In humans, undernutritional exposure in fetal and/or neonatal life is associated with an increased risk of adult diseases such as obesity, non-insulin-dependent diabetes and hypertension(Reference Hales and Barker1–Reference Reynolds, Walker and Phillips3). It has been suggested that low substrate availability during these critical periods of development represents a structural stressor producing long-term changes in different tissues. The capacity of the developing tissues to adapt to undernutrition causing permanent alterations has been termed ‘metabolic programming’(Reference Lucas4, Reference Vieau5). Several studies have reported that undernutrition during gestation alters both maternal and fetal levels of messengers such as insulin-like growth factors, growth hormone, insulin, glucocorticoids, leptin, catecholamines, thyroid hormones (TH) and placental hormones, all of which are closely associated with fetal growth and development(Reference Fowden, Giussani and Forhead6). In rats, the complete functionality of the hypothalamic–pituitary–thyroid (HPT) axis occurs mainly after birth. On the other hand, the thyroid gland begins to differentiate from fetal days 17–18 onwards when the colloid and follicular structures appear, and it reaches its full-grown structure and function on day 10 of lactation(Reference Pracyk, Seidler and McCook7, Reference Brown, Shalhoub and Coulter8). In this way, the critical period for the development and functional differentiation of the thyroid axis includes both pre- and post-perinatal periods. Extensive literature has demonstrated the crucial role of TH in the development and function of several tissues, besides in those of the brain. In metabolic terms, TH are crucial for maintaining the RMR and obligatory thermogenesis; they also play an important role in facultative thermogenesis, exerting a synergic effect with catecholamines in brown adipose tissue (BAT) by promoting heat production to maintain body temperature in a cold environment(Reference Silva9) and body weight during overfeeding(Reference Cannon and Nedergaard10). In the last few years, several studies have shown that nutritional conditions can determine thyroid status in humans(Reference LoPresti, Gray and Nicoloff11) and animals(Reference Quigley, Goya and Nachreiner12). In adults, severe food restriction suppresses the activity of the HPT axis, decreasing pituitary thyrotropin (TSH) content and plasma 3,5,3′-triiodothyronine (T3) and thyroxine (T4) levels(Reference van Haasteren, Linkels and van Toor13). In the perinatal period, modifications in thyroid status may depend on the period (prenatal or postnatal) and the time when the analysis is carried out, i.e. immediately after food restriction (weaning time) or in adulthood. In general terms, it is known that pups of food-restricted rat dams during gestation, lactation or both exhibit low plasma TSH, T3 and T4 levels at weaning(Reference Aláez, Calvo and Obregón14–Reference Fetoui, Bouaziz and Mahjoubi-Samet16), but if the analysis is carried out in the post-weaned period or adulthood, rats whose dams were fed protein-deficient diets during the neonatal period consistently exhibit alterations in the thyroid profile such as elevated or normal T3 levels, elevated TSH levels and/or a poor response of pituitary TSH release to in vitro thyrotropin-releasing hormone (TRH)(Reference Passos, Fonte Ramos and Dutra17–Reference Lisboa, Fagundes and Denolato19). In humans, it is difficult to establish nutritional conditions during pregnancy, though low birth weight and small body size at birth are the markers of fetal nutritional deficiencies. These factors have been reported to be associated with an increased risk for developing thyroid dysfunction in adulthood(Reference Kajantie, Phillips and Osmond20, Reference Phillips, Osmond and Baird21). The aim of the present study was to analyse the functionality of the HPT axis in the adult offspring of undernourished rat dams during gestation and lactation, in resting conditions and in response to a functional challenge (cold exposure). The present results showed that undernourished rats exhibited transitional changes in thyroid function on postnatal day 21, manifested as decreased T3 levels and normal T4 and TSH levels, but displayed a significant flip on day 90, which consisted of normalised T3 (total and free) and total T4 levels, but lower free T4 and persistently higher TSH levels, which were maintained even on postnatal day 140. This profile was accompanied by a scarce fat depot, a lower RMR and an exacerbated sympathetic BAT tone in basal conditions and attenuated thyroid axis responses to cold exposure, suggesting permanent alterations in both thyroid function and HPT axis.
