Iron-deficiency anaemia (IDA) is still one of the most common nutritional disorders globally. Furthermore, it is regarded as a public health problem in both developed and developing countries(Reference McLean, Cogswell and Egli1) with a global prevalence of more than 30 %(Reference Neymotin and Sen2) and Africa having the highest prevalence of IDA of 68·0 % in non-pregnant women(Reference McLean, Cogswell and Egli1). The high prevalence of IDA significantly affects the health and economy of developing countries: the median value of physical productivity losses per annum due to IDA was found to be 0·57 % of gross domestic product(Reference Zimmermann and Hurrell3). Another major global health concern is the increased prevalence of obesity. Although obesity and IDA represent opposite ends of the malnutrition spectrum(Reference Cepeda-Lopez, Aeberli and Zimmermann4), cross-sectional studies in developing countries have shown that obese people have an increased risk of Fe deficiency(Reference Cepeda-Lopez, Osendarp and Melse-Boonstra5–Reference Zafon, Lecube and Simó7). High serum ferritin levels are observed in the obese(Reference Zafon, Lecube and Simó7). Serum ferritin accurately reflects body Fe stores in healthy individuals, but ferritin is an acute-phase reactant and obesity is associated with a chronic, low-grade inflammatory status and thus elevated ferritin levels(Reference Lecube, Carrera and Losada8). The limited data available from small studies suggest that chronic adiposity-related inflammation, rather than decreased dietary intake or increased Fe requirements due to larger blood volume, may be the factor causing IDA in obese respondents(Reference Cepeda-Lopez, Osendarp and Melse-Boonstra5). IDA in obese individuals thus has multifactorial causes, including impairment to the intestinal Fe uptake and inadequate Fe bioavailability as a result of inflammation(Reference Zafon, Lecube and Simó7). Low dietary Fe intakes are usually caused by poverty, as low-income families consume mainly a carbohydrate-rich diet, based on plant staples, and often have limited dietary diversity(Reference Lynch9). Furthermore, obesity is also associated with poverty as a result of the higher intakes of energy-dense foods(Reference Townsend10).
South Africa is a country in nutrition transition that is characterised by a ‘double burden of malnutrition’, with under- and overnutrition occurring in the same population(Reference Cepeda-Lopez, Osendarp and Melse-Boonstra5, Reference Zimmermann, Zeder and Muthayya6). In South Africa, it was found that black African women in urban areas have a high prevalence of obesity and in 1990, 51 % of women aged 60 years and older were identified as being obese (BMI ≥ 30·0 kg/m2). Furthermore, the prevalence of anaemia in older adults has been reported to range from 14 % to 25 % with the most common cause of anaemia being the type associated with chronic disease(Reference Charlton, Ferreira and du Plessis11). The present study was conducted among an elderly community in Sharpeville, South Africa. Previous research in the same community found that the prevalence of obesity and IDA was 54·1 %(Reference Oldewage-Theron, Salami and Zotor12) and 13·2 %(Reference Oldewage-Theron, Samuel and Grobler13), respectively, in women. These studies did not examine the association between IDA and obesity. Research has shown that the combination of IDA and obesity may be more harmful to health than either of these conditions in isolation(Reference Zimmermann and Hurrell3). Furthermore, few studies have investigated the association between poor Fe status and obesity in nutrition transition countries, where both conditions are prevalent(Reference Cepeda-Lopez, Osendarp and Melse-Boonstra5). The objective of the present study was thus to determine the relationship between poor Fe status and overweight or obesity in an elderly community in Sharpeville, South Africa.
Methods
Research design
The present study was a cross-sectional, observational baseline survey. This study was conducted according to the ethics guidelines laid down in the Declaration of Helsinki and by the South African Medical Research Council(14) and all procedures involving human subjects were approved by the University of the Witwatersrand's Medical Ethics Committee for Research on Human Beings (M070826). Written informed consent was obtained from the respondents after an explanation of the objectives and study procedures.
The project was undertaken among the elderly attending a day-care centre in Sharpeville, situated in the Vaal region of South Africa, during October to November 2011. The day-care centre offers breakfast and lunch as well as skills training and religious activities aimed at the low-income elderly (aged 60 years and above) during weekdays. An earlier study had established the high prevalence of poverty, food insecurity, malnutrition and poor health in the Vaal region(Reference Oldewage-Theron, Salami and Zotor12).
