Malnutrition is a state resulting from the lack of uptake or intake of nutrients to meet physiological and mental function demands leading to altered body composition (decreased muscle and fat mass) and body cell mass with worse clinical outcomes from disease(Reference Cederholm, Bosaeus and Barazzoni1). In children, malnutrition is associated with enteral dysfunction, altered immunity and increased mortality(Reference Olofin, McDonald and Ezzati2). To detect children with an increased risk of death and in need of urgent treatment, the WHO defines severe acute malnutrition (SAM) among those aged 6 to 59 months as the presence of at least one of the following independent criteria: (i) weight-for-height Z-score (WHZ) less than -3; (ii) a mid-upper arm circumference (MUAC) less than 115 mm and (iii) the presence of bilateral pitting oedema(3). The use of either WHZ or MUAC as anthropometric criteria to define SAM is based on the observation that although WHZ and MUAC identify overlapping sets of children with severe conditions, these subsets are not identical(Reference Bern and Nathanail4,Reference Lindtjørn5,Reference Carter6,Reference Fernández, Delchevalerie and Van Herp7,Reference Laillou, Prak and Groot8,Reference Roberfroid, Huybregts and Lachat9,Reference Grellety and Golden10) and it is assumed that children with a high risk of death may be missed if only WHZ or only MUAC is used. Debate surrounding the appropriate anthropometric index or combination of indices to use continues within the nutrition community as there is a tension between the desire to use the best combination of indices to identify high-risk children and practical considerations regarding the reliability of complex measures and the need to maximise programme coverage.
Length (for children under the age of two) or height (for older children) is required for the calculation of WHZ, but these measures have important practical limitations. First, height/length boards are needed to measure children, but these are often not available in peripheral health facilities. Second, measuring length accurately requires the presence of two skilled health personnel(Reference Cashin and Oot11) who are not always available in commonly short-staffed health facilities in low- and middle-income countries. As such, measuring height/length in these contexts can have limited practicability for community outreach programmes as the reliability of the measures may be uncertain. Third, the calculation of WHZ from both weight and height/length can introduce an additional source of error(Reference Mwangome and Berkley12). In practice, WHZ can be reliably assessed to detect high-risk children only in some health facilities or, occasionally and mainly in humanitarian contexts, in mass community screenings that are usually supported by international non-governmental organisations. To avoid these constraints, MUAC is often used as the only anthropometric criterion for identifying children with SAM, sometimes increasing the case-defining threshold to 120 mm or 125 mm to maximise the likelihood of including children with WHZ < -3 and, particularly in highly food insecure contexts, to also include some moderately malnourished children to prevent transition into SAM(13,Reference Alé, Phelan and Issa14,Reference Daures, Phelan and Issoufou15) . This simple approach has the advantage of avoiding the confusion among both staff and caretakers that is created by having multiple criteria to identify different children as being at risk of dying.
Weight is easier to measure than height as it requires simple equipment (a functioning properly calibrated scale), which is widely available in most health facilities and requires only one trained health provider. Frequent weight measurements plotted against age to allow for an estimation of the weight-for-age Z-score (WAZ) are part of the standard package for the integrated management of childhood illness(16). This is included in the child growth monitoring and promotion programmes that are widely implemented in many countries. In a review of early studies, WAZ along with MUAC was found to offer a better compromise between sensitivity and specificity compared with using WHZ to identify children with a high risk of death(Reference Pelletier17). WAZ also selects children who are both wasted and stunted at the same time who have a higher risk of death(Reference Myatt, Khara and Schoenbuchner18,Reference Khara, Myatt and Sadler19) . However, WAZ calculation requires knowledge of age, which is problematic in some contexts, particularly in older children(Reference Frison, Kerac and Checchi20). Also, growth monitoring programmes are often poorly functioning, and their impact is uncertain. Their objective is to monitor growth, not to detect high-risk children and WAZ is not currently among the criteria recommended by the WHO to select malnourished children with a high risk of death in need of intensive treatment.
While the choice of anthropometric index has dominated the discourse around the most appropriate criteria for admission to therapeutic feeding programmes, the effect of the frequency of screening has rarely been considered. Nutritional status varies in individual children with age, feeding practices, seasons and shocks due to food insecurity and illness episodes. As a result, incident cases of SAM may be common and occasional detection (as is the case for mass screening sessions), even with the best diagnostic criteria, will tend to miss incident cases occurring in the months between screenings. Children with SAM are at a high risk of death without treatment and may die between screening sessions if these take place several months apart which is often the case owing to the high resource, logistics and manpower requirements involved. Early studies suggested that the prognostic values of MUAC and other anthropometric indices improved when used for shorter follow-up periods(Reference Briend and Zimicki21,Reference Pelletier, Low and Johnson22) .
