady, Kase and Wolf2007; Shen et al., Reference Shen, Avivi, Todaro, Spiro, Laurenceau, Ward and Niaura2008; Ye et al., Reference Ye, Muntner, Shimbo, Judd, Richman, Davidson and Safford2013), as well as with individual cardiovascular (CV) risk factors (Castaneda et al., Reference Castaneda, Buelna, Giacinto, Gallo, Sotres-Alvarez, Gonzalez and Talavera2016; Lopez-Leon et al., Reference Lopez-Leon, Aulchenko, Tiemeier, Oostra, van Duijn and Janssens2010; Scherrer et al., Reference Scherrer, Xian, Bucholz, Eisen, Lyons, Goldberg and True2003). Potential biological mechanisms to explain these relationships have been proposed, including altered hypothalamic-pituitary-adrenal axis function, circadian rhythms, inflammation, platelet activation, imbalance of neurotransmitters, health behaviors, or side effects of pharmacological treatment of depression (Amare et al., Reference Amare, Schubert, Klingler-Hoffmann, Cohen-Woods and Baune2017; Carney et al., Reference Carney, Freedland, Rich and Jaffe1995; McCaffery et al., Reference McCaffery, Frasure-Smith, Dube, Theroux, Rouleau, Duan and Lesperance2006). In addition, in meta-analysis studies of genetic epidemiology, a strong genetic influence was found for major depression (Sullivan et al., Reference Sullivan, Neale and Kendler2000), and significant familial aggregation was demonstrated for panic disorder, generalized anxiety disorder, phobias, and obsessive-compulsive disorder (Hettema et al., Reference Hettema, Neale and Kendler2001). In a systemic review of genome-wide and candidate gene studies, 24 potential pleiotroic genes and relevant pathways for the shared biological mechanisms explained the associations between cardiometabolic diseases risk and mood disorders (Amare et al., Reference Amare, Schubert, Klingler-Hoffmann, Cohen-Woods and Baune2017).
These studies support how genetic factors contribute to depressive and anxiety symptoms and CV risk factors, and that shared genetic factors in the associations between these traits could be a plausible mechanism (Amare et al., Reference Amare, Schubert, Klingler-Hoffmann, Cohen-Woods and Baune2017; Hettema et al., Reference Hettema, Neale and Kendler2001; Lopez-Leon et al., Reference Lopez-Leon, Aulchenko, Tiemeier, Oostra, van Duijn and Janssens2010; McCaffery et al., Reference McCaffery, Frasure-Smith, Dube, Theroux, Rouleau, Duan and Lesperance2006; Sullivan et al., Reference Sullivan, Neale and Kendler2000). Despite this evidence, the research on the role of shared genetic relationships in the association between depressive and anxiety symptoms, and CV risk is limited. In a study of the genetic correlation of depression symptoms with hypertension and heart disease in 2,731 male twins from the Vietnam Era Twin Registry (age 41.9 ± 2.7 years; non-Hispanic white, 94.6%), the genetic correlations were 0.19 between depression symptoms and hypertension and 0.42 between depression symptoms and heart disease, while there were no significant environmental correlations between these traits (Scherrer et al., Reference Scherrer, Xian, Bucholz, Eisen, Lyons, Goldberg and True2003). In the 2,383 individuals from the Erasmus Rucphen Family study (age 48.7 ± 15.1 years; men, 43.1%), a genetically isolated population in the Netherlands, the genetic correlations were 0.25–0.31 between scales of depressive symptoms and lipid levels and environmental correlations between these traits were −0.15~−0.16 after adjusting for age, sex, use of medication, and degree of consanguinity and sibship effects (Lopez-Leon et al., Reference Lopez-Leon, Aulchenko, Tiemeier, Oostra, van Duijn and Janssens2010).
