Introduction
Psychotic disorders affect several million people worldwide (Fleischhacker et al., Reference Fleischhacker, Arango, Arteel, Barnes, Carpenter, Duckworth, Galderisi, Halper, Knapp, Marden, Moller, Sartorius and Woodruff2014). The peak phase of first onset often falls during late adolescence and early adulthood (Kirkbride et al., Reference Kirkbride, Errazuriz, Croudace, Morgan, Jackson, McCrone and Murray2012). Many close relatives, predominately parents, will assume informal caregiving roles that can often be long-term (Onwumere et al., Reference Onwumere, Kuipers, Bebbington, Dunn, Fowler, Freeman, Watson and Garety2008; Boydell et al., Reference Boydell, Onwumere, Dutta, Bhavsar, Hill, Morgan, Dazzan, Morgan, Pararajan, Kuipers, Jones, Murray and Fearon2014; Lavis et al., Reference Lavis, Lester, Everard, Freemantle, Amos, Fowler, Hodgekins, Jones, Marshall, Sharma, Larsen, McCrone, Singh, Smith and Birchwood2015). The importance and value of carer support in psychosis has been extensively reviewed in the literature. The pattern of evidence highlights improved illness course (Norman et al., Reference Norman, Malla, Manchanda, Harricharan, Takhar and Northcott2005), mortality rates (Revier et al., Reference Revier, Reininghaus, Dutta, Fearon, Murray, Doody, Croudace, Dazzan, Heslin, Onyejiaka, Kravariti, Lappin, Lomas, Kirkbride, Donoghue, Morgan and Jones2015), treatment outcomes (Stowkowy et al., Reference Stowkowy, Addington, Liu, Hollowell and Addington2012) and facilitated access to relevant services for individuals with family support, when compared with peers without (Jansen et al., Reference Jansen, Gleeson and Cotton2015a).
Though many families will take on caregiving responsibilities and in many cases will live with their relative with psychosis (Garety and Rigg, Reference Garety and Rigg2001; Cotton et al., Reference Cotton, McCann, Gleeson, Crisp, Murphy and Lubman2013; Ran et al., Reference Ran, Chui, Wong, Mao, Lin, Liu and Chan2016), a large proportion will also report experiencing high levels of carer burden, social isolation and a poorer quality of life, as part of their role (Gupta et al., Reference Gupta, Isherwood, Jones and Van Impe2015; Poon et al., Reference Poon, Harvey, Mackinnon and Joubert2016; Sadath et al., Reference Sadath, Muralidhar, Varambally, Gangadhar and Jose2017). Psychosis can impact negatively on carer health and wellbeing, and lead to feelings of loss, burnout, worry, shame, self-stigma and psychological distress, which are already firmly established soon after first onset (Addington et al., Reference Addington, Coldham, Jones, Ko and Addington2003; Patterson et al., Reference Patterson, Birchwood and Cochrane2005; McCann et al., Reference McCann, Lubman and Clark2011; Boydell et al., Reference Boydell, Onwumere, Dutta, Bhavsar, Hill, Morgan, Dazzan, Morgan, Pararajan, Kuipers, Jones, Murray and Fearon2014; Onwumere et al., Reference Onwumere, Lotey, Schulz, James, Afsharzadegan, Harvey, Chu Man, Kuipers and Raune2017). Approximately 30–40% of carers report clinical depression and other indicators of psychological distress and morbidity (Kuipers and Raune, Reference Kuipers, Raune, Birchwood, Fowler and Jackson2000; Hayes et al., Reference Hayes, Hawthorne, Farhall, O'Hanlon and Harvey2015; Jansen et al., Reference Jansen, Haahr, Harder, Trauelsen, Lyse, Pedersen and Simonsen2015b) and reports of distress and burden can persist (Brown and Birtwistle, Reference Brown and Birtwistle1998; Lee et al., Reference Lee, Barrowclough and Lobban2014; Poon et al., Reference Poon, Harvey, Mackinnon and Joubert2016).
Caregiving relationships and outcomes
Caregiving relationships characterised by elevated criticism, hostility and intrusive behaviours, and commonly described as high expressed emotion (EE), are typically predictive of a poorer illness course and outcomes in psychosis, including higher rates of patient relapse and rehospitalisation (Bebbington and Kuipers, Reference Bebbington and Kuipers1994). This is particularly evident with reports of criticism that can have different underlying predictors and correlates (Alvarez-Jimenez et al., Reference Alvarez-Jiménez, Gleeson, Cotton, Wade, Crisp, Yap and McGorry2010; Cechnicki et al., Reference Cechnicki, Bielańska, Hanuszkiewicz and Daren2013). Carers reporting higher levels of patient-focused criticism are more inclined to blame their relative for their illness and perceive illness symptoms and related behaviours as something their relative could control, if they chose to (Bentsen et al., Reference Bentsen, Notland, Boye, Munkvold, Bjorge, Lersbryggen, Uren, Oskarsson, Berg-Larsen, Lingjaerde and Malt1998; Barrowclough and Hooley, Reference Barrowclough and Hooley2003; McNab et al., Reference McNab, Haslam and Burnett2007; Vasconcelos et al., Reference Vasconcelos, Wearden and Barrowclough2013).