Exclusion criteria included conditions that could influence body Fe stores, namely: reporting of haemorrhage in the preceding 6 months, Fe supplementation during the previous year, blood donation in the preceding 6 months and alcohol consumption of more than 50 g/d.
Sampling
The sample size calculation(15) used in the present study was as follows:
where Z is the Z value (e.g. 1·96 for a 95 % confidence interval); p is the percentage picking a choice, expressed as a decimal (p = 0·50 was used for sample size needed); and c is the confidence interval, expressed as a decimal (8·5).
A total of 100 respondents were thus needed for the present cross-sectional survey to obtain statistically representative data for this community. However, 104 out of 400 elderly persons (26 %) attending this faith-based day-care centre gave their written consent to participate in the study.
Blood analysis
To determine Fe status, fasting (>8 h) venous blood samples were drawn by two nursing sisters and a haematologist with a Vacutainer needle and minimal use of tourniquets. Blood sampling was carried out at the care centre between 07.00 and 10.00 hours after participants had been sitting for 15 min. The blood was placed on ice until separation, within 2 h of blood collection. Serum was harvested by low-speed centrifugation at 4°C and aliquoted into individual tubes. Serum and plasma were stored at −80°C for 2 weeks. The following analyses were performed: serum Fe (colorimetric; KonelabTM, Thermo Scientific, Waltham, MA, USA), ferritin (immunoturbidometric method; AIA FERR™, Tosoh Corporation, Yamaguchi, Japan), Hb (cyanomethaemoglobin colorimetric method; Sysmex, Randburg, South Africa), haematocrit (numeric integration; Sysmex), red blood cell count (cell-counting auto analyser; Sysmex), transferrin (immunoprecipitation; Konelab™), mean corpuscular volume (MCV; cell-counting auto analyser; Sysmex) and high-sensitivity C-reactive protein (hs-CRP; immunoprecipitation; Konelab™).
Dietary intakes
It is a known fact that measuring dietary intake data accurately is a challenge; therefore, when 24 h recall questionnaires are used to determine dietary intakes, the optimum practice would be to have at least three randomised days’ records to cover weekday variations and also because associated eating patterns exist for consecutive days(Reference Gibson16). In the present study, three consecutive days were selected from Sunday to Tuesday. The reason for this choice was that the elderly respondents receive lunch at the care centre and the majority of the respondents attend from Mondays to Fridays because of cultural and religious activities being presented on these days. On Thursdays and Fridays, only specific small groups such as the choir or management team meet at the centre. A menu is planned two weeks in advance by the centre management to prevent menu fatigue and assure dietary variety. Furthermore, no leftovers are recycled in the next day's menu. A Sunday was included to represent the dietary intakes on weekends and measured on a Monday. Saturdays were excluded due to potential memory degradation. This may result in potential selection bias; however, these were the only days on which it would be practical to cover as many of the respondents as possible for the dietary intake measurements. A four-stage, multiple-pass interviewing procedure described by Gibson(Reference Gibson16) was used for the 24 h recall questionnaire data collection over a period of three consecutive days (Sunday to Tuesday). Trained fieldworkers used food models and household measuring tools (cups, plates, spoons) to assist the respondents in estimating portion sizes. Dietary intake data were analysed by a registered dietitian using the Foodfinder® version 3 software program, developed by the Medical Research Council and based on the South African food composition tables(Reference Langenhoven, Kruger and Gouws17). The median nutrient intakes of the three days was calculated for total energy (kJ/d), total protein (g/d), total Fe, haem and non-haem Fe (mg/d), Ca (mg/d) and vitamin C (mg/d).
Anthropometry
Anthropometric measurements included body weight and height, measured according to standard procedures(Reference Lohman, Roche and Martorell18) with a calibrated Philips electronic scale (Amsterdam, The Netherlands), model HF350 (135 kg/100 g), and a Scales 2000 (Durban, South Africa) stadiometer, respectively. All measurements were taken twice and the average of the two measurements recorded. BMI was calculated using weight (kg) divided by height squared (m2) and categorised according to the WHO cut-off points(19).