However, to the best of our knowledge, there are no published studies on the shared genetic influence between depressive and anxiety symptoms and estimated CV risk. In the current study, we aimed to assess shared genetic correlations of depressive and anxiety symptoms at a baseline visit with estimated CV risk score at the baseline and follow-up visits among Korean twins and their family members enrolled in the Healthy Twin study. We used the Adult Treatment Panel III risk estimate, the most commonly used CHD risk score (Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, 2001) to estimate risk for an individual of having a ‘hard’ CHD (myocardial infarction or CHD death).
Materials and Methods
Study Population
The study subjects were participants in the Healthy Twin study, a multi-center cohort study established in 2005 for South Korean adult (> 30 years of age), same-sex twins and their first-degree adult family members (Gombojav et al., Reference Gombojav, Song, Lee, Yang, Kho, Hwang and Sung2013; Sung et al., Reference Sung, Cho, Lee, Ha, Choi, Choi and Song2006). Of the total 3,479 study participants, the trait measurement records for 1,991 individuals at baseline (monozygotic twin individuals, 706; men, 995; women, 996; age 44.4 ± 11.0 years) and 1,059 individuals at follow-up (monozygotic twin individuals, 451; men, 517; women, 542; age 47.2 ± 10.4 years) who were aged from 34 to 74 years and had complete assessment data for depressive and anxiety symptoms were used for the relationship with 10-year CV risk. The rate of recruitment success rate was 53.2%. The interval between baseline and follow-up visits was 3.2 ± 1.2 years. This study was carried out in accordance with the Declaration of Helsinki. Informed consent along with conflict of interest disclosure was obtained from the study participants. All study procedures were approved by the respective institutional review boards of the participating institutions.
Measurements of the Depressive and Anxiety Symptoms
The assessment of measures in the Healthy Twin study has been described in detail elsewhere (Gombojav et al., Reference Gombojav, Song, Lee, Yang, Kho, Hwang and Sung2013; Sung et al., Reference Sung, Cho, Lee, Ha, Choi, Choi and Song2006). Depressive and anxiety symptoms at baseline visit were assessed using scales included in a self-administered questionnaire. Depressive symptoms in the past week were assessed with a scale that used the 20-item Center for Epidemiological Studies Depression Scale (CES-D; Radloff, Reference Radloff1977), which was translated to Korean (Chon et al., Reference Chon, Choi and Yang2001). For the CES-D, participants were asked how often they have felt each of the feelings listed below during the past week. Each item was scored as 0 (rarely or none of the time, < 1 day), 1 (some or a little of the time, 1–2 days), 2 (occasionally or a moderate amount of the time, 3–4 days), or 3 (most or all of the time, 5–7 days). Anxiety symptoms were assessed using the State-Trait Anxiety Inventory (STAI) developed by Spielberger et al. (Reference Spielberger, Gorsuch and Lushene1970). We used the Korean version of STAI adapted by Kim (Reference Kim1978). Each section consists of 20 questions, totaling 40 questions. The State Anxiety Inventory scale (SAI) reflects fluctuating emotional state and severity, which depends on the situation and time, while the Trait Anxiety Inventory scale (TAI) evaluates a constant behavioral tendency. Each measure is validated as a predictor of objective health outcomes in cardiac populations (Shibeshi et al., Reference Shibeshi, Young-Xu and Blatt2007; Wassertheil-Smoller et al., Reference Wassertheil-Smoller, Shumaker, Ockene, Talavera, Greenland, Cochrane and Dunbar-Jacob2004). In the present sample, internal consistency value (Cronbach's α) was 0.89 for the CES-D, 0.89 for the TAI, and 0.92 for the SAI.
Measurement of Cardiovascular Risk Estimate and Covariates
CV risk was estimated at baseline and follow-up visits using the Adult Treatment Panel III risk estimate (Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, 2001). This risk estimate is based on a modified Framingham risk model for predicting an individual's risk for developing CVD over the next 10 years in those aged 30–74 years, and includes age, total cholesterol, high density lipoprotein cholesterol (HDL-C), systolic blood pressure, and smoking status as components (Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, 2001). Smoking status (current smoker vs. non-current smoker) was assessed using a self-reported questionnaire. Other components of CV risk estimates were measured for participants who fasted overnight for at least 12 hours. Blood pressure was assessed manually using a standard mercury sphygmomanometer under standardized conditions. The levels of total cholesterol and HDL-C (measured using an enzymatic or homogeneous assay) were measured using ADVIA 1650 (Siemens, Germany) or Hitachi 7600-210/Hitachi 7180 (Hitachi, Tokyo, Japan) equipment. The analyses were conducted in a central laboratory authorized by the Korean Association of Quality Control for Clinical Laboratory Examination.
