Vitamin D and folate are critical for cell signalling and functioning and can influence processes related to cancer risk and prognosis( Reference Ulrich 1 , Reference Lamprecht and Lipkin 2 ). The anti-carcinogenic effects of vitamin D are mediated through its diverse effects on cellular differentiation, proliferation, apoptosis and angiogenesis( Reference Deeb, Trump and Johnson 3 ). Folate and other B vitamins such as vitamin B12 play important roles as co-factors in one-carbon metabolism, which is essential for nucleotide synthesis and DNA methylation( Reference Ulrich 1 , Reference Lamprecht and Lipkin 2 ). Disruption of these processes leads to abnormalities in DNA methylation and imbalance of DNA precursors, leading to aberrant DNA synthesis and repair( Reference Ulrich 1 , Reference Lamprecht and Lipkin 2 ).
Globally, colorectal cancer is the second most commonly diagnosed cancer in women and the third one in men( Reference Jemal, Bray and Center 4 ). Studies have demonstrated that a higher pre-diagnostic vitamin D status is associated with a decreased colorectal cancer risk( Reference Jenab, Bueno-de-Mesquita and Ferrari 5 – Reference Yin, Grandi and Raum 7 ) and there is emerging evidence that higher plasma vitamin D concentrations can also improve survival in patients with colorectal cancer( Reference Ng, Meyerhardt and Wu 8 , Reference Ng, Sargent and Goldberg 9 ). On the other hand, folate appears to have a dual effect on colorectal carcinogenesis, depending on the timing and dosage( Reference Kim 10 – Reference Song, Medline and Mason 13 ). In normal colorectal epithelial cells, folate deficiency predisposes to colorectal carcinogenesis whereas in established neoplasms, folate deficiency appears to have an inhibitory effect on colorectal carcinogenesis and high folate levels may promote the growth of an existing cancer( Reference Kim 10 , Reference Ulrich and Potter 11 , Reference Song, Medline and Mason 13 , Reference Ulrich and Potter 14 ). Vitamin B12 is an important co-factor in folate metabolism but studies relating plasma vitamin B12 to colorectal cancer risk and prognosis are relatively very few and results equivocal( Reference Dahlin, Van Guelpen and Hultdin 15 , Reference Eussen, Vollset and Hustad 16 ). Because there is paucity of information on how these nutritional biomarkers impact on colorectal cancer survival, more studies are needed to discern their effects.
Plasma vitamin biomarker levels may vary based on dietary and other health-related practices in different countries. However, studies comparing this wide range of vitamin biomarker levels across geographical regions are scarce. A characterisation of this variability may be important for studying the association of these biomarkers with colorectal cancer prognosis. In order to discern geographical differences in plasma 25-hydroxyvitamin D3 (25(OH)D3), folate and vitamin B12, we evaluated levels and predictors among colorectal cancer cases and controls in an international pilot study with participants from Heidelberg (Germany), Seattle (WA, USA), and Tampa (FL, USA).
Methods
Study groups
The study participants from the USA are members of a colorectal cancer prognosis study (ColoCare), with sites at the Fred Hutchinson Cancer Research Center, Seattle (WA, USA), H. Lee Moffitt Cancer Center and Research Institute, Tampa (FL, USA), and the National Center for Tumor Diseases, German Cancer Research Center, Heidelberg (Germany). The objective of our consortium is to identify the factors that determine short-term survival in a prospective cohort of colorectal cancer patients. These include inherited genetic and tumour characteristics that affect early detection, treatment response and prognosis as well as health behaviours that patients adopt that may have health outcomes. German samples included in this pilot study were obtained from participants of the Darmkrebs Chancen der Verhütung durch Screening (DACHS) study at the German Cancer Research Center in Heidelberg (Germany)( Reference Brenner, Chang-Claude and Seiler 17 ). Samples included from Tampa were collected as part of Moffitt's Total Cancer Care Program( Reference Fenstermacher, Wenham and Rollison 18 ).
Eligible for the study were colorectal cancer patients, at least 18 years of age, with a first diagnosis of invasive colon or rectal cancer (stages I–IV) receiving care at any of the ColoCare consortium/DACHS sites. This pilot study involved 149 colorectal cancer cases and ninety-one age- and sex-matched controls (Heidelberg, forty cases and forty controls; Seattle, ninety-one cases and thirty-three frequency-matched controls; Tampa, eighteen cases and eighteen controls), recruited between the years 2006 and 2010 and whose biospecimens were obtained within the same time frame with identical blood-draw protocols at each of the sites. Blood samples were obtained at the time of recruitment into the study. For the cases, the samples were pre-treatment samples obtained at the time of surgery. In the DACHS study, control subjects were community-based and randomly selected from population registries. They were frequency-matched to cases on age, sex and county of residence. We excluded controls with a history of colorectal cancer; otherwise eligibility criteria were the same as for the cases. Controls were contacted through mail and follow-up calls, and interviews were conducted at their homes. The participation rate among eligible control participants was a little higher than 50 %( Reference Brenner, Chang-Claude and Seiler 17 ). The controls in Moffitt, Tampa were frequency matched to the cases on age (5-year intervals), sex and race (white non-Hispanic). Controls in Seattle were drawn from participants in a screening and surveillance colonoscopy programme who had no evidence of pathology within the colon and were matched to the cases for age, sex and race.