Materials and methods
Induction of undernourishment during gestation and lactation
Female Wistar rats initially weighing 240 (sem 20) g were housed in individual cages in a room under regulated temperature (22 ± 2°C) and 12 h dark–12 h light cycles. After 2 weeks of adaptation, the rats were randomly assigned to consume a commercial diet (Rodent Laboratory Diet 5001 containing 1 μg iodine/g, which ensures the recommended iodine daily intake in both the groups(Reference Obregón, Ruiz de Oña and Calvo22)) and water ad libitum (control group) or a diet representing only 60 % of the mean food intake of the control group (restricted group). All rats were mated with male Wistar rats, and the food regimen was started on this day and continued during gestation and lactation. The experiment was carried out using ten control dams and twelve restricted dams. On partum day, considered as the first lactation day, only eight pups (four females and four males) were retained per dam. After weaning on the 21st day of age, the offspring of the control and restricted dams were housed in individual cages under conditions that were the same as those described above and allowed ad libitum access to water and the commercial diet. Body weight, body length and food intake (24 h) were measured three times per week until 90 d of age. On postnatal days 21 and 90, blood samples from the tail tip of female pups were collected and centrifuged at 5000 rpm for 10 min to obtain plasma, and the plasma was stored at − 20°C until TSH and TH (total and free T3 and T4) analyses. At 90 d of age, after measuring the RMR, the female offspring of the control dams and restricted dams were euthanised (40 mg/kg pentobarbital, intraperitoneally), and the carcasses and livers were processed for biochemical analysis. Handling and euthanasia of the rats were reviewed and approved by the ad hoc ethics committees of UNAM and IPN and complied with the Guide for the Care and Use of Laboratory Animals of the Mexican Council for Animal Care (NOM-062-ZOO-1999) and International Committee guidelines.
Determination of carcass contents
The carcasses were obtained by discarding heads, limbs, tails, skins and viscera of the rats and stored at − 20°C until the analysis of their components. Protein content was determined using Kjeldahl's method with automatic digestion (Kjeltec auto sampler system 1035, Analyzer Tekator; Foss Tecator, Foss North America Inc.); lipid content was measured using a dried powder of the samples with a Soxhlet apparatus by extraction with petroleum diethyl ether. Retroperitoneal adipose depot was extracted and weighed.
Determination of RMR
The RMR of the offspring was determined using an Oxymax System (Columbus Instruments) with connected Opto-Varimex Mini Devices for estimating motor activity. The rats were fasted overnight for 15 h. In each recording, 3 min events were measured for 6 h, and the data of VO2 (litres/kg0·75× h), CO2 production (litres/kg0·75× h), resulting respiratory quotient and metabolic rate (kJ/kg0·75× h) were entered into a computer, which also recorded activity given as the number of pulses. For more details, see Ayala et al. (Reference Ayala, Racotta and Hernández-Montes23).
Determination of hormone concentrations
The circulating levels of leptin were measured using a specific ELISA (Millipore Corporation). TSH levels were measured using RIA (Biotrack assay; Amersham Corporation). Total T3 and T4 levels were measured using a homologous RIA standardised in our laboratory(Reference Valverde and Aceves24), and free T3 and T4 levels were measured using ELISA (Diagnostica Internacional).
Determination of cold exposure responses of adult rat offspring of the control dams and restricted dams
On postnatal day 140, the male rats were exposed to room (22 ± 1°C) or low (4 ± 1°C) temperature for 24 h. Blood samples were collected before and after cold exposure and plasma leptin, TSH, T4 and T3 levels were measured. The rats were euthanised, and their tissues (ventral lobe of the liver and interscapular BAT) were dissected, weighed and stored at − 70°C. Hepatic 5′-deiodinase (HD1) activity was measured using the radioiodide release method(Reference Aceves, Escobar and Rojas-Huidobro25). BAT weight, catecholamine content and type 2 deiodinase (Dio2) mRNA expression were determined in the BAT as indicators of sympathetic response.
Determination of hepatic 5′-deiodinase activity
Liver samples were homogenised at 1:10 (w/v) in a buffer (pH 7·0) containing 10 mm-HEPES, 0·32 m-sucrose, 5 mm-dithiothreitol and 1·0 mm-EDTA. Crude homogenates were centrifuged at 12 000 rpm for 2 min. 125I-labelled reverse T3 was purified by passage through a column (Sep-Pak, C-18 Cartridges; Millipore Waters). In the standard assay, 50 μl of the homogenate (5 μg of protein) and 50 μl of a radiolabelled mix (2 nm-125I-labelled reverse T3, 0·5 μm-non-radiolabelled reverse T3 and 5 mm-dithiothreitol) in a final volume of 100 μl were used. After incubation (1 h at 37°C), the released acid-soluble 125I was isolated by chromatography on Dowex 50 W-X2 columns (Bio-Rad) and measured using a gamma counter. Protein content was measured using the Bradford method (Bio-Rad). Results are given as nmol I released/mg protein per h.