The covariates considered were baseline measurements such as body mass index (BMI), educational attainment (< graduated high school, graduated high school, or > graduated high school), income level (< 2 × 106 won vs. ≥ 2 × 106 won), alcohol consumption (current user vs. current non-user), and exercise (yes vs. no to engaging in regular moderate to high-intensity exercise). BMI was calculated as measured weight (kg) divided by measured height squared (m2) using a digital balance (Tanita Co., Seoul, Korea) and stadiometer (Samwha Co., Seoul, Korea), respectively. Self-reported questionnaires were used to assess other covariates.
Statistical Analysis
Chi-square tests or t-tests were conducted to assess differences in the characteristics between those included in the follow-up analysis and those who were excluded in the follow-up analysis. The absolute 10-year CV risk and CV risk scores at baseline and follow-up, and the CES-D, TAI, and SAI scores at baseline between men and women were compared using t-tests. Spearman correlations were applied to the relationships among the CES-D, TAI, and SAI scores. Mixed linear models were used to determine the associations of the CV risk score at baseline and follow-up with the CES-D, TAI, and SAI scores, BMI, socioeconomic status, and health behaviors at baseline. Due to skewed distribution of absolute 10-year CV risk, the CV risk score was used for these relationships. In this model, the correlation structures from family and twin relationships were included as random effects, and the CES-D, TAI, and SAI scores, and other characteristics (e.g., education, income, BMI, alcohol consumption, exercise, and the CV risk score at baseline for the follow-up analysis) were included as fixed effects. The analyses were conducted in all subjects as well as separately for men and women on the basis of previous findings of sex-specific associations between psychosocial distress and CVD development (Haukkala et al., Reference Haukkala, Konttinen, Uutela, Kawachi and Laatikainen2009). To evaluate whether the associations of the CES-D, TAI, and SAI scores with the CV risk score were not related to genetic and shared familial factors, a co-twin control analysis in pairs of monozygotic twins was conducted. The risk for having a higher CV risk score at baseline and follow-up between monozygotic twin pairs was assessed using conditional logistic regression analysis for the CES-D, TAI, and SAI scores, education, income, BMI, alcohol consumption, exercise, and the baseline CV risk score (in the analysis for follow-up CV risk score). A p value < .05 was considered statistically significant. Data were analyzed using IBM Statistical Package for the Social Sciences (SPSS) software version 23 (IBM Corp., Armonk, NY, USA).
The genetic analysis was conducted using the Sequential Oligogenic Linkage Analysis Routines (SOLAR) package (http://solar.sfbrgenetics.org, version 6.6.2). Heritabilities were calculated for the CES-D, TAI, and SAI scores, and the CV risk score and its components (i.e., total cholesterol, systolic blood pressure, and HDL-C) at baseline and follow-up. Heritabilities were calculated as the proportion of phenotypic variance explained by additive genetic effects. To find any evidence of common genetic and environmental regulation between the CES-D, TAI, and SAI scores, and the CV risk score with its components (baseline and follow-up), we partitioned the phenotypic correlations into correlations explained by genetic sharing (ρG) and environmental sharing (ρE). Regarding the bivariate analysis for the CV risk score and its components at follow-up, we adjusted for baseline value for each trait. If ρG or ρE significantly deviated from zero, we regarded it as evidence of a genetic or environmental association between the two traits, respectively.