Patient perceptions of negative caregiving relationships (i.e. perceived EE) are themselves also linked to poorer patient functioning and outcomes (Onwumere et al., Reference Onwumere, Kuipers, Bebbington, Dunn, Freeman, Fowler and Garety2009; Hesse et al., Reference Hesse, Kriston, Mehl, Wittorf, Wiedemann, Wolwer and Klingberg2016), which are observable at first episode (Von Polier et al., Reference Von Polier, Meng, Lambert, Strauss, Zarotti, Karle, Dubois, Stark, Neidhart, Zollinger, Burgin, Felder, Resch, Koch, Schulte-Markwort and Schimmelmann2014; Haidl et al., Reference Haidl, Rosen, Schultze-Lutter, Nieman, Eggers, Heinimaa, Juckel, Heinz, Morrison, Linszen, Salokangas, Klosterkötter, Birchwood, Patterson and Ruhrmann2018).
Carer burden is complex and multi-dimensional, and we know that higher levels are positively linked with greater levels of carer distress and negative caregiving relationships (Raune et al., Reference Raune, Kuipers and Bebbington2004). Carer burden is also influenced by several clinical and demographic factors that hitherto have included carer age, the type of caregiving relationship (e.g. being a parent carer v. other carers) and illness beliefs (Kuipers and Bebbington, Reference Kuipers, Bebbington, Sartorius, Leff, L'opez-Ibor, Maj and Okasha2005; Gonclaves-pereira et al., Reference Gonclaves-pereira, Xavier, van Wijngaarden, Papoila, Schene and Caldas-de-Almedia2013; Patel et al., Reference Patel, Chawla, Krynicki, Rankin and Upthgrove2014). EE and burden are long-term risk factors for poorer illness outcomes (Bebbington and Kuipers, Reference Bebbington and Kuipers1994). Hence, the inclusion of evidence-based psychosocial interventions for individuals with psychosis and their families in several treatment guidelines across the globe (Kreyenbuhl et al., Reference Kreyenbuhl, Buchanan, Dickerson and Dixon2010; National Institute for Health and Care Excellence (NICE), 2014; Galletly et al., Reference Galletly, Castle, Dark, Humberstone, Jablensky, Killackey, Kulkarni, McGorry, Nielssen and Tran2016; Norman et al., Reference Norman, Lecomte, Addington and Anderson2017). Traditionally, the interventions integrate different components such as psychoeducation, problem-solving, emotional processing, each designed to facilitate a better understanding about psychosis, a more relaxed family atmosphere and greater use of adaptive coping strategies.
The current study
The predictors of outcome across treatment groups can provide valuable prognostic information by helping to clarify which participants will respond more favourably to treatment in general, whereas treatment moderators provide prescriptive information about optimal treatment selection. Although there are clinical benefits in establishing baseline predictors of overall treatment success, identifying treatment moderators (i.e. who will do better in which treatment) may have more important clinical and cost-effectiveness implications.
There is, however, a very limited evidence base on treatment predictors in carer populations in psychosis. Further, where there is available data, they are rarely based on epidemiological representative samples compared with controls, which invariably increases the risk of underestimating the complexities of treating families in real-world services. Likewise, the literature is also scarce on moderators of treatment outcomes in carers. Despite the value of identifying the subgroups of caregivers and the circumstances associated with the effectiveness of early multi-element psychosocial interventions for psychosis, there is, as yet, little information about moderators of outcome. These findings would be extremely relevant in order to clarify generalisability issues of the experimental intervention effectiveness. The present study aims to address this gap in the literature.
As part of the GET UP (Genetics, Endophenotypes, Treatment: Understanding early Psychosis), PIANO (Psychosis: early Intervention and Assessment of Needs and Outcome) multi-element psychosocial intervention cluster trial in first-episode psychosis (FEP) (Ruggeri et al., Reference Ruggeri, Bonetto, Lasalvia, De Girolamo, Fioritti and Rucci2012), the current study sought to identify, among pre-treatment characteristics, predictors and moderators of caregiver burden and emotional distress as measured by the General Health Questionnaire-12 (GHQ-12) self-report screen for psychiatric disturbance at 9 months post baseline. It aimed to understand: (a) which caregivers’ characteristics, among pre-treatment variables at baseline, are associated with a better treatment response regardless of treatment type (non-specific predictors); and (b) which characteristics are associated with a better response defined in terms of reduced levels of carer burden and emotional distress to the specific treatment provided in the GET UP PIANO trial (moderators). Based on the existing literature, we hypothesised that, regardless of treatment, improvement in carer burden and emotional distress at 9 months would be associated with non-parental caregivers, fewer hours per week spent between carers and patients and patients’ greater perception of positive care from carers (Bebbington and Kuipers, Reference Bebbington and Kuipers1994; Kuipers and Bebbington, Reference Kuipers, Bebbington, Sartorius, Leff, L'opez-Ibor, Maj and Okasha2005; Awad and Voruganti, Reference Awad and Voruganti2008; Poon et al., Reference Poon, Harvey, Mackinnon and Joubert2016; Sadath et al., Reference Sadath, Muralidhar, Varambally, Gangadhar and Jose2017). Given the lack of available information, no specific a priori hypotheses were offered about moderators; thus, moderator analyses will be exploratory and utilise the same set of variables analysed as predictors.