Determination of brown adipose tissue type 2 deiodinase expression
Dio2 and β-actin mRNA expression was determined using a semi-quantitative RT-PCR. Total RNA was isolated using the TRIzol reagent (Invitrogen). Single-strand complementary DNA was synthesised using 20 μg of total RNA using oligo d(T) as the primer. The reverse transcription product was amplified in 50 μl of PCR buffer containing 10 pmol of each oligonucleotide primer, 02 μm of dNTP and one unit of DNA polymerase. The sequences of the oligonucleotides used were (s) act cgg tca ttc, tgc, tgc tca ag and (as) ttc aaa ggc tac ccc ata ag for Dio2 and (s) aca gag tac ttg cgc tca gga and (as) cca tca tga agt gtg acg ttg for β-actin. The samples were subjected to twenty-eight or forty cycles (β-actin and Dio2, respectively), consisting of 45 s at 95°C, 45 s at 55°C and 45 s at 72°C. The last extension was carried out for 10 min. As a control, a reaction mixture containing an RNA sample with the appropriate primers, but without the RT, was included. The reaction products were analysed by 2 % agarose gel electrophoresis, and the resulting bands were visualised by ethidium bromide staining. Band sizes were confirmed with a 1 Kb DNA ladder (Invitrogen). Relative changes in Dio2 expression were normalised to those in β-actin (housekeeping gene) expression. Polaroid pictures were taken; the pictures were digitised using a Hewlett Packard Scanner Jet 11CX (Hewlett-Packard Company), and the signals were analysed using an editing version of the NIH-ImageJ 14.7 m program.
Determination of catecholamine content in brown adipose tissue
Noradrenaline (NA), adrenaline and dopamine concentrations were determined according to the method of Villanueva et al. (Reference Villanueva, Piñón and Quevedo-Corona26), using HPLC and an electrochemical detector (ESA Coulochem II). Briefly, BAT was homogenised (1:10, w/v) in ice-cold 0·4 m-perchloric acid and centrifuged, and the supernatant was frozen until catecholamine content determination. Catecholamines were extracted by adsorption on acid-washed alumina at pH 8·6, washed with deionised water several times and eluted in a 0·1 m-perchloric acid solution.
Statistical analysis
All results are expressed as the means with standard errors. Data were analysed using Student's two-tailed t test or two-way ANOVA with Tukey's post hoc test. Plasma TH levels, HD1 activity and BAT deiodinase expression evaluated in the cold exposure study were analysed using a two-way ANOVA with temperature and state of nutrition as factors. P< 0·05 was considered to be statistically significant. Data were analysed with the Sigma Stat software version 3.5 (Systat Software, Inc.).