Results
Characteristics of Subjects
The 10-year CV risk, the CV risk score, the CES-D, TAI, and SAI scores, age, BMI, education, alcohol use, exercise at baseline in men and women who were included in the follow-up analysis were not significantly different from those characteristics in excluded subjects in the follow-up analysis. However, there were more monozygotic twins in the included subjects in the follow-up analysis compared to the excluded subjects (Table 1). Men were more likely to have higher CV risk scores, while also having lower CES-D, TAI, and SAI scores compared to women at baseline and follow-up (p < .01).
TABLE 1 Comparison of Characteristics by Sex and Follow-Up Status in Subjects Who Did Not Have a History of Stroke and Coronary Heart Diseases at Baseline
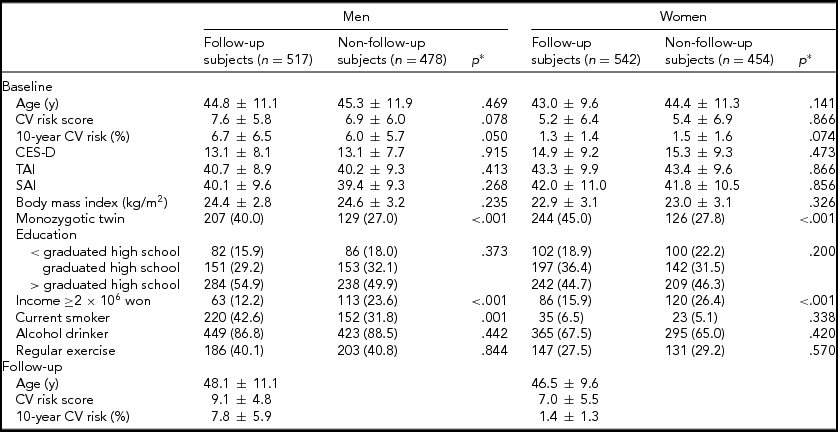
Values represent mean ± SD or n (%). CV = cardiovascular; CES-D = Center for Epidemiological Studies Depression scale score; TAI = Trait Anxiety Inventory score; SAI = State Anxiety Inventory score.
* Using t-test or chi-square test.
Relationships of CV Risk Score with Depression and Anxiety Symptoms and Other Characteristics
The correlation coefficients were 0.69 for the relationship between the CES-D and the TAI scores, 0.63 for the relationship between the CES-D and the SAI scores, and 0.79 for the relationship between the SAI and the TAI scores in all subjects at baseline (all p < .001).
Table 2 presents the associations of the CV risk score with CES-D, TAI, and SAI scores, BMI, socioeconomic status, and health behaviors after taking into account for random and fixed effects (as described in Methods). Higher CES-D scores were associated with higher CV risk scores at baseline in men, while higher TAI score was associated with higher CV risk scores at follow-up in women. There were differential associations of the CV risk score with BMI, education, income, alcohol use, and exercise according to sex and time.
TABLE 2 The Associations of the Cardiovascular Risk Score at Baseline and Follow-Up With the CES-D, TAI, and SAI scores, BMI, Socioeconomic Status, and Health Behaviors (n = 1,059)
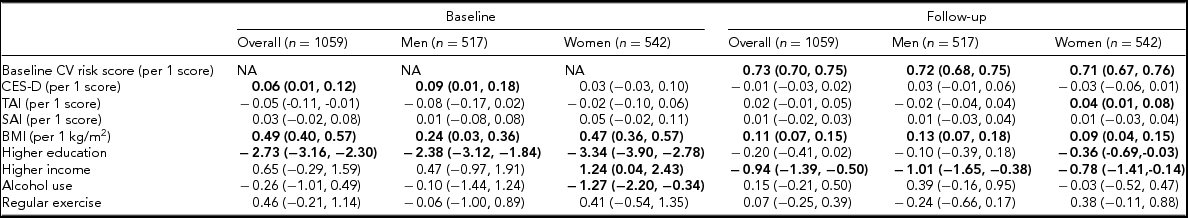
Bold type represents significant coefficients. CES-D = Center for Epidemiological Studies Depression scale score; TAI = Trait Anxiety Inventory score; SAI = State Anxiety Inventory score; BMI = body mass index; NA = not applicable.