Data analysis
Data were analysed using the statistical software package IBM SPSS Statistics 21·0. Measured BMI was used to categorise the respondents into normal weight (≥18·5 to <25·0 kg/m2), overweight (≥25·0 to <30·0 kg/m2) and obese (≥30·0 kg/m2) groups(19). Descriptive statistics were determined for the different BMI groups (medians for not normally distributed data, means and standard deviations for normally distributed data), and the two-tailed independent t test was conducted to determine significant differences (P ≤ 0·05) between the groups. Two-tailed paired t tests were used to determine significant differences (P ≤ 0·05) within the groups. Pearson correlations (P ≤ 0·05) were used to determine significant relationships between dietary intakes or BMI and haematological and biochemical parameters, as well as between the various haematological and biochemical parameters. Only significant relationships are reported in the results. Linear regression analysis was done to determine the predictors of BMI and hs-CRP. The first step in the linear regression model was to explore possible predictor variable(s) by using the Automatic Linear Modelling whereby all blood variables were posted as possible predictors (n 16), with model accuracy prediction of 75·4 %. Further factor analysis was carried out to reduce variables to remain with high predictors, filtering out the confounding variables, as well as minimising redundancy effects. Thereafter the remaining variables (n 6) with prediction accuracy of 89·0 % for hs-CRP were then analysed through linear regression analysis. The same procedure was followed for BMI linear regression modelling.
Results
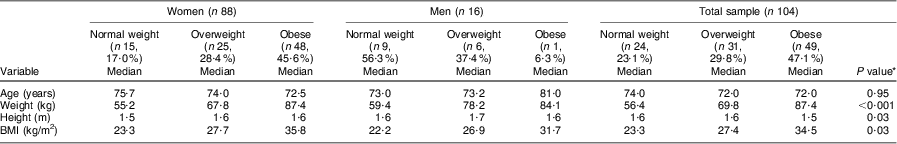
The majority of the women were overweight (28·4 %) or obese (54·6 %), whereas 50·0 % of the men were normal weight with only 37·4 % overweight and 6·3 % obese (Table 1). Only one man and one woman were underweight; for statistical significance, these values were omitted for the biochemical and dietary intake results.
Table 1 BMI category of the elderly participants aged ≥60 years by gender, Sharpeville, South Africa, October–November 2011

The results in Table 2 show that significant differences were observed for both weight (P < 0·001) and height (P = 0·03) and BMI (P = 0·03) in the different BMI groups for both the women and men. The women's age decreased progressively with increasing BMI compared with the men, whose age increased progressively with increasing BMI; however, this was not significant.
Table 2 Comparison of different BMI categories of the elderly participants aged ≥60 years with respect to median age and anthropometric parameters, Sharpeville, South Africa, October–November 2011

*Significance of the difference between BMI categories.
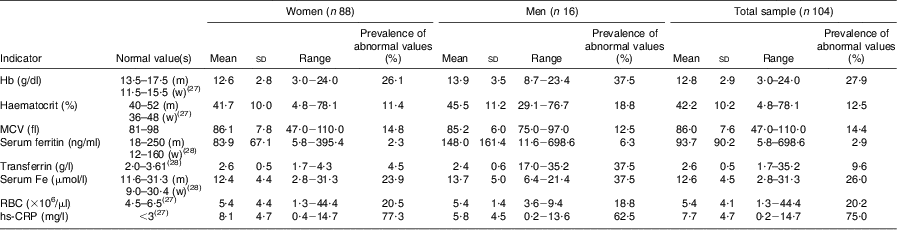
The results in Table 3 show normal mean serum levels for all Fe status parameters in both the women and men; however, 26·1 % and 37·5 % of the women and men, respectively, had low Hb levels and 11·4 % of women and 18·8 % of men had decreased haematocrit. Based on serum Fe, 23·9 % and 37·5 % of the women and men respectively had poor Fe status, compared with 2·3 % and 6·3 % respectively based on serum ferritin and 4·5 % and 37·5 % respectively based on transferrin. The prevalence of microcytosis (decreased MCV; an indication of Fe deficiency) was 14·8 % in women and 12·5 % in men. For the total sample, 58 % of the respondents had normal Fe status, 15 % were classified as Fe depleted (based on decreased ferritin, transferrin or serum Fe levels), 9 % were Fe deficient (based on decreased Fe parameters + decreased MCV but normal Hb) and 13% were Fe-deficient anaemic (based on low serum Fe parameters + low MCV + low Hb). Ten per cent of the respondents had low Hb levels with no other low Fe status parameters, and were thus anaemic due to other causes. The mean hs-CRP levels were higher than the normal level in both the women and men, and 77·3 % and 62·5 %, respectively, had hs-CRP levels higher than 3 mg/l.