Some SAM children have associated complications which worsen their prognosis and require inpatient treatment. In in-patient settings, clinical signs associated with a poor outcome have a predictive value which is highest at the moment of assessment and declines rapidly over time, which is an argument for frequently assessing the clinical status of each child admitted for treatment(Reference Wen, Brals and Bourdon23). This also raises the issue of a need for frequent clinical and nutritional screening in outpatient settings.
The global number of deaths associated with SAM is unknown, but prudent estimates suggest that it is likely to be several hundreds of thousands every year(Reference Black, Victora and Walker24). The choice of criteria used to admit children to therapeutic programmes should, therefore, be made carefully in order to include as many children as possible with a high risk of death. However, to avoid overloading programmes, minimise overcrowding at treatment facilities and reduce treatment costs, the criteria must also be specific, i.e. minimising the number of false positives who are selected who would likely survive in the absence of treatment. The objective of the current study was to assess the prognostic value of the different anthropometric indices, WHZ, WAZ and MUAC, and their combinations over different follow-up periods. The aim was to determine the optimal frequency of screening needed to detect malnourished children with a high risk of death using various anthropometric indices. The implications of the optimal frequency of screening on the choice of anthropometric criteria were also examined.
Methods
Data sources
The current analysis was based on a data set that combined twelve cohorts to examine the relationship between anthropometry and mortality. These cohorts were selected based on two criteria. First, they should examine the relationship between anthropometry and mortality in cohorts of populations of children with no access to a community-based programme of management of severe malnutrition. The objective of these programmes is to improve the prognosis of children with SAM and make populations where such programmes are present not suitable to look at the risk of death in absence of treatment. As these community-based programmes became more widespread in recent years, most cohorts fulfilling these criteria were old, the oldest being based on data collected in 1977, which is a limitation of the study. Second, the principal investigator of these cohort should agree to share the data for this analysis.
Ten of these cohorts, previously described in detail by McDonald et al.(Reference McDonald, Olofin and Flaxman25), were from Bangladesh, Ghana, Guinea-Bissau, India, Indonesia, Nepal, Peru, Philippines, Senegal and Sudan. Two additional cohorts from the Democratic Republic of Congo (DRC) and Niger were also analysed. Four of the twelve cohorts included MUAC measurements: Senegal, DRC, Nepal and Niger.
The first additional data set was from Bwamanda, in the north western province of Equateur, DRC(Reference Van Den Broeck, Eeckels and Vuylsteke26). In brief, an open cohort of over 5000 children aged less than 59 months was followed for about 2 years. Children were measured up to six times at 3-month intervals from October 1989 to February 1991. Mortality surveillance was extended to April 1992. Each visit included anthropometric measurements (weight, height and MUAC). Weight was measured with spring scales to the nearest 100 g, and length or height was measured using locally designed and constructed measuring boards of standard design to the nearest millimetre (mm). MUAC measurements were taken by one medical doctor to the nearest mm.
The second additional cohort came from a randomised controlled trial in Niger on the effect of mass treatment with azithromycin on trachoma and other outcomes(Reference O’Brien, Amza and Kadri27). In randomly selected communities, children received a single oral dose of azithromycin (20 mg/kg of body weight up to a maximum dose of 1 g). As part of the trial, a subset of children (maximum 62 per community; total 1023) had anthropometry assessed at 12 months and 36 months after enrolment into the trial. The children were weighed standing or in the arms of a caregiver. Weight was recorded to the nearest 100 g using digital tared scales. MUAC was measured to the nearest 1 mm using a tape developed by Johns Hopkins University (Baltimore, MD, USA). All measurements were conducted three times, and the mean value was used for the analysis presented here. The study personnel referred children with MUAC < 115 mm or illness to the local health post.
In all the studies, deaths were monitored for up to 4 to 6 months following measurements. Dates were used to calculate the time between anthropometric measures and death to assess the effect of time on the relationship between each anthropometric index and death. All but one of these cohorts dated from a period when the community management of SAM was not implemented and should reflect mortality in the absence of such programmes. In the study from Niger, children with an MUAC < 115 mm were referred to local healthcare facilities for treatment(Reference O’Brien, Amza and Kadri27).