Results
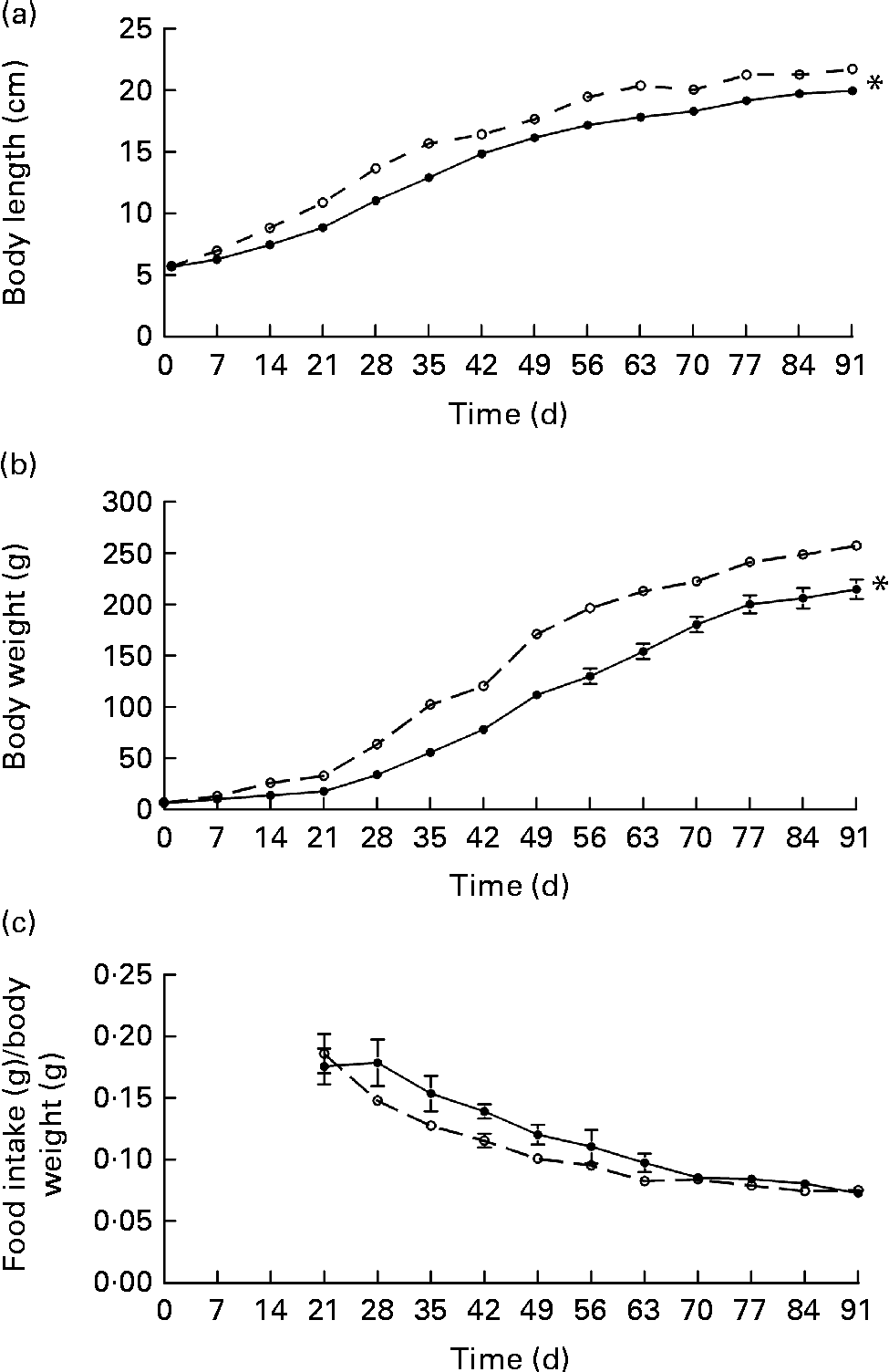
The first consequences observed in the offspring of undernourished dams were changes in their body weight and length in comparison with the control group. Although both the groups of rats had the same body length, body weight of the restricted rats was significantly lower on the day of birth. These results are in agreement with the lower body-weight gain observed in the restricted dams (88 (sem 6·6) g) in comparison with the control dams (168 (sem 3·7) g) during gestation. Although lower body weight and length (since day 7) were maintained in the restricted offspring at 90 d of age (Fig. 1), the slope in the growth rate was similar in both the groups. In addition, carcass protein contents were not different between the two groups, but relative lipid content (9·8 (sem 0·6) v. 7·7 (sem 0·6) %, P= 0·035) and retroperitoneal fat content were significantly lower in the restricted offspring (Table 1). Since this metabolic phenotype showed no sex differences, subsequent analyses were carried out in the male or female offspring indistinctly.

Fig. 1 (a) Body length, (b) body weight and (c) food intake/body weight in the female offspring of the control (–○–) or food-restricted dams during gestation and lactation (–●–). After day 21, the offspring were fed ad libitum. Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different from those of the control group (P< 0·05; two-way repeated-measures ANOVA).
Table 1 Metabolic parameters in adult female offspring (postnatal day 90) of the control dams or restricted dams during gestation and lactation (Mean values with their standard errors of offspring of the control and restricted rat dams per group (n 5 and n 6, respectively))
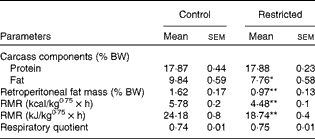
% BW, percentage of body weight.
Mean values were significantly different from those of the control group: * P< 0·05, ** P< 0·01 (Student's t test).
RMR
The respiratory quotient – the relationship between VO2 and CO2 production – of the fasted rats showed similar values (0·75) in both the groups, but resting energy expenditure was significantly lower in the restricted rats (24·18 v. 18·74 kJ/kg0·75× h (5·78 v. 4·48 kcal/kg0·75× h), P< 0·001) (Table 1).