Table 3 Prevalence of abnormal biochemical and iron status indicators of the elderly participants aged ≥60 years by gender, Sharpeville, South Africa, October–November 2011

MCV, mean corpuscular volume; RBC, red blood cell count; hs,-CRP, high-sensitivity C-reactive protein; m, men; w, women.
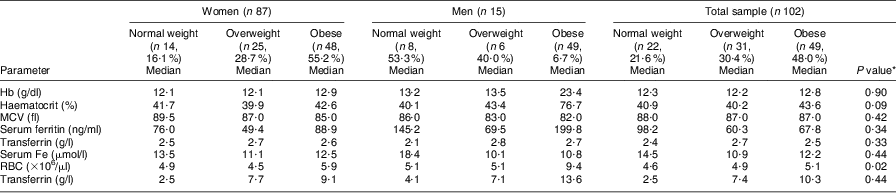
The results in Table 4 show that higher hs-CRP levels were observed in the overweight and obese categories of the total sample as well as for both genders; however, these differences were not significant. No significant differences were observed in any of the serum Fe status parameters or hs-CRP between the BMI categories and between genders. Most of the serum Fe parameters showed normal levels across all BMI groups except for serum Fe, which showed marginally lower than normal levels in the overweight and obese men. Median Hb levels increased progressively with increased BMI; however, 21·4 %, 20·0 % and 40·0 % of the women in the normal-weight, overweight and obese groups had Hb < 11·5 g/dl, respectively, compared with 37·5 % and 66·7 % of the men in the normal-weight and overweight groups, respectively, with Hb < 13·5 g/dl.
Table 4 Comparison of different BMI categories of the elderly participants aged ≥60 years with respect to median levels of biochemical and haematological parameters, Sharpeville, South Africa, October–November 2011

MCV, mean corpuscular volume; RBC, red blood cell count; hs,-CRP, high-sensitivity C-reactive protein.
*Significance of the difference between BMI categories.
Depleted Fe stores (based on serum ferritin levels) occurred in 4·0 % (n 1) and 2·1 % (n 1) of the women in the overweight and obese groups, respectively, compared with 1·7 % (n 1) of the men in the overweight group. For the total sample, 6·5 % of the overweight and 2·0 % (n 1) of the obese group had depleted Fe stores. On the other hand, the prevalence of women with elevated ferritin levels was 21·4 %, 4·0 % and 30·0 % in the normal-weight, overweight and obese categories, compared with only 12·5 % of men in the normal-weight category. Elevated ferritin levels were observed in 18·8 % of the normal-weight group compared with 3·2 % (n 1) in the overweight and 28·6 % in the obese group among the total sample of respondents. The prevalence of microcytosis (based on MCV < 81 fl) was 14·3 % in the normal-weight, 12·0 % in the overweight and 20·0 % in the obese women, whereas 12·5 % (n 1) of the normal-weight and 16·7 % (n 1) of the overweight men presented with microcytosis. For the total sample of elderly respondents, the prevalence of microcytosis was 13·6 %, 12·9 % and 20·4 % in the normal-weight, overweight and obese group, respectively. Serum Fe levels decreased progressively with increasing BMI, whereas transferrin showed higher levels in the overweight and obese groups compared with the normal-weight group.