Statistical analysis
For all cohorts, the analysis was limited to children aged between 6 and 59 months at the time of screening. These age limits were used as the criterion of MUAC < 115 mm is recommended by the WHO only in this age group and because therapeutic and supplementary feeding programmes focus on this age group. Analysis of MUAC used the four cohorts that had MUAC data available.
WHZ and WAZ were calculated using the 2006 WHO growth standards(28) and version 0·3·1 of the ‘Z-scorer’ library for the R language and environment for statistical computing(Reference Myatt and Guevarra29,30) . The analysis was performed for intervals of different durations, all starting at the time of anthropometric assessment and ending 1, 3 and 6 months after the assessment or at the death of the child.
In the first stage of the analysis, the sensitivity of each anthropometric variable to identify children with a high risk of death was examined. Sensitivity was defined as the proportion of children below the threshold of case definition who died during follow-up. Specificity was defined as the proportion of children above the threshold of case definition who survived during follow-up. False positives were defined as children below the threshold of case definition who survived. Sensitivity was plotted against the false-positive ratio (1 – specificity) to obtain receiver operating characteristic (ROC) curves(Reference McNeil, Keller and Adelstein31).
Sensitivity and the false-positive ratio for predicting death for each measure were calculated at steps of 0·1 Z-scores (WAZ, WHZ) or steps of 1 mm (MUAC) over the range of the measurements with a false-positive ratio of less than 25 % recorded in each cohort calculated from fourfold (i.e. 2-by-2) contingency tables. This upper limit for the false-positive ratio was chosen as beyond this many low-risk children are selected which would overload treatment programmes. A Haldane–Anscombe correction (i.e. adding 0·5 to all cells in the contingency table) was applied only to tables with one or more zero cells prior to calculating sensitivity and false-positive ratios for subsequent analysis(Reference Weber, Knapp and Ickstadt32). The resulting estimates were combined using a random-effects meta-analysis, and pooled ROC curves (sensitivity v. false-positive ratio) were plotted. A random-effects analysis was used as there was no compelling reason to assume that a common effect size underlay all the studies in the analysis since the cohorts came from different settings at different times, addressed different research questions, employed different protocols and were performed by different teams. Under the random-effects model, we assume that there is a distribution of true effects. The resulting summary effect is the estimated mean of this distribution of effects.
The current analysis was first performed for a 6-month interval following measurement. The analysis was repeated for intervals of 3 months and 1 month following measurement. Areas under the curve (AUC) for each ROC curve were estimated using the trapezoidal rule(Reference Atkinson33). Standard errors for each of the AUC estimates were determined using a bootstrap procedure (i.e. as the SD of the distribution of the bootstrap replicates of the AUC, with r = 999 replicates used in each bootstrap analysis). The statistical significance of the differences in the AUCs was assessed using a single-tailed two-sample Z-test (i.e. treating each estimated AUC as the proportion of the unit square lying below the ROC curve). The sensitivity and false-positive ratios of different nutritional indices or of the combinations of MUAC < 115 mm or WHZ < -3 and of MUAC < 115 mm or WAZ < -3 were estimated for each cohort for different follow-up periods and pooled using random-effects meta-analyses. The combination of MUAC < 115 mm or WHZ < -3 is currently recommended by the WHO to identify children in need of intensive treatment(3). The association MUAC < 115 mm or WAZ < -3 has been shown to be the most sensitive combination to identify children with a severe anthropometric deficits with a high risk of death(Reference Myatt, Khara and Dolan34,Reference Khara, Myatt and Sadler19) . The current analysis was repeated using MUAC with a 120 mm and a 125 mm cut-off as these cut-offs have been proposed for use in simplified programmes using MUAC as the sole screening and admission criteria(Reference Daures, Phelan and Issoufou15,Reference Bailey, Opondo and Lelijveld35,Reference Maust, Koroma and Abla36) .
The performance of each anthropometric case definition at different lengths of follow-up was compared by calculating the relative change in sensitivity and false-positive ratio. For example:
 $$Relative\;chang{e_{Sensitivity}} = {{{{Sensitivit{y_1} - \;Sensitivit{y_2}}}}\over{{{{{Sensitivit{y_2}}}}}}} \times 100$$
$$Relative\;chang{e_{Sensitivity}} = {{{{Sensitivit{y_1} - \;Sensitivit{y_2}}}}\over{{{{{Sensitivit{y_2}}}}}}} \times 100$$
The standard errors for these ratios were calculated using the Delta method(Reference Hirschberg and Lye37,Reference Guillot38) . Estimates from each cohort were pooled using a random-effects meta-analysis.