Plasma thyroid hormone levels in the offspring
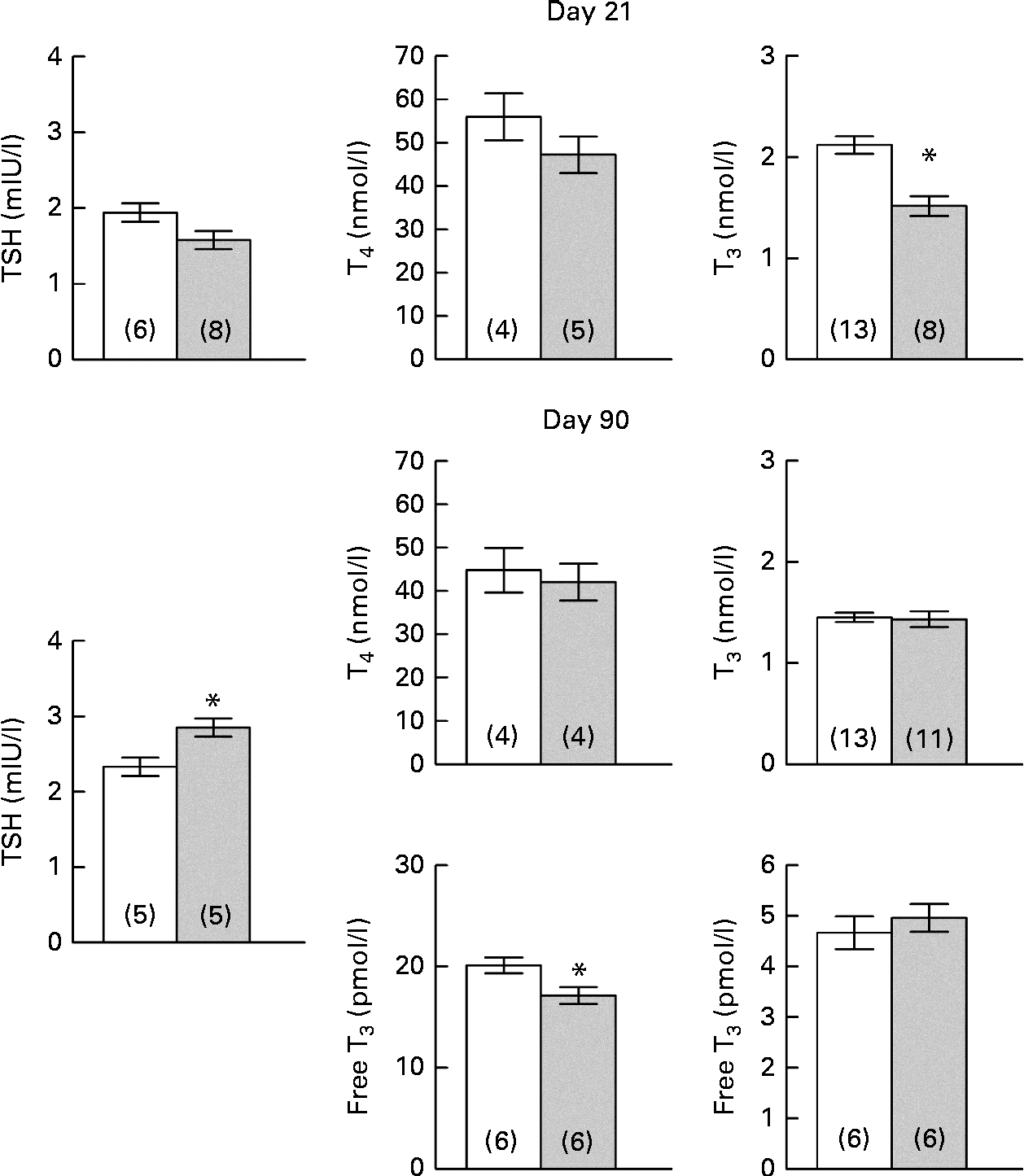
Pups whose dams were restricted during gestation and lactation compared with those of the control dams exhibited no changes in T4 or TSH levels, but significantly lower T3 levels (28·5 %, P< 0·001) at weaning (postnatal day 21). In adulthood (postnatal day 90), the pattern of T3 and TSH levels was reversed, i.e. normal T3 and T4 levels with elevated concentrations of TSH were observed in the restricted rats (22·1 %, P< 0·05) (Fig. 2). To analyse the availability of TH, levels of free T3 and free T4 were measured on day 90. The restricted rats exhibited lower plasma free T4 levels in comparison with the control rats (P= 0·029), whereas both the groups exhibited no differences in plasma free T3 levels (Fig. 2).