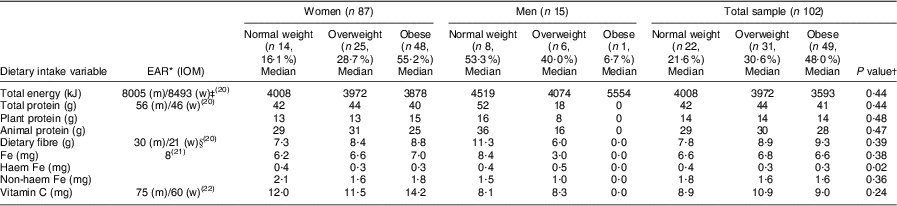
Regarding the dietary assessment, the main source of food intake was starchy foods that provided carbohydrates with little vegetable and fruit intakes. Chicken, beef and milk appeared as protein sources, but in small portion sizes. The nutrient intakes are presented in Table 5. The men showed consistently higher total energy, total protein and dietary fibre intakes than the women, although in both genders total energy intakes were low compared to the estimated energy requirements of 8493 kJ and 8005 kJ for healthy moderately active women (aged ≥51 years) and men (aged ≥70 years), respectively(20). For the total group, the energy intakes decreased progressively with an increased BMI. Using the Estimated Average Requirements, the total protein, dietary fibre(20), Fe(21) and vitamin C(22) intakes were low for both genders. However, no significant differences were observed in nutrient intakes between the BMI categories for both women and men, except for haem Fe intake that was significantly (P = 0·02) lower in the overweight and obese groups of women compared with the normal-weight group. The opposite was observed in the men, where the overweight group showed significantly higher haem Fe intakes in the overweight and obese (n 1) groups compared with the normal-weight group. The total protein, animal protein and vitamin C intakes decreased progressively with higher BMI in both women and men; however, dietary Fe intake increased progressively with higher BMI among women (Table 5).
Table 5 Comparison of different BMI categories of the elderly participants aged ≥60 years with respect to median nutrient intake levels, Sharpeville, South Africa, October–November 2011

EAR, Estimated Average Requirement; IOM, Institute of Medicine; m, men; w, women; EER, estimated energy requirement; PAL, physical activity level.; AI, Adequate Intake.
*EAR(20–22) for women aged 51–70 years (mean age 67·0 (sd 21·7) years) and men aged ≥70 years (mean age 73·4 (sd 5·6) years).
†Significance of the difference between BMI categories.
‡EER for women were calculated based on mean age (67·0 (sd 21·7) years), mean height (1·6 (sd 0·1) m) and weight (75·0 (sd 17·7) kg) for the women and moderate activity levels. The EER of 8493 kJ was thus calculated as 448 – (7·95 × age (67))+PAL for low active (1·5) × (11·4 × weight (75) × height (1·6)) × 4·18 kJ(20). EER for men were calculated based on mean age (73·5 (sd 5·6) years), mean height (1·7 (sd 0·1) m) and mean weight (67·4 (sd 12·7) kg) for the men and moderate activity levels. The EER of 8005 kJ was thus calculated as 448 – (7·95 × age (75))+PAL for low active (1·5) × (11·4 × weight (67·4) × height (1·7)) × 4·18 kJ(20).
§AI where no EAR is available.
A significant positive relationship was observed between BMI and hs-CRP levels. hs-CRP was negatively correlated to serum Fe levels. A significant positive correlation was found between the respondents with IDA (low Hb levels) and those with low Fe stores (low serum ferritin levels) and microcytosis (low MCV levels). No significant relationships existed between BMI and the Fe status parameters and dietary intake or between the Fe status parameters and dietary intake (Table 6). A significant relationship between serum ferritin and hs-CRP was expected, but both parameters are reactive proteins and can be influenced by acute and chronic inflammation. The regression analyses (R = 0·491, R 2 = 0·241) carried out on the predictors of hs-CRP from a range of variables (BMI, dietary intake and Fe status parameters) showed that only serum Fe (P < 0·001) and BMI (P = 0·00) were predictors of hs-CRP in the present study, where the model fitness was significant (P < 0·001). Similarly, the only predictor of BMI was hs-CRP (R = 0·354, R 2 = 0·125, P = 0·00) with model fitness significance of P < 0·001.
Table 6 Significant correlations between BMI and haematological and biochemical parameters among the elderly participants aged ≥60 years, Sharpeville, South Africa, October–November 2011

hs,-CRP, high-sensitivity C-reactive protein; MCV, mean corpuscular volume.