Methods
The GET UP PIANO trial
The GET UP PIANO trial (Ruggeri et al., Reference Ruggeri, Bonetto, Lasalvia, De Girolamo, Fioritti and Rucci2012) is a large multi-centre randomised controlled cluster trial comparing an add-on multi-element psychosocial early intervention with ‘routine care’ for patients with FEP and their caregivers provided within Italian public general mental health services. It was designed to assess early multi-element psychosocial interventions in epidemiologically representative samples of patients and families treated in routine generic mental health settings. Of the 126 community mental health centres (CMHCs) located in two northern Italian regions (Veneto and Emilia-Romagna) and the urban areas of Florence, Milan and Bolzano, 117 (92.8%) participated, covering an area of 9 304 093 inhabitants. The assignment units (clusters) were the CMHCs, and the units of observation and analysis were patients and their families. The trial received approval by the ethics committees of the coordinating centre (Azienda Ospedaliera Universitaria Integrata di Verona) and each participating unit and was registered with ClinicalTrials.gov (NCT01436331). Full details on the protocol of the GET UP PIANO study and on the main findings of the GET UP PIANO trial are given elsewhere (Ruggeri et al., Reference Ruggeri, Bonetto, Lasalvia, De Girolamo, Fioritti and Rucci2012, Reference Ruggeri, Bonetto, Lasalvia, Fioritti, de Girolamo and Santonastaso2015).
Participants
During the index period, all CMHCs participating in the GET UP PIANO trial were asked to refer potential cases of psychosis at first contact to the study team. Inclusion eligibility comprised patients aged 18–54 years; residence within specified CMHC catchment area; presence of at least one of the following: hallucinations, delusions, qualitative speech disorder, qualitative psychomotor disorder, bizarre or grossly inappropriate behaviour, or two of the following: loss of interest, initiative and drive, social withdrawal, episodic severe excitement, purposeless destructiveness, overwhelming fear or marked self-neglect (as rated by the World Health Organisation (WHO) Screening Schedule for Psychosis (WHO, 1992), and first contact with CMHC). Exclusion criteria comprised a 3-month or greater history of use of anti-psychotic medication for treatment of the same or similar mental health problem; presence of other mental health condition(s) due to general medical condition; other International Classification of Diseases-10 psychiatric diagnosis (apart from psychosis); moderate–severe learning disability confirmed by clinical functional assessment.
Across both study arms, patients meeting inclusion criteria were invited to undertake standardised assessments as soon as possible, once they achieved clinical stabilisation and provision of informed written consent. They were provided with information detailing the nature, scope and possible consequences of participation in the trial and informed that they could withdraw consent at any time. Patient participants were also asked to give consent for family member contact; family members who agreed to participate provided written informed consent. There were no inclusion or exclusion criteria for relatives, beyond that all eligible patient participants were required to provide consent to involve a key family member in the assessments. The data are based on one identified carer per household.
Treatments
The experimental treatment consisted of a multi-element psychosocial intervention, adjunctive to routine care. It included the delivery of cognitive behavioural therapy (CBT) for psychosis to patients (Kuipers et al., Reference Kuipers, Fowler, Garety, Chisolm, Freeman, Dunn, Bebbington and Hadley1998; Garety et al., Reference Garety, Fowler, Freeman, Bebbington, Dunn and Kuipers2008), and of psychosis-focused family intervention (FI) (Kuipers et al., Reference Kuipers, Leff and Lam2002) to families, together with case management (Burns and Firn, Reference Burns and Firn2002), involving both patients and their families. The family-based, psychosis-focused intervention treatments were provided by psychiatrists and psychologists in the participating teams. They had completed specialist therapy training and had their competencies assessed with a specified minimum threshold level required to offer treatment to patients. The interventions typically include psychoeducation, emotional support, coping strategies, problem solving, emotional processing and relapse prevention (crisis planning) components that vary according to the presenting needs and agreed goals. The interventions comprised an optimal 10–15 sessions (typically six sessions in the initial 3 months followed by monthly sessions over the remaining 6 months) delivered over a 9-month period. Interventions were for individual families and delivered in accordance with the Kuipers et al.’s (Reference Kuipers, Leff and Lam2002) evidence-based treatment manual. To support treatment fidelity and avoid therapy drift, therapists attended regular supervision with external therapy experts that included written session summaries. An independent team of raters assessed random samples of audiotaped therapy recordings against therapy checklist. Control arm CMHCs provided only treatment as usual (TAU), which, in Italy, comprises personalised outpatient psychopharmacological treatment and non-specific supportive clinical management by the CMHC (Ferrannini et al., Reference Ferrannini, Ghio, Gibertoni, Lora, Tibaldi, Neri and Piazza2014). FIs in TAU consisted of non-specific informal support sessions.
Measures
Carers
Carer outcomes (i.e. burden and emotional distress) were assessed by the Involvement Evaluation Questionnaire (IEQ-EU, van Wijngaarden et al., Reference van Wijngaarden, Schene, Koeter, Vazquez-Barquero, Knudsen, Lasalvia and Mcrone2000) and the GHQ-12 (Goldberg and Williams, Reference Goldberg and Williams1988) at baseline (before treatment was initiated) and at 9-month follow-up, by independent researchers, blind to treatment allocation.