WAZ < -3, WHZ < -3 and MUAC < 115 mm, 120 mm or 125 mm have different false-positive ratios and their respective capacity to identify children who are going to die is, therefore, difficult to directly assess with ROC curves which compare anthropometric indices with cut-offs at the same false-positive level. Performance may be better illustrated using Venn diagrams that directly display the case numbers. Therefore, in the second stage of the analysis, the ability of different anthropometric criteria to identify children with a severe anthropometric deficit (WAZ < -3 or WHZ < -3 or MUAC < 115 mm) who died during follow-up was compared using Venn diagrams(Reference Venn39). This part of the analysis was performed with all the individual study data sets concatenated (i.e. joined end-to-end) into a single data set (i.e. a crude pooled analysis). Venn diagrams were also drawn for each cohort separately.
Data management and data analysis were performed using R language and environment for statistical computing (version 4.0.3) scripts were managed using the R-AnalyticFlow scientific workflow system (version 3.1.8)(30).
Results
The numbers of 6-month follow-up intervals and deaths for each cohort and during each period of follow-up are reported in Table 1. A total of 56 559 children giving 193 266 child intervals were available for the analysis. For analyses involving MUAC, a total of 16 579 children giving 56 857 child intervals were available.
Table 1 Characteristics of the twelve cohort studies with numbers of children included in each study and number of deaths observed during different follow-up periods
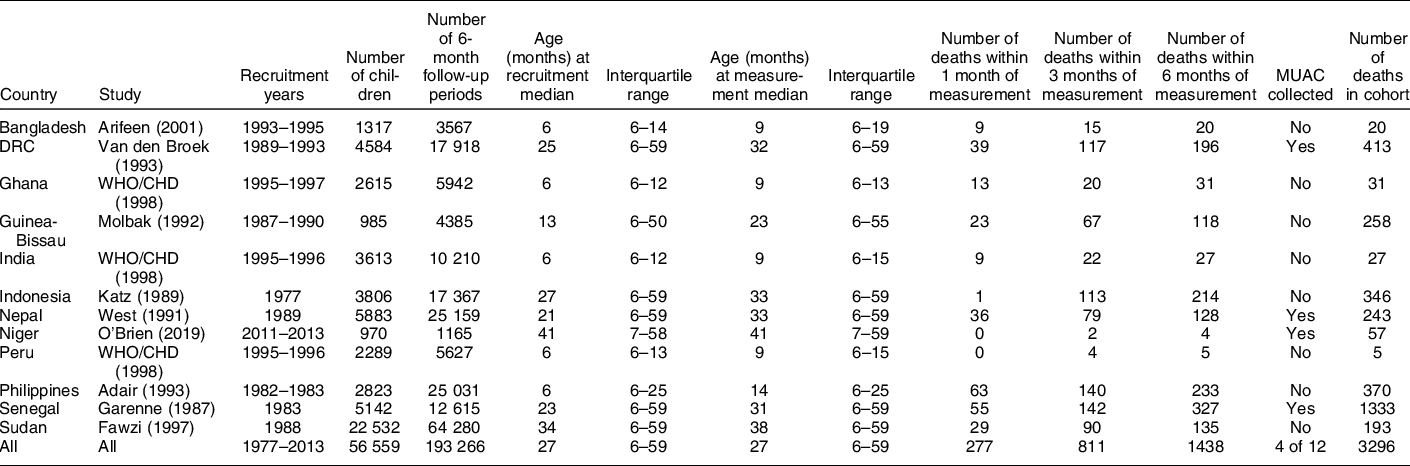
Refers to subjects/interval events used in the current analysis (i.e. after censoring records with extreme measurements/values and children aged below 6 month or above 59 months). Only deaths occurring within 6 months of measurement were included in the analysis.
The ROC curves (AUC) of MUAC, WHZ and WAZ were higher for shorter duration of follow-up indicating a better sensitivity for each specificity level (Fig. 1(a), (b) and (c)). AUC were not significantly different when compared over their whole range of false-positive ratios. However, when only the parts of the ROC curve with false-positive ratios at or below 25 % were considered, the AUC were significantly higher for all indices for 1-month intervals than for 3- and 6-month intervals (Table 2).

Fig. 1 Receiver operating characteristic (ROC) curves of different anthropometric indices with death as outcome for different duration of follow-up. (a) Weight-for-age. (b) Weight-for-height. (c) Mid-upper arm circumference (MUAC)
Table 2 AUC (point estimate, se) of different receiver operating characteristic (ROC) curves for false-positive ratios ≤ 25 % for follow-up duration of 1, 3 and 6 months (random-effects meta-analysis)

MUAC, upper arm circumference; WHZ, weight-for-height Z-score; WAZ, weight-for-age Z-score.