Fig. 2 Effects of undernutrition on plasma thyrotropin (TSH), thyroxine (T4) and 3,5,3′-triiodothyronine (T3) levels in the female offspring (postnatal day 21) and free T4 and free T3 levels in adult rats (postnatal day 90). Values are means of the control (□) and 40 % food-restricted (![]() ) rats, with their standard errors represented by vertical bars. Numbers of rats per group are shown in parentheses. * Mean values were significantly different from those of the control group (P< 0·05; Student's t test).
) rats, with their standard errors represented by vertical bars. Numbers of rats per group are shown in parentheses. * Mean values were significantly different from those of the control group (P< 0·05; Student's t test).
Cold exposure response
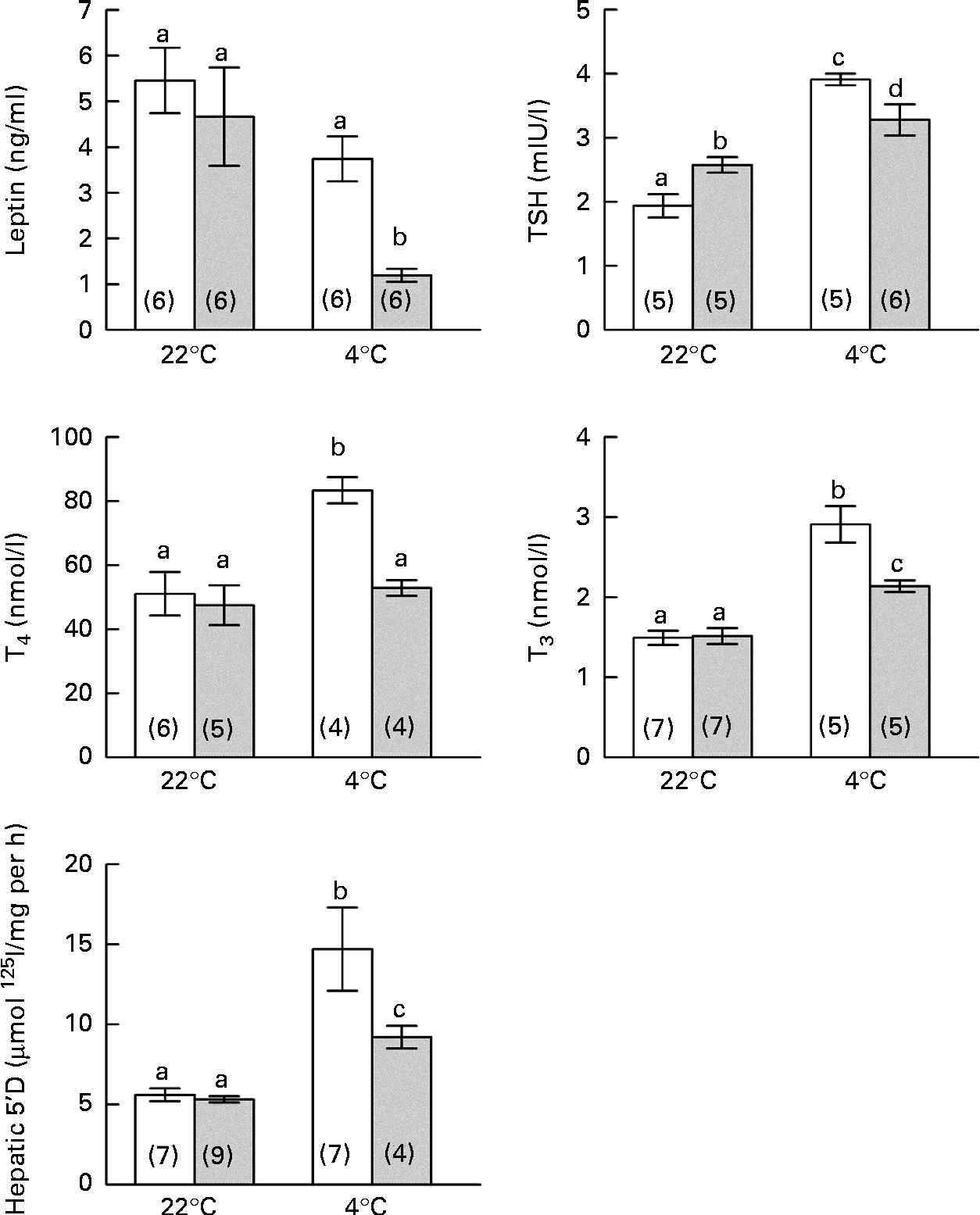
As shown in Fig. 3, leptin levels were not different between the groups at room temperature, but a significant reduction in leptin levels was observed in the restricted rats during cold exposure. The control rats exhibited classical thyroid activation with increases in circulating TSH, T4 and T3 levels, as well as a rise in HD1 activity. Conversely, the restricted rats exhibited a partial and dissociated response, showing significant but attenuated increases in TSH and T3 levels and HD1 activity, but no changes in T4 levels.