Values were estimated coefficients (95% confidence interval) using a mixed linear model including random effects (twin and family effects) and fixed effects (baseline CV risk in the analysis for follow-up CV risk, CES-D, TAI, SAI, alcohol use, exercise, BMI, education, and income).
Heritability, Bivariate Analysis, and Co-Twin Control Analysis
Heritability estimates were 0.245~0.326 for the CV risk scores, 0.320 for the CES-D score, 0.367 for the TAI score, and 0.482 for the SAI score. Bivariate analysis resulted in significant common genetic correlations in the relationships of the CES-D, TAI, and SAI scores with the CV risk score at baseline and follow-up (after adjusting for baseline CV risk score). Shared common environmental correlations were observed in the relationships of the CES-D and SAI scores with the CV risk score at baseline; the SAI score with the CV risk score at follow-up (Table 3).
TABLE 3 Heritability and Genetic and Environmental Correlations for the Relationships of CES-D, TAI, and SAI Scores With the CV Risk Score With Its Components (n = 1,059)
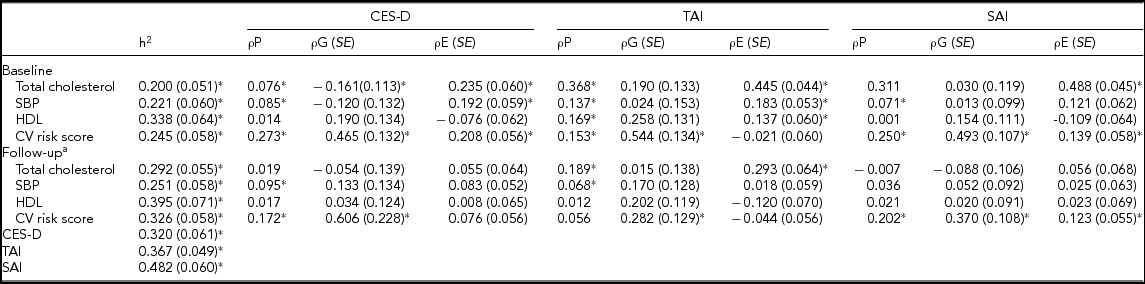
CES-D = Center for Epidemiological Studies Depression scale score; TAI = Trait Anxiety Inventory score; SAI = State Anxiety Inventory score; SBP = systolic blood pressure; HDL = high density lipoprotein cholesterol; h2 – heritability; ρP = common phenotypic correlation; ρG = common genetic correlation; ρE = common environmental correlation.
a adjusting for baseline value in bivariate analysis with each trait (i.e., sum of CV risk score at baseline for sum of CV risk at follow-up).
* p < .05.
In the multivariate conditional logistic analysis for within-monozygotic twin pairs to assess non-shared environmental factors, there were no significant associations of the CES-D, TAI, and SAI scores with the CV risk score at baseline and follow-up. Monozygotic twins with higher CV risk scores at baseline were 1.39 times more likely to have higher BMI compared to their co-twin pairs with lower CV risk scores at baseline. Monozygotic twins with higher CV risk scores at baseline or follow-up were respectively 52% and 53% less likely to exercise regularly compared to their co-twin pairs with lower CV risk scores at baseline or follow-up (Table 4).
TABLE 4 Risk Estimation for Having Higher Cardiovascular (CV) Risk Scores at Baseline and Follow-Up: Co-Twin-Control Analysis in Monozygotic Twins (n = 159 pairs)
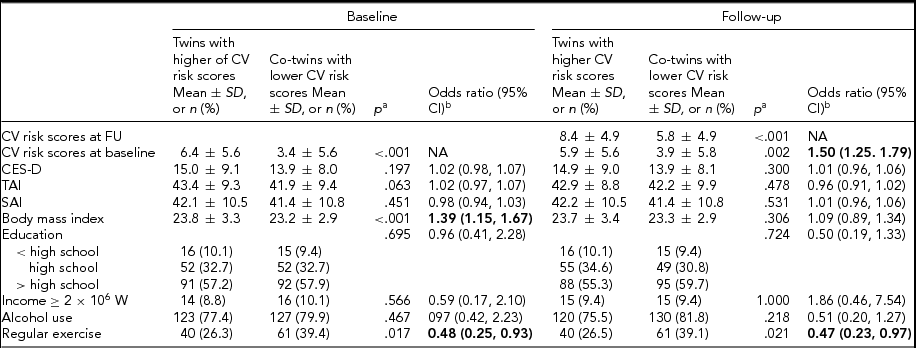
Bold type represents significant odds ratios. SD = standard deviation; CI = confidence interval; NA = not applicable.