One-sided comparisons for assessing the effect of duration of follow-up.
Comparisons are based on twelve cohorts except those with MUAC which were based on cohorts from DRC Nepal, Niger and Senegal.
When the ROC curves for the same duration of follow-up were pooled together on the same graph for 6-month follow-up, MUAC and WAZ had higher ROC curves compared with WHZ (Fig. 2(a)). For 1-month follow-up, MUAC and WHZ had higher ROC curves than WAZ (Fig. 2(b)). However, only the AUC for MUAC was significantly higher than that for WHZ for 1-month follow-up for the part of the curve with false-positive ratios at or below 25 % (Table 2).

Fig. 2 Receiver operating characteristic (ROC) curves of different anthropometric indices with death within 6 months and within 1 month as outcome. (a) Death within 6 months. (b) Death within 1 month
Table 3 shows the sensitivity and specificity of the three individual indices WAZ, WHZ < -3 and MUAC < 115 mm and the combinations of these indices to detect children with a high risk of death for 6-month, 3-month and 1-month follow-up. Sensitivity was significantly higher with 1-month follow-up compared with 6-month follow-up for all indices. The sensitivity was 29 % for MUAC < 115 mm with 1-month follow-up compared with 18 % for 6-month follow-up, corresponding to a 49 % relative increase. For WHZ < -3, the sensitivities were 22 % compared with 12 % corresponding to a 48 % relative increase. For WAZ < -3, the sensitivities were 42 % compared with 31 % corresponding to a 28 % relative increase (Table 3).
Table 3 Increase in the false-positive ratios and sensitivity when duration of follow-up is reduced from 6 months to 3 or 1 month sensitivity

MUAC, upper arm circumference; WHZ, weight-for-height Z-score; WAZ, weight-for-age Z-score.
* Results from a random-effects meta-analysis of sensitivity calculated in each cohort.
† Results from a random-effects meta-analysis of relative change in sensitivity calculated in each cohort.
‡ Analysis of results from four cohorts with MUAC data.
§ Analysis of results from twelve cohorts.
The false-positive ratio was significantly higher for MUAC < 115 mm, WHZ < -3 and WAZ < -3 for 1 and 3 months compared with 6 months, but the effect size was small (Table 4). The highest proportional increase was for MUAC < 115 mm with a false-positive ratio which increased from 3·5 % to 3·6 % corresponding to a relative increase of 3 %. This increase in the false-positive ratio was due to the classification as survivors of children who died after 1- or 3-month follow-up.
Table 4 Increase in the false-positive ratios and sensitivity when duration of follow-up is reduced from 6 months to 3 or 1 month false-positives ratio (FPR)

MUAC, upper arm circumference; WHZ, weight-for-height Z-score; WAZ, weight-for-age Z-score.
* Results from a random-effects meta-analysis of FPR calculated in each cohort.
† Results from a random-effects meta-analysis of relative change in FPR calculated in each cohort.
‡ Analysis of results from four cohorts with MUAC data.
§ Analysis of results from twelve cohorts.
Combining indices resulted in an increase in sensitivity but also in false-positive ratios. Overall, this increase in sensitivity was smaller than that observed when shortening follow-up. For a 6-month follow-up, adding WHZ < -3 to MUAC < 115 mm increased the sensitivity from 17·8 % to 20·4 %, a non-significant relative increase of 19 %. In contrast, the corresponding false-positive ratio increased from 3·5 % to 4·7 %, corresponding to a relative increase of 55 % (P < 0·01) and an absolute increase of 1·2 %. Adding WAZ < -3 to MUAC < 115 mm increased sensitivity from 17·8 % to 36·3 % corresponding to a relative increase of 127 % (P = 0·01) but the false-positive proportion increased to 16·4 %, equivalent to a 5·9-fold increase compared with MUAC < 115 mm alone.