The IEQ-EU (van Wijngaarden et al., Reference van Wijngaarden, Schene, Koeter, Vazquez-Barquero, Knudsen, Lasalvia and Mcrone2000) is a widely used measure of carer burden that taps broad domains of caregiving experience and easy to complete. It is a 31-item four subscale questionnaire. The subscales relate to the encouragement and care that the caregiver has to give to the patient (urging); to personal problems between patient and caregiver (tension); to the caregiver's worries (worrying); and burden and monitoring patients about their medication, sleep and any dangerous behaviours (supervision). All items are scored on a five-point Likert scale. Higher scores indicate greater burden of care as an overall scale and within each domain. The measure has been translated into several different languages and culturally validated, including for use with Italian populations. It is psychometrically sound with proven reliability and validity data (Van Wijngaarden et al., Reference van Wijngaarden, Schene, Koeter, Vazquez-Barquero, Knudsen, Lasalvia and Mcrone2000). Across different studies, internal consistency ratings (Cronbach's α) across the separate subscales have ranged from 0.68 to 0.86 and for the total scores has been 0.87–0.90. The test–retest reliability ratings are at least at 0.70 (Van Wijngaarden et al., Reference van Wijngaarden, Schene, Koeter, Vazquez-Barquero, Knudsen, Lasalvia and Mcrone2000).
The GHQ-12 (Goldberg and Williams, Reference Goldberg and Williams1988) is a global, widely used and cross-culturally validated measure to screen and identify minor psychiatric disorders. Each item assesses the severity of a mental health problem over the past few weeks using a four-point Likert scale. Higher scores indicate more psychological(emotional) distress. Its application as a unidimensional measure of distress has been most common through multidimensional approaches and focus on the individual factors (Graetz, Reference Graetz1991). It has extensive, worldwide published data attesting its reliability and validity in different groups (e.g. Werneke et al., Reference Werneke, Goldberg, Yalcin and Ustun2000; Chandra Kashyap and Kant Singh, Reference Chandra Kashyap and Kant Sing2017) including those from Italy (Politi et al., Reference Politi, Piccinelli and Wilkinson1994), which yielded Cronbach's α ratings of 0.81. In its original form, the GHQ-12 was 60 items that were subsequently reduced to 30 items, 24 items and then 12 items. The 12-item version yields comparable reliability ratings to longer forms and has good validation against standardised mental health interviews (Goldberg et al., Reference Goldberg, Gater, Sartorius, Ustun, Piccinelli, Gureje and Rutter1997; Politi et al., Reference Politi, Piccinelli and Wilkinson1994).
Patients
The 25-item Parental Bonding Instrument (Parker et al., Reference Parker, Tupling and Brown1979) measures an adult's retrospective account of the parenting they received up to the age of 16 years. The measure is completed separately for care received from the mother and father. It yields two scales: ‘care’ and ‘overprotection’ (or ‘control’). Higher scores reflect a greater recollection of that parenting style. Optimal parenting is typically expressed by participant reports of high care and low control.
The Level of Expressed Emotion Scale (LEE, Cole and Kazarian, Reference Cole and Kazarian1988; Cole and Kazarian, Reference Cole and Kazarian1993) is a 60-item self-report measure designed to assess patient perceptions of carer EE. It was originally conceived as a reliable and expedient alternative to the Camberwell Family Interview (Vaughn and Leff, Reference Vaughn and Leff1976), the gold standard measurement of carer EE. It comprises four subscales: emotional response (e.g. high emotional response to illness (e.g. anger)), negative attitude (e.g. doubt patient is genuinely ill, blame patient for illness), intrusiveness (e.g. offering unsolicited often critical advice and frequent attempts to have contact) and low tolerance and high expectations (e.g. intolerance of illness behaviour and impairments). Respondents are required to read through a set of brief statements and indicate to what degree the statement accurately represents their carer's behaviour towards them during the preceding 3 months on a Likert scale of 1 (untrue) to 4 (true). An overall EE and subscale scores are generated.
As a global measure of patient symptomatology, the Positive and Negative Syndrome Scale (PANSS, Kay et al., Reference Kay, Fiszbein and Opler1987) was used. The PANSS is a 30-item semi-structured interview used to rate psychotic symptomatology and comprises three subscales related to positive symptoms, negative symptoms and general psychopathology. Interview items are rated on a seven-point Likert scale that reflects increasing levels of psychopathology with higher scores indicating higher levels of symptomatology.
The Childhood Experience of Care and Abuse Questionnaire (CECAQ, Bifulco et al., Reference Bifulco, Brown and Harris1994) is a self-report questionnaire that taps adverse childhood experiences including reports of physical and sexual abuse and neglect. A single item that assesses patient perceptions of caregiver criticism was used as an additional method to assess relationship quality.
Before starting the assessments, independent evaluators received formal training in the use and administration of instruments, with measurement of their knowledge, skills and assessment of inter-rater reliability to assess competency. Assessments followed once patients had achieved clinical stabilisation (i.e. sufficient mental state stability to engage in a brief clinical assessment), provided written informed consent and prior to commencement of interventions.