Discussion
Fe is found in every living cell and is an essential element for maintaining health, and although Fe is conserved efficiently through the body's homeostatic mechanism, poor Fe status can still arise. IDA is still considered the most common nutritional deficiency in the world. The elderly is one of the most at-risk populations for poor Fe status due to insufficient dietary intake, malnutrition, reduced gastric acid production caused by ageing, occult gastrointestinal bleeding, inflammatory bowel disease or malignant conditions. Increased mortality has been associated with mild IDA in the elderly and it is thus important to make correction of poor Fe status a priority in this population group(Reference Clark23). The present study is a follow-up to previous published data in the same elderly community(Reference Oldewage-Theron, Samuel and Grobler13). In the present study, the men were found to have a higher prevalence of IDA (37·5 %) than the women (26·1 %). This is the opposite of what was found in the previous study where the women had a higher prevalence of IDA (13·2 %) than the men (12·5 %)(Reference Oldewage-Theron, Samuel and Grobler13). Microcytic anaemia is mostly associated with IDA(Reference Oldewage-Theron, Samuel and Grobler13) and in this elderly community, the prevalence of microcytic anaemia was 14·8 % and 12·5 % in the women and men, respectively. In both women and men, the prevalence of IDA and microcytic anaemia has increased since 2008.
The anthropometric data showed that 50·0 % of the men had normal BMI values; only 15·9 % of the women had normal BMI values, while 28·4 % and 54·6 % were overweight or obese, respectively. Mean BMI was significantly higher for women than men (P = 0·01). These findings are similar to those in 2008, when 30·5 % of the women were overweight and 47·2 % were obese, with 65·8 % of the men having normal weight and 18·4 % and 15·8 % being overweight and obese, respectively. Both the anthropometric and Fe status results confirmed that South Africa, a country in nutrition transition, is consistently characterised by a ‘double burden of malnutrition’ with under- and overnutrition occurring in the same population(Reference Cepeda-Lopez, Osendarp and Melse-Boonstra5, Reference Zimmermann, Zeder and Muthayya6). It is further known that hunger and obesity can exist within the same household(Reference Townsend10, Reference Scheier24). This was previously confirmed in this elderly community(Reference Oldewage-Theron, Salami and Zotor12) and it seems as if it has remained unchanged.
It has recently been found that obesity is associated with chronic, low-grade systemic inflammation which may be associated with IDA, specifically raised ferritin, low serum Fe and low Hb levels(Reference Ausk and Ionnauou25). In the present study, higher hs-CRP levels were observed in the overweight and obese categories of both women and men. Furthermore, the positive relationship between hs-CRP and BMI indicated chronic inflammation in the higher BMI groups, which is consistent with recent research developments(Reference Cepeda-Lopez, Osendarp and Melse-Boonstra5, Reference Ausk and Ionnauou25). The negative relationship between hs-CRP and serum Fe indicated that lower serum Fe levels were related to the inflammation linked with higher BMI. Fe status parameters have been found to be significantly related to BMI(Reference Neymotin and Sen2). This relationship was confirmed by the regression analyses indicating that serum Fe and BMI were the only predictors of hs-CRP levels in the present study. Although no direct significant relationships between BMI and the other Fe status parameters were observed, it can be concluded that obesity-linked inflammation may have an effect on Fe status, specifically serum Fe levels, in these elderly respondents. Obesity-related inflammation is further linked to elevated ferritin levels; however, no significant relationship between serum ferritin and BMI or hs-CRP was found despite the elevated ferritin levels observed in 21·4 %, 4·0 % and 30·0 % of women in the normal-weight, overweight and obese categories, respectively.
In the present study, consistently poor dietary diversity resulting in low nutrient intakes was reported when compared with the Dietary Reference Intakes. Dietary Fe intakes were similar across the BMI categories of both men and women, except for haem Fe intake that was significantly (P = 0·02) lower in the overweight and obese groups of women compared with the normal-weight group. The opposite was observed in the men, where significantly higher haem Fe intake was found in the overweight and obese groups compared with the normal-weight group. The intakes of total protein, animal protein and vitamin C decreased progressively with higher BMI in both women and men, but no significant differences across the BMI categories or between men and women were observed. These results were similar to those observed in a study conducted in Mexican women(Reference Cepeda-Lopez, Osendarp and Melse-Boonstra5). Similarly to the Mexican study, lower energy intakes were reported in the overweight and obese women than in the normal-weight women; however, the same was not observed in the men. No significant relationships between the dietary intake variables and Fe status parameters were observed and thus it cannot be concluded that the poor dietary intakes were responsible for the poor Fe status in this elderly community.