This study was conducted according to the guidelines laid down by the Declaration of Helsinki and all procedures involving human subjects were approved by the Institutional Review Boards of the German Cancer Research Center (DKFZ), the Fred Hutchinson Cancer Research Center, Seattle (WA, USA) and H. Lee Moffitt Cancer Center and Research Institute, Tampa (FL, USA). Written informed consent was obtained from all the subjects.
Laboratory analysis
Plasma 25(OH)D3 was measured by reverse-phase HPLC in the Biochemistry and Biomarkers Laboratory of the Division of Preventive Oncology, German Cancer Research Center, DKFZ (Dr Owen). The intra-assay and inter-assay CV of 25(OH)D3 at a concentration of 50 ng/ml were 2·3 and 3·1 %, respectively. Plasma folate and vitamin B12 were quantified using SimulTRAC® B12/FOLATE – SNB 57Co/125I Radioassay Kit (MP Biomedicals) in the Biomarker Laboratory (Dr Song) at the Fred Hutchinson Cancer Research Center with intra-assay CV of 3·1 and 3·5 % and inter-assay CV of 7·0 and 5·1 % for folate and vitamin B12, respectively.
Statistical analysis
To account for skewed distributions, Wilcoxon or Kruskal–Wallis tests were used to test for the differences in biomarker concentration by age group (18–59, 60–69 and 70–80 years), case status, tumour stage (I–IV), study site (Heidelberg, Seattle and Tampa), and season of blood draw (spring: March–May; summer: June–August; autumn: September–November; winter: December–February). Because the samples were unequally distributed across seasons at the three study sites, we computed season-corrected levels for the descriptive analyses of 25(OH)D3 in order to give equal weight to all four seasons. Linear regression analysis (using both forward stepwise and enter methods) with log-transformed outcome parameters was used for multivariate analyses. All statistical analyses were conducted using SAS (V9.1.2, SAS Institute Inc.). Statistical significance was defined as P < 0·05 and all statistical tests were two-sided.
Results
Cases and controls were, on average, aged 60·0 (sd 13·3) and 60·9 (sd 13·1) years, respectively. The majority of tumours were of stages III (39·6 %) and IV (26·2 %). Among the cases, the mean 25(OH)D3, folate and vitamin B12 concentrations were 25·6 (sd 13·5) ng/ml, 21·6 (sd 14·0) ng/ml, and 638 (sd 597) pg/ml, respectively, whereas among the controls, they were 25·9 (sd 14·0) ng/ml, 18·0 (sd 13·7) ng/ml, and 515 (sd 241) pg/ml (Table 1).
Table 1. Baseline characteristics of colorectal cancer cases and controls

25(OH)D3, 25-hydroxyvitamin D3.
In multivariate modelling, significant predictors for the biomarkers included season for 25(OH)D3, study site for 25(OH)D3, folate, vitamin B12, and tumour stage for vitamin B12 (Table 2). Probably because of the limited sample size of this pilot study, case status was not statistically significant as a predictor; hence, cases and controls were combined in subsequent analyses. Also, the results from models excluding case-status were identical to those including case-status in the model. The association from controls only was similar, yet did not reach statistical significance. Plasma 25(OH)D3 concentrations were, on average, higher in Heidelberg (31·7 ng/ml; range 11·0–83·0 ng/ml) than in Seattle (23·3 ng/ml; range 4·0–80·0 ng/ml) and Tampa (21·1 ng/ml; range 4·6–51·6 ng/ml) (Table 3). Hypovitaminosis D (plasma 25(OH)D3 <20 ng/ml) was more prevalent in Tampa (54 %) and Seattle (50 %) than in Heidelberg (23 %) (data not shown). On the other hand, plasma folate concentrations were, on average, lower in Heidelberg (11·1 ng/ml; range 0·3–48·2 ng/ml) than in Seattle (25·3 ng/ml; range 3·5–70·3 ng/ml) and Tampa (23·8 ng/ml; range 6·8–69·7 ng/ml). No participants from Seattle and Tampa had folate deficiency (folate <3 ng/ml), while 10 % of those from Heidelberg had folate deficiency. Plasma vitamin B12 concentrations followed the same pattern as folate with lower average values recorded in Heidelberg (395 pg/ml; range 96–1641 pg/ml) than in Seattle (740 pg/ml; range 183–3750 pg/ml) and Tampa (522 pg/ml; range 94–1085 pg/ml).