Statistical analyses
Analyses were conducted using an intention-to-treat approach. IEQ-EU and GHQ-12 scores were analysed separately in mixed-effects random regression models. In order to take into account the trial design in which caregivers (level 1) were nested within CMHCs (level 2) (CONSORT guidelines for cluster randomised trials; Campbell et al., Reference Campbell, Piaggio, Elbourne and Altman2012), the individual CMHCs were included in the models as a random effect. In order to identify predictors and moderators of treatment outcome according to MacArthur's approach (Kraemer et al., Reference Kraemer, Wilson, Fairburn and Agras2002), we selected, a priori, on clinical or empirical grounds and derived from the literature, pre-treatment caregivers’ variables. Specifically, we investigated age and gender of caregiver, family relationship shared with patient (parents v. others), hours per week spent with patient (<32 v. ⩾32), mother's criticism and father's criticism (assessed by CECA-Q item 6; yes v. no), PBI (care and protection (mother), care and protection (father)), LEE (emotional response, negative attitude, intrusiveness, tolerance and expectations) and IEQ-EU tension at baseline (this last variable considered only for GHQ-12). Each model included treatment allocation (T coded as +1/2 for caregivers in the Experimental Treatment Group and −1/2 for those in the TAU Group), one predictor/moderator (M standardised), their interaction (T × M) and the baseline score of the outcome investigated (B standardised). When the main effect of a variable was significant, but the interaction was not, the variable was considered a non-specific predictor of outcome. When the interaction was significant (regardless of the significance of main effects), the variable was considered as a moderator.
In a secondary analysis, missing data on outcomes were estimated using a multiple imputation approach by chained equations (MICE), which generate 50 different plausible imputed data sets and combines results from each of them. Multiple imputations by chained equations were applied because it enables different variable types to be handled; specifically, we used predictive mean matching to deal with possible non-normality when imputing continuous variables.
The α level was set to 0.05 for all main effects and interactions. No correction for multiple testing was applied due to the exploratory nature of the study. All statistical analyses were carried out using the STATA software package, version 13 (Stata Corp, 2013)
Results
Overall, 380 relatives (230 experimental; 150 TAU) out of 444 FEP patients were available for assessment at baseline. In the experimental arm, 16 patients did not have an identified relative; six patients declined consent to contact their relative; seven relatives declined consent to engage in the FI; and 13 patients refused to engage with the individual CBT, so the matched relative was excluded. In the TAU arm, ten patients did not have an identified relative and 12 patients declined consent to contact their relative (see Fig. 1 for relative's trial profile).

Fig. 1. Trial profile for relatives.
At baseline, 185 experimental arm and 75 TAU arm relatives were assessed. Demographic and pre-treatment characteristics of the 260 caregivers examined as potential predictors or moderators of outcome are presented in Table 1 and have been previously published elsewhere (Lasalvia et al., Reference Lasalvia, Bonetto, Lenzi, Rucci, Lozzino and Cellini2017; Ruggeri et al., Reference Ruggeri, Lasalvia, Santonastaso, Pileggi, Leuci and Miceli2017).
Table 1. Pre-treatment characteristics of caregivers examined as potential predictors/moderators of carer treatment outcome (EXP n = 185; TAU n = 75)

No significant differences with respect to socio-demographics of relatives and link with patient variables were found between the two trial arms. At follow-up, 60 (32.4%) caregivers in the experimental group and 15 (20.0%) in the TAU group dropped out from assessment. There were no significant differences in demographics and outcome variables at baseline between completers and non-completers, with exception only of the GHQ-12 total score in the experimental group (completers: 14.27 s.d. 6.00 v. non-completers: 16.39 s.d. 7.84; p = 0.044).
By considering burden of care (IEQ-EU), both groups had similar baseline scores (t-test; p > 0.05). Specifically, we observed the following scores: Total EXP 2.07 s.d. 0.69 v. TAU 1.98 s.d. 0.63; Tension EXP 1.70 s.d. 0.66 v. TAU 1.57 s.d. 0.50; Supervision EXP 1.75 s.d. 0.99 v. TAU 1.58 s.d. 0.79; Worrying EXP 2.81 s.d. 1.15 v. TAU 2.69 s.d. 0.98; Urging EXP 2.09 s.d. 0.85 v. TAU 2.10 s.d. 0.89. Both groups experienced an improvement at follow-up, however no dimension reached statistical significance (Total EXP 1.79 s.d. 0.93 v. TAU 1.80 s.d. 0.64; Tension EXP 1.60 s.d. 1.02 v. TAU 1.58 s.d. 0.64; Supervision EXP 1.54 s.d. 1.13 v. TAU 1.38 s.d. 0.71; Worrying EXP 2.14 s.d. 0.96 v. TAU 2.31 s.d. 1.12; Urging EXP 1.81 s.d. 1.02 v. TAU 1.88 s.d. 1.02). Emotional distress (GHQ-12) differed significantly between the two groups at baseline (EXP 15.06 s.d. 6.82 v. TAU 12.97 s.d. 5.69; p = 0.023 t-test), while both groups experienced significant improvement at the 9 months follow-up – this proved more so for the experimental group (see Table 2) (EXP 10.88 s.d. 4.58 v. TAU 11.65 s.d. 6.03; (FU-BL) EXP v. TAU −1.71, p = 0.029).
Table 2. Relatives’ outcomes: IEQ and GHQ assessed at baseline and at 9-month follow-up, together with regression coefficients of experimental treatment v. treatment as usual (95% CI)

a p = 0.023, t-test.