Conclusions and recommendations
It can be concluded that poor Fe status as well as overweight and obesity are persistent public health problems in this elderly community, as the prevalence of IDA, based on Hb and serum ferritin levels, has increased since 2008 and the prevalences of overweight and obesity have remained similar to the results in 2008. Because obesity is characterised by chronic low-grade inflammation, it may be related to the characteristics of anaemia inflammation such as raised serum ferritin, low serum Fe and low Hb levels(Reference Ausk and Ionnauou25). hs-CRP was positively associated with BMI, thus inflammation was associated with overweight and obesity in the present study. BMI was negatively associated with serum Fe levels in the present study and thus proves the hypothesis that Fe status is affected by overweight and obesity and its associated inflammation in the elderly. A relationship between obesity-related chronic, low-grade inflammation and poor Fe status has been found in adults(Reference Cepeda-Lopez, Osendarp and Melse-Boonstra5, Reference Ausk and Ionnauou25), specifically women(Reference Neymotin and Sen2, Reference Zimmermann, Zeder and Muthayya6), as well as in children(Reference Cepeda-Lopez, Osendarp and Melse-Boonstra5, Reference Zimmermann, Zeder and Muthayya6), but the significance of the present study is that this relationship was also confirmed for elderly black persons in South Africa. The elderly group is especially at risk of impaired nutritional status with increasing age and the many side-effects of malnutrition may exacerbate one another(Reference Cowan, Roberts and Fitzpatrick26). Furthermore, the elderly are also at risk of poor Fe status due to many factors including low dietary Fe intakes, Fe malabsorption due to altered gastrointestinal conditions, medication (aspirin or non-steroidal anti-inflammatory drugs) usage, inflammation and chronic diseases(Reference Clark23); and overweight and obesity thus exacerbate the situation, leading to a more progressive chronic inflammation. Other studies have shown that the link between Fe depletion and obesity may be due to increased circulating hepcidin levels due to inflammation. A limitation of the present study was that hepcidin levels were not measured and other confounding factors that may influence Fe status, body weight and inflammation were not included. Another limitation was the small sample size of the men. Furthermore, the number of respondents per group was not proportionately distributed due to actual measurement outcomes, resulting in small sample sizes per BMI group. The results should thus not be generalised to other elderly communities. Furthermore, measuring dietary intake over three consecutive days may pose another limitation.
It has been reported that dietary diversification is the optimal solution for addressing poor Fe status, but poverty and dietary preferences of people remain the main barriers to this approach(Reference Lynch9). Furthermore, the increasing prevalence of obesity globally is also recognised as a major public health concern(Reference Neymotin and Sen2). In the present study, however, the poor dietary intakes were not related to IDA. It is thus recommended that the association between obesity and Fe status in the elderly be further investigated with a focus on the potential cause-and-effect mechanisms for this relationship.
Acknowledgements
Source of funding: This study was funded by the National Research Foundation (NRF) and the Vaal University Research and Innovation Committee. NRF had no role in the design, analysis or writing of this article. Conflict of interest: There is no conflict of interest in this study. Ethics statement: All research procedures were approved by the University of the Witwatersrand's Medical Ethics Committee for Research on Human Beings (M070826). The research was conducted according to the Declaration of Helsinki and the South African Medical Research Council's guidelines for research on human beings. Written informed consent was obtained from the respondents after an explanation of the objectives and study procedures. Authors’ contributions: W.H.O.-T. was responsible for the research question formulation and study design. The dietary intake and anthropometric data were collected by W.H.O.-T. and A.A.E.; the biochemical and haematological data were collected and analysed by C.J.G. Data capturing, cleaning and analyses were done by W.H.O.-T. and A.A.E. All three authors participated in all stages of writing the manuscript. Acknowledgements: The authors acknowledge the University of the Witwatersrand's Medical Ethics Committee for Research on Human Beings for approving the study, as well as the NRF and the tertiary institution for funding this project. The management of the care centre and participants in this study are also acknowledged.