Table 2. Predictors of plasma 25-hydroxyvitamin D3 (25(OH)D3), vitamin B12 and folate among colorectal cancer cases and controls†
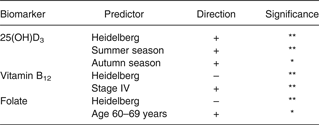
* P < 0·10, ** P < 0·05.
† Cases and controls were combined because case status was not statistically significant.
Table 3. Nutritional biomarker concentrations among colorectal cancer cases and controls in the three study locations
(Mean values and ranges)

25(OH)D3, 25-hydroxyvitamin D3.
Seasonal differences in plasma 25(OH)D3 concentrations were evident in Heidelberg, where the highest plasma 25(OH)D3 concentrations were observed in summer (37·0 ng/ml) and the lowest in spring (21·3 ng/ml), but no such seasonal variation was noted in Seattle and Tampa (Fig. 1).

Fig. 1. (a) Plasma vitamin D (25-hydroxyvitamin D; 25(OH)D) concentration by study site. (b) Plasma folate concentration by study site. (c) Plasma vitamin B12 concentration by study site. The horizontal bars represent medians and the circles represent potential outliers in the data. (d) Seasonal plasma 25(OH)D by study site: ![]() , spring;
, spring; ![]() , summer;
, summer; ![]() , autumn;
, autumn; ![]() , winter. Values are means.
, winter. Values are means.
Discussion
To the best of our knowledge, this is the first study to compare a range of nutritional biomarkers (25(OH)D3, folate and vitamin B12) among colorectal cancer patients and controls in different geographical regions. Our international pilot study revealed differences in biomarker concentrations between the US sites and Germany.
Because dietary sources of vitamin D are very limited, cutaneous synthesis of vitamin D (within the UVB wavelength of 290–315 nm) is a very important source of plasma 25(OH)D3 ( 19 ). The UV radiation required for the synthesis of vitamin D in the skin is more abundant and more functional during summer than during winter( 19 , Reference Kimlin 20 ). Hence, among people who do not use adequate vitamin D supplements or consume vitamin D-fortified foods, season is one of the most important determinants of plasma 25(OH)D3 concentrations.
Thus, the seasonal variations in plasma 25(OH)D3 observed in Heidelberg are in line with expectations. Somewhat surprising is the lack of seasonal variation and the low plasma 25(OH)D3 concentrations observed in Seattle and Tampa. Of the three sites, Tampa has the highest daytime temperatures during summer but the participants from Tampa had the lowest plasma 25(OH)D3 concentrations during summer. The differences in plasma 25(OH)D3 concentration during summer among participants in our study could reflect the extent of outdoor activities and possible sun-avoidance behaviours (including sunscreen use) in the three cities. In Heidelberg, people are more likely to commute with bicycles or walk than use cars, whereas people are more likely to practise sun-avoidance in Florida( Reference Levis, Gomez and Jimenez 21 ).
Few studies have examined the impact of season on 25(OH)D3 concentrations in lower altitude regions in the USA. Although a previous study in South Florida reported a seasonal variation in serum 25(OH)D3 concentrations( Reference Levis, Gomez and Jimenez 21 ), the magnitude (14 %) is much smaller than what is observed (38 %) among residents of northern latitudes in the USA( Reference Levis, Gomez and Jimenez 21 , Reference Woitge, Knothe and Witte 22 ). Compared with the previous study from South Florida, the winter 25(OH)D3 concentrations among participants from Tampa in our study are identical (23·3 v. 23·1 ng/ml, respectively) but the summer concentrations were lower (17·7 v. 26·8 ng/ml). Thus, the low summer 25(OH)D3 concentrations in Tampa could be a chance finding due to the low number of participants from Tampa in our study.
Studies have revealed that current food fortification practices in the USA may not be adequate to prevent vitamin D deficiency among vulnerable people during winter( Reference Yetley 23 , Reference Calvo, Whiting and Barton 24 ). Likewise, it has been shown that although supplement use increases serum 25(OH)D3 concentrations, it does not make up for insufficient sun exposure( Reference Yetley 23 ), and current supplement recommendations are not adequate to prevent vitamin D deficiency( Reference Ginde, Liu and Camargo 25 ). As vitamin D supplementation is discussed in the context of improving survival in colorectal cancer patients( Reference Ng, Meyerhardt and Wu 8 , Reference Ulrich and Holmes 26 ), our study shows that high plasma vitamin D concentrations can be achieved in populations with substantial outdoor activity during summer (such as Heidelberg). Nevertheless, vitamin D fortification in the USA did not prevent low levels of serum 25(OH)D3 in Tampa or Seattle, cities that practise sun-avoidance or reduced sun exposure.