Most families in the experimental group engaged in at least one family session (91.1%, n = 170); the majority receiving five or more FI sessions (90.6%, n = 154), and from these, 72.7% (n = 112) attended ten or more sessions.
Predictors
Of the predictors examined, only patient reports of early maternal criticism (i.e. during the first 16 years) predicted lower caregiver worrying as measured by IEQ-EU at 9 months (b = –0.36, p = 0.019), regardless of treatment assignment (see Table 3 Main effect column). However, multiple imputation analysis did not confirm this result.
Table 3. Pre-treatment characteristics as potential predictors/moderators of treatment outcome in caregivers. Mixed-effects random regression models estimated on caregivers who were assessed at both baseline and follow-up (EXP n = 125; TAU n = 60) (only variables significant at p < 0.05) are shown)

*Predictors/moderators which remained significant (p < 0.05) after applying multiple imputation procedure by chained equations (MICE).
Moderators
Differential effects of pre-treatment IEQ-EU Tension on GHQ-12 (b = –0.37, p = 0.044) were found (see Table 2 Interaction with treatment column). Moreover, the LEE tolerance and expectations dimension moderated IEQ-EU Tension domain (b = + 0.48, p = 0.021), while age of caregiver was a moderator of IEQ-EU Worrying (b = + 0.35, p = 0.017). When analyses were rerun using multiple imputation of missing data, all these findings were confirmed (b = –0.38, p = 0.003; b = + 0.42, p = 0.034 and b = + 0.34, p = 0.022, respectively).
In order to determine the pre-treatment IEQ-EU Tension level cut-off at which the experimental treatment started to be significantly superior to usual care, the domain was categorised using different cut-offs in a sensitivity analysis. This analysis showed that starting from 2.0 there was a significantly higher beneficial effect of experimental treatment at 9 months, in terms of reduction in GHQ-12 total scores (Fig. 2). Carers with IEQ-EU Tension levels below 2 showed similar reduction of GHQ-12 in both experimental and usual treatment.
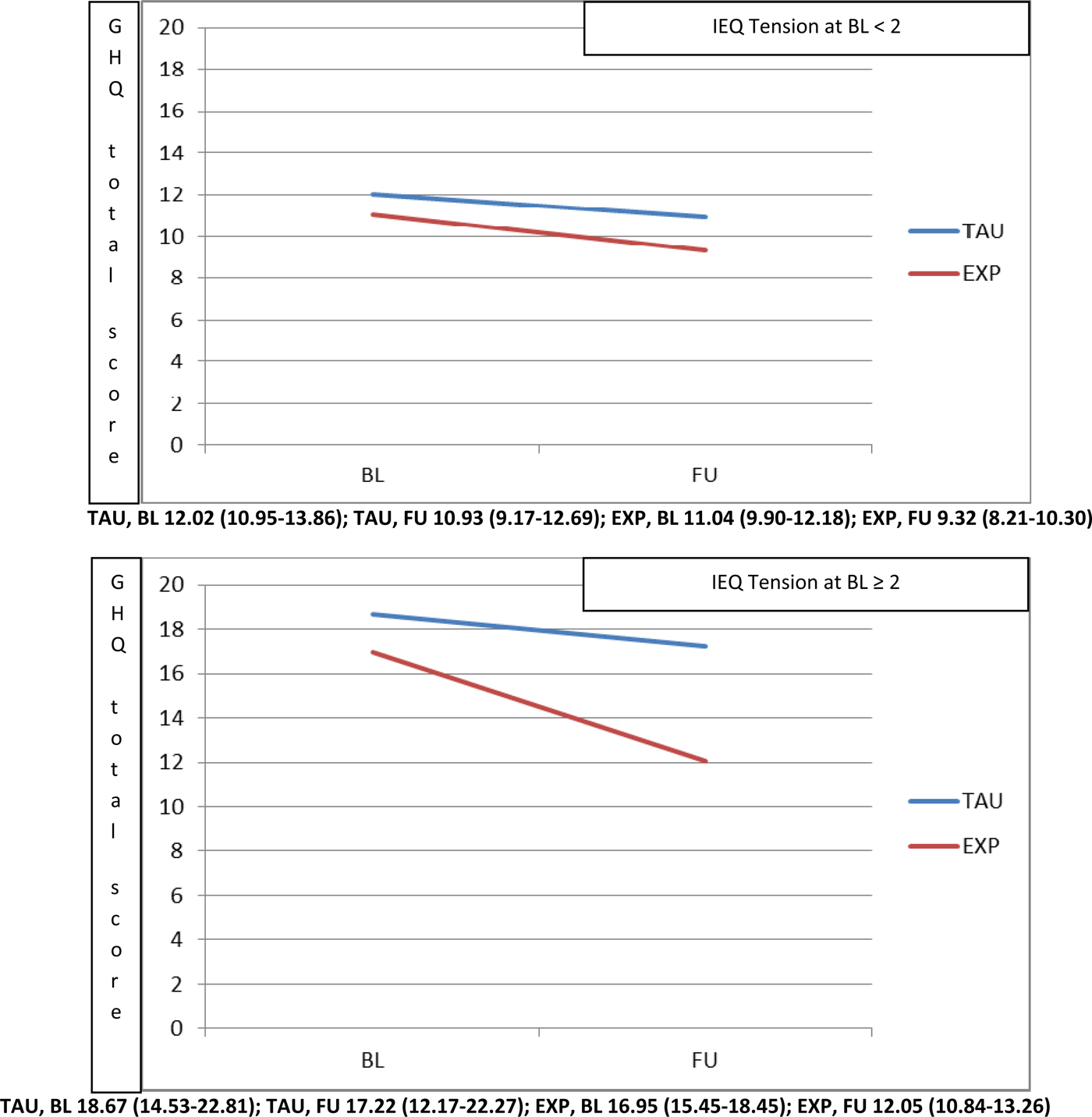
Fig. 2. Moderation played by pre-treatment IEQ tension domain (top panel <2, bottom panel ⩾2) on the effect of intervention (Experimental v. TAU) on the GHQ-12 total score.