aPaired t-test or conditional logistic regression analysis. bMultivariate conditional logistic regression analysis including the CES-D, TAI, and SAI scores, body mass index, education level, income level, alcohol use, regular exercise, and CV risk score at baseline (in the analysis for follow-up CV risk score) as predicting variables.
Discussion
In the Korean twins and their family members who did not have history of CVD, we found a positive association between depressive symptoms and concurrently estimated CV risk scores in men but not in women after adjusting for anxiety symptoms, BMI, education, income, alcohol use, and exercise habit. In addition, there was a positive association between TAI scores and future CV risk scores in women after adjusting for baseline CV risk scores, depressive symptoms, SAI scores, BMI, education, income, alcohol use, and exercise. Despite these less consistent relationships according to sex, scales of anxiety assessment, and assessment time, current findings could be interpreted that increased depressive or anxiety symptoms could occur with higher CV risk estimates and predict higher CV risk estimates in the future. The observation would support previous knowledge about the associations between depressive and anxiety symptoms and CVD and CV risk factors (Batelaan et al., Reference Batelaan, Seldenrijk, Bot, van Balkom and Penninx2016; Castaneda et al., Reference Castaneda, Buelna, Giacinto, Gallo, Sotres-Alvarez, Gonzalez and Talavera2016; de Miranda Azevedo et al., Reference de Miranda Azevedo, Roest, Hoen and de Jonge2014; Lopez-Leon et al., Reference Lopez-Leon, Aulchenko, Tiemeier, Oostra, van Duijn and Janssens2010; Moise et al., Reference Moise, Khodneva, Richman, Shimbo, Kronish and Safford2016; Rutledge et al., Reference Rutledge, Linke, Krantz, Johnson, Bittner, Eastwood and Merz2009; Salaycik et al., Reference Salaycik, Kelly-Hayes, Beiser, Nguyen, Brady, Kase and Wolf2007; Scherrer et al., Reference Scherrer, Xian, Bucholz, Eisen, Lyons, Goldberg and True2003; Shen et al., Reference Shen, Avivi, Todaro, Spiro, Laurenceau, Ward and Niaura2008; Ye et al., Reference Ye, Muntner, Shimbo, Judd, Richman, Davidson and Safford2013).
Despite evidence that depressive and anxiety symptoms and CVD are heritable, the research for common genetic influence on the associations between these traits is very limited. In genetic studies for depressive and anxiety symptoms including self-reported questionnaires, CVD, and CV risk factors, the estimated heritability was moderate to high (Amare et al., Reference Amare, Schubert, Klingler-Hoffmann, Cohen-Woods and Baune2017; Carney et al., Reference Carney, Freedland, Rich and Jaffe1995; Hettema et al., Reference Hettema, Neale and Kendler2001; McCaffery et al., Reference McCaffery, Frasure-Smith, Dube, Theroux, Rouleau, Duan and Lesperance2006; Silberg et al., Reference Silberg, Heath, Kessler, Neale, Meyer, Eaves and Kendler1990). The estimated heritability in our study was in line with previous findings. Furthermore, we observed shared genetic factors between depressive and anxiety symptoms and CV risk estimate even after adjusting for baseline CV risk scores for the bivariate analysis with CV risk scores at follow-up. These findings could enhance the case for shared genetic vulnerability in the association between depressive or anxiety symptoms and CVD or CV risk factors.