The Venn diagrams presented in Fig. 3 show the number of children who died within 1 month of assessment with at least one severe anthropometric deficit detected with WAZ < -3, WHZ < -3 and MUAC < 115 mm (Fig. 3(a)) and also with MUAC < 120 mm (Fig. 3(b)) and MUAC < 125 mm (Fig. 3(c)) with data from all studies having MUAC pooled together. The corresponding Venn diagrams for each country are presented in Supplemental Fig. 1. WAZ < -3 was the most sensitive criterion as it identified a total of fifty-one children among those with severe anthropometric deficits at assessment who subsequently died (n 170), corresponding to 39 % of total deaths (Fig. 3(a)). MUAC < 115 mm identified thirty-three children who died during follow-up (25 % of total deaths) and WHZ < -3 identified twenty of them (15 % of total deaths). Only three children who died with a WHZ < -3 were not identified by MUAC < 115 mm, corresponding to 2·3 % of total deaths. The three children not detected by MUAC < 115 mm had a WAZ < -3 which means that a combined criterion of MUAC < 115 mm or WAZ < -3 would identify all deaths associated with WHZ < -3.
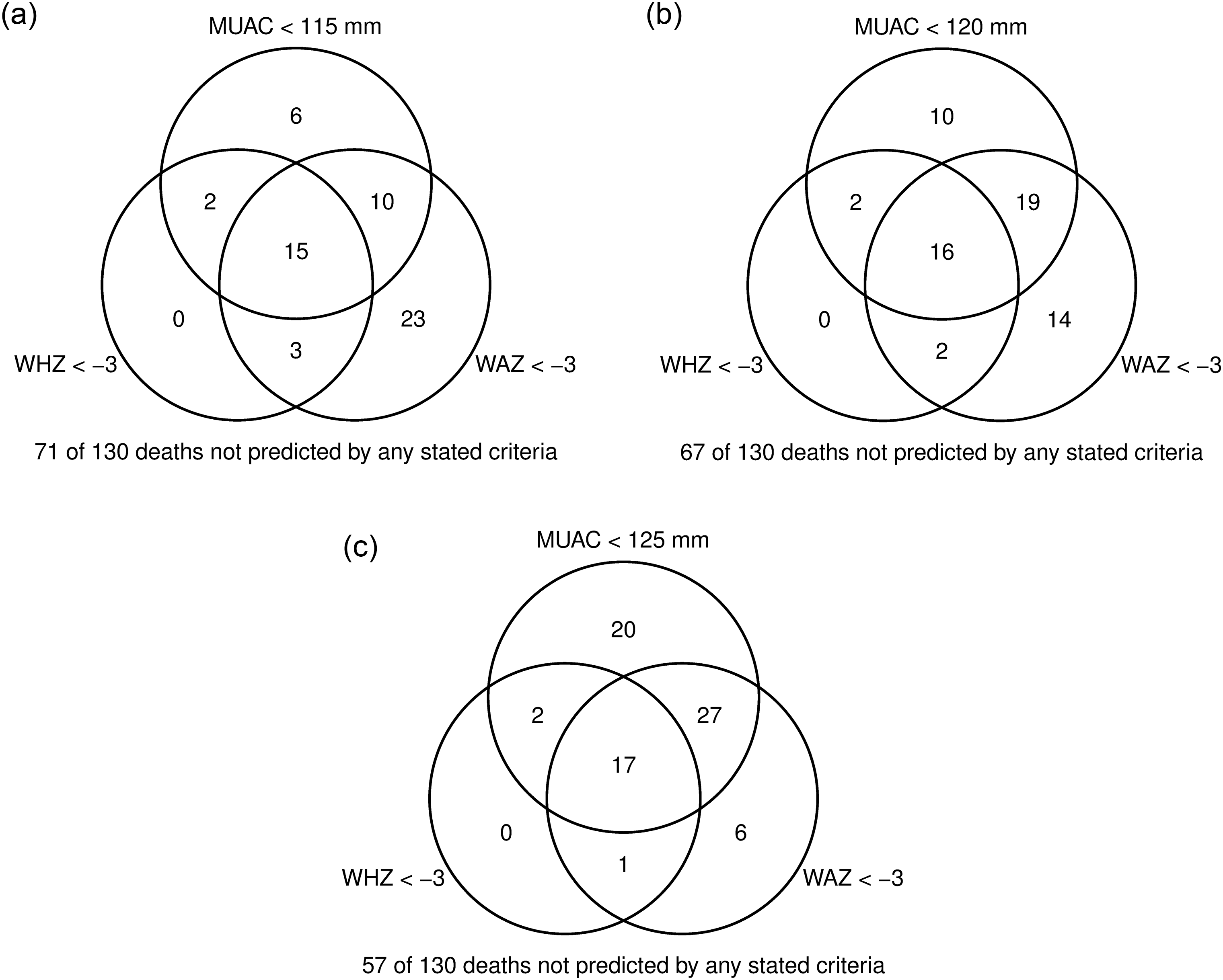
Fig. 3 Venn diagram showing the overlap of children identified by different anthropometric indices among children who died within 1 month of nutritional assessment. (a) Mid-upper arm circumference (MUAC) < 115 mm. (b) MUAC < 120 mm. (c) MUAC < 125 mm
The implications of increasing the MUAC cut-off in programmes using this as the sole criterion for admission, as is commonly the case with simplified protocols, were also explored. Increasing the MUAC cut-off from 115 mm to 120 mm and 125 mm increased the number of detected deaths from 33 to 47 and 66, respectively, for a total of 130 deaths (Fig. 3(b) and (c)). Higher MUAC cut-offs decreased the number of children only identified by WAZ < -3 but not by MUAC. However, even with MUAC < 125 mm, seven children with a WAZ < -3 were missed by MUAC, representing 7 % of total deaths. The increase in MUAC was associated with increasing proportions of false-positives from 3·6 % for MUAC < 115 mm to 7·5 % and 14·3 % for MUAC < 120 mm and 125 mm, respectively.