The same approach was applied in order to explore the moderation due to the LEE tolerance and expectations domain on IEQ-EU Tension. We found that where patients reported LEE tolerance and expectations levels below 8 (i.e. where patient perceptions of carer tolerance towards the patient were low), carers showed a significantly higher beneficial effect of experimental treatment at 9 months, in terms of reduction in IEQ-EU Tension scores (Fig. 3, top panel).

Fig. 3. Moderation played by LEE tolerance and expectations domain (top panel <8, bottom panel ⩾8) on the effect of intervention (Experimental v. TAU) on the IEQ tension domain.
Finally, carers aged <51 years (at the top of Fig. 4) experienced a higher beneficial effect of experimental treatment in terms of reduction in IEQ-EU Worrying, while carers aged 51 years and above experienced at 9 months, a similar reduction of IEQ-EU Worrying in both the experimental and usual treatment arms.

Fig. 4. Moderation played by age of caregiver (top panel <51, bottom panel ⩾51) on the effect of intervention (Experimental v. TAU) on the IEQ worrying domain.
Discussion
The FEP can be a traumatic and stress-provoking period for individuals with psychosis and their families (McCann et al., Reference McCann, Lubman and Clark2011; Bendall et al., Reference Bendall, Alvarez-Jimenez, Hulbert, McGorry and Jackson2012). The illness course can fluctuate with elevated levels of relapse and poor social and vocational functioning (Robinson et al., Reference Robinson, Woerner, Alvir, Bilder, Goldman, Geisler, Koreen, Sheitman, Chakos, Mayerhoff and Lieberman1999; Velthorst et al., Reference Velthorst, Fett, Reichenberg, Perlman, van OS, Bromet and Kotov2017). The impact of care (i.e. carer burden) is often recorded at its highest levels during the first episode (Addington et al., Reference Addington, Coldham, Jones, Ko and Addington2003). Access to evidence-based psychosocial interventions, designed to improve understanding, uptake of adaptive coping and address the negative impact of illness on functioning and relationships, is increasingly proposed and implemented in several different countries (Mueser et al., Reference Mueser, Penn, Addington, Brunette, Gingerich, Glynn, Lynde, Gottlieb, Meyer-Kalos, McGurk, Cather, Saade, Robinson, Schooler, Rosenheck and Kane2015; Marwaha et al., Reference Marwaha, Thompson, Upthegrove and Broome2016). This is the first study to investigate in a FEP ‘real-world’ setting which caregiver characteristics: (a) predict carer burden and emotional distress at 9 months regardless of treatment assignment (non-specific predictors) and (b) moderate differential response of treatment (moderators).
The results identified only one significant treatment predictor, which was patient perception of early maternal criticism. It predicted carer burden, specifically in terms of carer reports of worry. The significance of this finding, however, was not maintained after multiple imputation analysis for missing data. Thus, overall, the current findings did not identify pre-treatment predictors for carer outcomes. It is unclear why there was a failure to identify any significant predictors of treatment outcome for carers. It could be argued that the predictors, themselves, were not the most suitable. However, as reported, the selected predictors were drawn up based on indications from the relevant literature. The absence of significant findings suggest a greater complexity of factors potentially impacting carer treatment outcomes at FEP and highlighted the need for further work to isolate these key variables. It would seem important to note that it was only until very recently that family-based interventions recorded carer outcomes in their own right (Lobban et al., Reference Lobban, Postlethwaite, Glentworth, Pinfold, Wainwright, Dunn, Clancy and Haddock2013), and highlighted the importance of looking at carer outcomes.
In contrast, our exploratory analyses identified three significant moderators of carer burden and distress. Higher pre-treatment levels of carer burden, specifically in terms of tension (i.e. strained and difficult relations between carer and relative), moderated effects of treatment on carer outcomes to yield greater reductions on emotional distress levels in carers. Patient perceptions of greater carer intolerance of the patient and their illness symptoms moderated greater reductions in carer burden in terms of tension; and younger age of caregiver (<51 years old) moderated greater reductions in carer burden, specifically in terms of worry. It could be suggested that carers expressing interpersonal difficulties with their relative which, in some circumstances, might have predated the psychosis onset, will also be the groups to derive the greatest benefits from the multi-component interventions. Whilst their elevated levels of burden could serve as a marker of those carers who are most in need and struggling with their understanding and adaptation to the illness. It could also simply be the case that given their elevated burden levels, there is more room to demonstrate improvements. However, the importance of not assuming that carers who present in a less overtly distressed manner or report less relationship difficulties with their relative do not require input from services is acknowledged (Treanor et al., Reference Treanor, Lobban and Barrowclough2013).