The lower plasma folate concentrations among participants from Heidelberg compared with those from the USA are not unexpected considering the fact that mandatory fortification of staple foods with folic acid commenced in the USA in 1998( Reference Aufreiter, Gregory and Pfeiffer 27 ) while fortification is not practised in Germany( Reference Herrmann and Obeid 28 ). The mandatory fortification programme has resulted in a substantial increase in plasma folate concentrations in the USA, where the prevalence estimates for low serum folate concentrations (<3 ng/ml) declined from 20·6 % in the period 1988–1994 to 0·6 % in 2004( Reference Pfeiffer, Johnson and Jain 29 ).
However, we also observed large intra-site differences in plasma folate and vitamin B12 concentrations at each study site. This may be due mostly to the use of folic acid-containing supplements. It has been shown that supplement and multivitamin use is higher among cancer patients compared with the general population( Reference Velicer and Ulrich 30 ). Between 64 and 81 % of cancer patients use any vitamin or mineral supplement, compared with 50 % in the general population( Reference Velicer and Ulrich 30 ). In a large study in Washington state, USA, colorectal cancer patients were 33 % more likely to use folic acid supplements compared with cancer-free controls( Reference Greenlee, White and Patterson 31 ) and such a supplement use is associated with higher plasma folate and vitamin B12 concentrations( Reference Yang, Cogswell and Hamner 32 ). Therefore, the use of folic acid-containing supplements is an important determinant of plasma folate concentrations in colorectal cancer patients. This may be of potential concern considering the fact that very high plasma folate concentrations that can be achieved with supplement use may accelerate the growth of already existing colorectal cancers( Reference Kim 10 ). In a randomised controlled trial, folic acid given at a dosage of 1 mg/d was associated with a 1·7-fold increased risk of advanced colorectal cancers( Reference Cole, Baron and Sandler 12 ), thus excluding its utility and safety as a secondary or tertiary prevention agent for colorectal cancer. However, this is an area of ongoing research and further studies are needed to elucidate the relationship between folate and survival in patients with colorectal cancer.
A particular strength of our study is the comparison of biomarkers across international study sites with identical laboratories for assays and consistent blood-draw protocols. However, our sample size as well as variable set was limited and we had no information on supplement use; hence we could not investigate the association of supplement use with nutritional biomarker concentrations.
In conclusion, we observed substantial differences in nutritional biomarker concentrations across international study sites, probably reflecting dissimilarities in supplement use, food fortification, and sun-seeking behaviour, among other health practices. However, intra-site differences at each study location were larger than between-location variability, suggesting that individual health behaviours play a significant role. As we aim to understand predictors of colorectal cancer prognosis, there is much to learn from international cohorts, which offer a broad range of exposure and health behaviours that cannot be obtained at a single site.
Acknowledgements
We thank all the participants of the DACHS study and the interviewers, physicians and recruiting hospitals who have been involved in the study. We also acknowledge the excellent technical assistance rendered by Ute Handte-Daub, Renate Hettler-Jensen, Belinda Su Kapereit and Muhabbet Celik.
The DACHS study was supported by grants from the German Research Council (Deutsche Forschungsgemeinschaft, grant numbers BR 1704/6-1, BR 1704/6-3, BR 1704/6-4 and CH 390 117/1-1), and the German Federal Ministry of Education and Research (grant numbers 01KH0404 and 01ER0814).
The study was conceived of and designed by C. M. U., E. S., H. B., J. C. C., C. A., M. H., K. M., T. A. S. and W. G. Funding was obtained by C. M. U., E. S., H. B., J. C. C., D. S., K. M., T. A. S. and W. G. Data were acquired by C. M. U., E. S., H. B., J. C. C., C. A., X. S., R. W. O., M. H., D. S., K. V., S. K. R., G. W. and R. H. In charge of quality control of data and algorithms were H. B., J. C. C., C. A., X. S., R. W. O., M. H., K. V., S. K. R., G. W. and R. H. Data analysis and interpretation were performed by C. M. U., A. T. T., E. S., H. B., J. C. C., M. H., H. B., K. M., T. A. S. and W. G. Statistical analysis was done by J. K. and H. B. The manuscript was prepared by A. T. T., C. A., J. K. and S. K. R. The manuscript was edited by C. M. U., E. S., X. S., M. H., H. B., D. S., K. V., K. M., T. A. S. and W. G. The manuscript was reviewed by C. M. U., E. S., H. B., J. C. C., R. W. O., G. W. and R. H.
There is no conflict of interest.