There are two studies regarding the genetic association between depressive symptoms and CVD or CV risk factors. In a male twin study of the Vietnam Era Twin Registry, the genetic correlation between depressive symptoms and hypertension was 0.19 and the genetic correlation between depression and heart disease was 0.42 (Scherrer et al., Reference Scherrer, Xian, Bucholz, Eisen, Lyons, Goldberg and True2003). In the Erasmus Rucphen Family study, the genetic correlations between depressive symptoms (assessed using the CES-D scale and the Hospital Anxiety and Depression Scale) and serum lipid levels ranged from 0.25 to 0.31. In that study, the genetic correlations of depressive symptoms with blood pressure and serum glucose level were not significant (Lopez-Leon et al., Reference Lopez-Leon, Aulchenko, Tiemeier, Oostra, van Duijn and Janssens2010). While these two studies revealed evidence for a genetic connection between depressive symptoms and CV risk factors and concurrent heart disease, we did not find any study that demonstrated a genetic correlation between depressive or anxiety symptoms and estimated CV risk scores, a composite value of several CV risk factors. We also found that the genetic correlation with estimated CV risk scores were stronger than the genetic correlation with individual CV risk factors. Our results, along with previous studies, support the possibility of the presence of pleiotropic (common) genes influencing the concurrent as well as future associations between depressive and anxiety symptoms and CV risk estimates in middle-aged men and women. Our observations could be in line with an overlap of depression and CVD linkage signals that suggest the potential for common candidate genes related to inflammation or serotonin that contribute to both depression and CVD (Lopez-Leon et al., Reference Lopez-Leon, Aulchenko, Tiemeier, Oostra, van Duijn and Janssens2010; McCaffery et al., Reference McCaffery, Frasure-Smith, Dube, Theroux, Rouleau, Duan and Lesperance2006). Further research is necessary to investigate genes involved in predicting future CVD in individuals with depressive or anxiety symptoms.
Of note, we found significant shared environmental correlations between depressive and anxiety symptoms and the CV risk score, whereas we did not observe non-shared environmental factors in those relationships by the analysis of within-monozygotic twin pairs after adjusting for covariates. At least, those shared environmental factors may be explained by uncontrolled factors such as dietary habit of families. We also demonstrated the importance of education, BMI, and exercise as modifiable risk factors related to higher CV risk scores.
However, there are limitations that should be addressed. First, the assessment of depressive and anxiety symptoms was based on self-reported questionnaires. While these scales are validated tools for predicting CV outcomes (Shibeshi et al., Reference Shibeshi, Young-Xu and Blatt2007; Wassertheil-Smoller et al., Reference Wassertheil-Smoller, Shumaker, Ockene, Talavera, Greenland, Cochrane and Dunbar-Jacob2004), the Korean version of these questionnaires has not been validated for predicting CV outcomes. Second, the tools for estimating 10-year CV risk may under- or overestimate observed CVD risk according to population characteristics (Lloyd-Jones et al., Reference Lloyd-Jones, Huffman, Karmali, Sanghavi, Wright, Pelser and Goff2016). Based on a previous study, The Adult Treatment Panel III- based risk estimation may overestimate the risk of CHD in the Korean population (Jee et al., Reference Jee, Jang, Oh, Oh, Lee, Park and Woodward2014). Third, the associations were not fully adjusted for other potential confounding factors and therefore residual confounding factors may influence current associations. Finally, the subjects may be more concerned with their health compared with the general population, and consequently, volunteer bias cannot be excluded. Nevertheless, the current findings were compatible with established associations between depressive and anxiety symptoms and CV risk factors. Furthermore, the current findings could provide more evidence for the genetic correlations between those traits.
In conclusion, we found shared genetic and environmental influences on the relationship of depressive and anxiety symptoms with concurrent and future CV risk estimates. Further studies are needed to better elucidate the genetic origin and pathway explaining the associations between the psychological distress and CVD development and outcome.
Acknowledgments
This study was supported by the 2016 Inje University Research grant. None of the authors have any conflicts of interest to report.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.