The uneven number of carers across the treatment conditions was noted. It possible that the relatives were more attracted by the description of the FI provided in the experimental arm as compared with the usual non-specific informal support sessions proposed in the control group. This phenomenon occurred on a naturalistic basis as all staff members received formal training in describing both treatments as efficacious.
The difficulties observed in the wellbeing and functioning of carers of long-term psychosis populations can typically emerge soon after the first episode. We know that family environment at FEP offers important implications for the quality and direction of patient outcomes (Domínguez-Martínez et al., Reference Domínguez-Martínez, Medina-Pradas, Kwapil and Barrantes-Vidal2014; Koutra et al., Reference Koutra, Trilivac, Roumeliotaki, Basta, Simos, Lionis and Vgontzas2015; Haidl et al., Reference Haidl, Rosen, Schultze-Lutter, Nieman, Eggers, Heinimaa, Juckel, Heinz, Morrison, Linszen, Salokangas, Klosterkötter, Birchwood, Patterson and Ruhrmann2018). Our results are encouraging and suggest multi-element psychosocial treatment approaches delivered during the FEP phase in routine mental health services, does appear to exert a specific and additional beneficial effect on caregivers (Penn et al., Reference Penn, Waldheter, Perkins, Mueser and Lieberman2005), and we now have an awareness of potential factors that can moderate enhanced outcomes.
Strengths and limitations
To the best of our knowledge, this study represents the first exploration of predictors and moderators of carer outcomes in FEP following multi-element treatments or TAU treatment. It extends similar work exploring general outcomes of psychosocial interventions in patients (Penn et al., Reference Penn, Waldheter, Perkins, Mueser and Lieberman2005) and compliments developments in identifying treatment predictors and moderators in patients (Lasalvia et al., Reference Lasalvia, Bonetto, Lenzi, Rucci, Lozzino and Cellini2017). The sample size, prospective design methodology and rationale underpinning the study in a large catchment area and in a highly representative cohort of participants remain notable strengths. The study, however, does have limitations. First, the sample was drawn from specified Northern Central Italian regions, which means caution is required before generalising findings to groups from other socio-economic areas. The number of relatives that did not provide their consent to complete baseline assessments and the proportion of relatives classified as non-completers at follow-up might also serve as a limitation. Thus, it is possible that these relatives might have held specific appraisals regarding how they perceive their relative, the illness and the nature of their family relationship that limits the generalisability of findings to the wider group of carers. Second, we previously acknowledged that our moderator analyses were exploratory, with the primary aim of providing useful information for designing future studies. This is likely to improve with time following a greater focus on carer outcomes. We are aware that we performed a high number of statistical tests without correction. Multiple testing corrections are applied in order to reduce the number of false positives, but this correction may increase the number of false negatives, where there is an effect but we do not detect it as statistically significant. Due to the exploratory nature of this study, we did not apply multiple testing because the cost of a false negative could be that we have missed out on an important result to be confirmed in future larger studies.
Implications
Our findings are encouraging but require replication and employment of samples drawn from other geographical contexts. Future considerations of the underlying mechanisms or key therapeutic components that give rise to the positive changes are indicated. We already know that in an unselected group of FEP carers in routine services, multi-element psychosocial interventions can yield more positive outcomes on carer distress and burden of care than TAU (Ruggeri et al., Reference Ruggeri, Bonetto, Lasalvia, Fioritti, de Girolamo and Santonastaso2015). In services where resources might be limited and access to support triaged and prioritised, it would appear that younger aged carers exhibiting higher levels of burden, interpersonal difficulties with the patient and struggling to acknowledge that the identified patients do have a recognisable mental health problem that is likely to impact on their functioning and behaviour are also those most likely to exhibit the greatest gains from the interventions.
Conclusion
Following the increasing and globalised focus on early intervention in psychosis (e.g. Marwaha et al., Reference Marwaha, Thompson, Upthegrove and Broome2016), the results offer some helpful guidance on resource allocation and prioritisation. Though the evidence base for targeting recommended evidence-based interventions in psychosis to those identified to derive greatest benefit remains limited (Harveyet al., Reference Harvey, Lewis and Farhall2018), our preliminary findings support the approach. The important role played by carers in helping to improve the scale and quality of patient outcomes in psychosis is well established, as is the need to provide comprehensive care packages to support them in their role (Mueser et al., Reference Mueser, Penn, Addington, Brunette, Gingerich, Glynn, Lynde, Gottlieb, Meyer-Kalos, McGurk, Cather, Saade, Robinson, Schooler, Rosenheck and Kane2015). However, far more evidence is required to improve our understanding of the benefits of interventions and key determinants of optimal carer outcomes in FEP.
Availability of data and materials
The data that support the findings of this study are available, upon reasonable request and with permission, from Professor Mirella Ruggeri and The Get Up Group.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2045796019000155
Acknowledgements
We are grateful to the patients and their family members who participated in this study.
Financial support
This work was supported by the Ministry of Health, Italy – Ricerca Sanitaria Finalizzata, Code H61J08000200001.
Conflict of interest
None.