Discussion
The current study shows that the value of different anthropometric indices for predicting death rapidly and significantly decreases with the increasing length of follow-up. Our results suggest that with a 1-month follow-up sensitivity of different anthropometric indices to detect children with a high risk of dying is higher than with a 6-month follow-up, with the largest relative increase for MUAC < 115 mm and WHZ < -3 of 48 % and 49 %, respectively. In contrast to what happens when several indices are used in combination, this increased sensitivity associated with an increased frequency of follow-up is obtained with a minimal increase in the number of false positives who will survive in the absence of treatment. This increase in false-positive ratios seen when shortening follow-up intervals has little practical impact as it is small and is due to children who died between 1 and 3 or 6 months and who would benefit from treatment when included in programmes. Limiting the number of false positives is important to avoid an increase in programme costs, which are directly related to the number of children to be treated. This suggests that increasing the frequency of screening may be a more promising approach than combining two indices to detect children in need of treatment. This result is in line with the better prediction of clinical danger signs when used frequently as has been reported in clinical settings(Reference Wen, Brals and Bourdon23).
MUAC < 115 mm in our data set identified 85 % (seventeen children out of twenty) of children with WHZ < -3 who died during the month following assessment. Our results suggest that for identifying high-risk children there is a greater and more important overlap between the MUAC and WHZ indices than is suggested by population surveys and analyses of programme data(Reference Grellety and Golden10). Concerns about missing many high-risk children when using MUAC < 115 mm alone seem to be less relevant for monthly screening than for longer intervals between screenings.
MUAC can be measured by mothers at the household level after a short training session(Reference Blackwell, Myatt and Allafort-Duverger40) and has been used successfully to detect high-risk children in large-scale projects(13,Reference Alé, Phelan and Issa14) . The use of ‘family MUAC’ is rapidly expanding(41) and it could be a potential way to implement very frequent measurements of MUAC to improve the detection of high risk children. However, MUAC measure by caregivers adds to their workload and is subject to measurement errors and this approach should be rigorously evaluated. Increasing the cut-off to 120 mm or 125 mm as has been done in some programmes increases the sensitivity of the detection of high-risk children but also the proportion of false-positives referred to treatment.
In addition to better identification of children with a high risk of death, frequent screening has the advantage of identifying high-risk children at an earlier stage, possibly before the onset of complications. A study from Niger showed that in areas where mothers detect SAM themselves using a colour-banded MUAC tape, a smaller proportion of children who were identified and admitted had complications requiring inpatient treatment compared with areas where the screening was done by community health workers(Reference Alé, Phelan and Issa14). A study conducted in Kenya and South Sudan showed that a high MUAC on admission was associated with a lower risk of relapse which is another potential advantage of early case detection(Reference Lelijveld, Musyoki and Adongo42).
Our study also showed that even when used frequently, the combination of MUAC < 115 mm and WHZ < -3 failed to identify quite a few children with a WAZ < -3 who died during follow-up. Some children with a WAZ < -3 who died during follow-up were also missed when the MUAC cut-off was increased to 120 mm and even 125 mm. WAZ < -3 corresponds to a severe anthropometric deficit that combines wasting and stunting associated with a high risk of death(Reference Myatt, Khara and Schoenbuchner18). Adding WAZ < -3 to a screening scheme would increase the detection of the high-risk wasted and stunted children not identified by WHZ. However, the calculation of WAZ requires knowledge of age, which may limit its use in many settings particularly in older children. In addition, the increase in sensitivity obtained by adding WAZ < -3 is associated with an increase in false positives, which may have important implications in terms of programme costs. However, these additional costs could be reduced by providing less intensive treatment to some subsets of children with moderately increased risk of death.
No child who died during follow-up was identified by WHZ < -3 when MUAC < 115 mm or WAZ < -3 were used as the selection criterion. This suggests that the combination of WAZ < -3 or MUAC < 115 mm would eliminate the need for the use of WHZ. This is consistent with evidence for infants aged less than 6 months not included in the current analysis(Reference Mwangome, Ngari and Fegan43). A recent review of the literature concluded that, in infants less than 6 months, MUAC and WAZ were also the best criteria to identify infants with a high risk of death(Reference Lelijveld, Kerac and McGrath44).
The current study has some limitations. First, it is based on pooled data from historical cohorts from different regions with one data set going back to more than 30 years, and it can be argued that these cohorts are too different to be pooled together and have a mortality pattern very different from that which might be observed today. While it is true that the absolute level of mortality has changed dramatically over the past 20 years, previous studies have shown that the relative risks associated with different anthropometric deficits are rather stable across regions(Reference Pelletier17). In addition, any heterogeneity was taken into account during the part of the analysis based on sensitivity, false-positives or ROC curves which used a random-effects meta-analysis approach. Second, oedema was not recorded during the anthropometric assessment. Children with oedema have a high risk of death, but their WHZ can be high as a result of fluid accumulation. This may have impacted the prognostic value of WHZ. This, however, would also have impacted the prognostic value of WAZ and is, therefore, unlikely to change one major conclusion of the current study regarding the possible need to include WAZ to detect high risk children. In addition, oedema is a transient event, with oedematous children dying or recovering within a few days or weeks, and therefore is rarely seen in cross-sectional surveys or intermittent assessments(Reference Williams45). This reinforces our conclusion regarding the need for frequent nutritional screening. Eventually, while three of these studies were carried out before the development of community-based management of malnutrition and reflected the outcome of SAM children in the absence of treatment, the fourth took place in a setting where children with MUAC < 115 mm were referred for treatment. This may have improved the survival of these children as suggested by the absence of death in the month following anthropometric assessment. This may have led to an underestimation of the importance of frequent screening in this particular cohort.
It is worth noting that in these studies, the mortality results recorded are all-cause as information on the cause of death was not consistently recorded between and within data sets. This means it is not possible to specifically attribute cause of death to anthropometric status. We can only ascertain how anthropometric indices identify children with a high risk of death.
The current study also has a unique strength. It is based on the analysis of all known available data sets with all anthropometric indices including MUAC and mortality. A large sample size is needed to study the relationship between these indices and the risk of death which is, from a statistical point of view, a rare event, especially when examined over short time periods as in this study. As such, this data set is uniquely able to provide information regarding the need to use frequent anthropometric assessment to identify high-risk children.
Conclusions
The prognostic value of different anthropometric indices to predict death decreases over time. To optimise the detection of high-risk children, monthly screening is preferable where resources allow. The proportion of detected high-risk children who would die in the absence of treatment can also be increased by using several indices simultaneously. The most sensitive scheme is MUAC combined with WAZ. The addition of WHZ does not capture any additional children with a high risk of death than the combination of MUAC < 115 mm and WAZ < -3. However, combining MUAC and WAZ results in an increase in false positives, that is, of children who will survive in the absence of treatment compared with using MUAC only with important implications in terms of the number of children treated and the consequent programme costs.
Acknowledgements
Acknowledgements: The authors thank the principal investigators of the original cohort studies for providing permission for the use of their data for this analysis. We also extend our thanks to Natalie Sessions who supported us in the collation of comments for successive drafts. Financial support: This paper is made possible by the generous support of the American people through the United States Agency for International Development (USAID) (Award No. 720BHA23CA00001) and of Irish Aid (grant numbers HQPU/2021/ENN and HQPCR/2022/ENN). The ideas, opinions and comments herein are entirely the responsibility of its authors and do not necessarily represent or reflect the views of USAID or the United States Government or Irish Aid or the Irish Government. Authorship: The study was conceived and designed by A.B., M.M. and T.K. The data were analysed by M.M. with inputs from all authors. A.B. and M.M. wrote the first draft of the manuscript and are responsible for the final content. All authors contributed to the article and have read and approved the final manuscript. Ethics of human subject participation: All studies from which data was used received approval from appropriate ethical review committees. The analysis presented here did not involve new data collection and was undertaken using anonymised records and therefore did not require further ethical approval.
Conflicts of interest:
There are no conflicts of interest.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980023000149