Fig. 3 Effects of perinatal undernutrition (40 % food restricted) on leptin levels and thyroid response induced by cold exposure (4 ± 1°C for 24 h) in adult male offspring (postnatal day 140). Values are means, with their standard errors represented by vertical bars. Numbers of rats per group are shown in parentheses. a,b,c,dMean values with unlike letters were significantly different (P< 0·05; two-way ANOVA). □, Control; ![]() , restricted; TSH, thyrotropin; T4, thyroxine; T3, 3,5,3′-triiodothyronine; 5′ D, 5′-deiodinase.
, restricted; TSH, thyrotropin; T4, thyroxine; T3, 3,5,3′-triiodothyronine; 5′ D, 5′-deiodinase.
BAT Dio2 is highly sensitive to sympathetic stimulation and is considered as a marker of sympathetic cold exposure response. At room temperature, the restricted rats exhibited the highest values of BAT Dio2 expression, suggesting an exacerbated sympathetic basal tone. When both the groups of rats were exposed to cold, only the control group exhibited a typical BAT Dio2 response to cold, with raised values similar to those observed in the restricted rats (Fig. 4). During cold exposure, the restricted rats also exhibited significantly high NA concentrations and a clear tendency of increasing BAT weight and dopamine and adrenaline concentrations (Table 2).
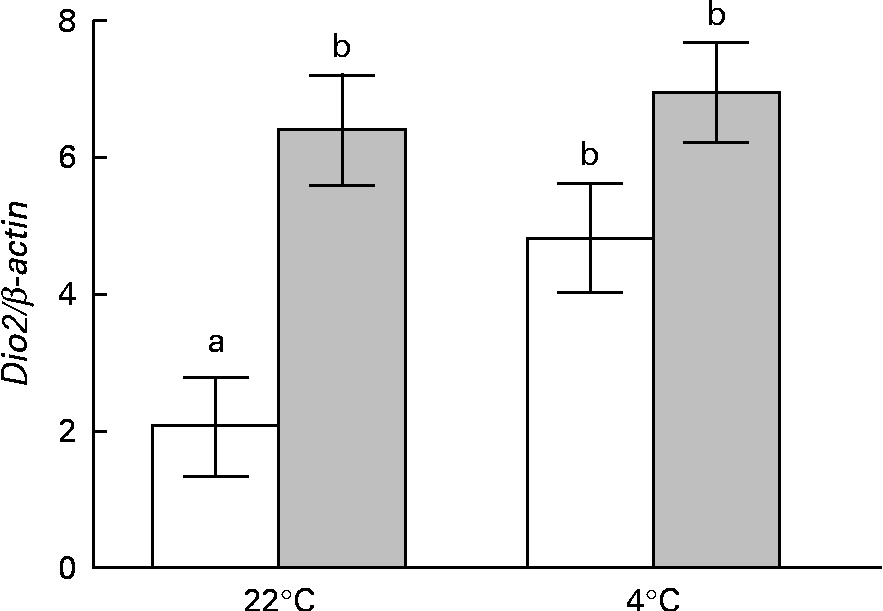
Fig. 4 Effects of perinatal undernutrition (40 % food restricted) on Dio2 expression in the brown adipose tissue obtained from the male offspring (postnatal day 140) before and after being exposed to cold (4 ± 1°C for 24 h). Values are means (n 5 rats per group), with their standard errors represented by vertical bars. Dio2 expression is normalised to β-actin expression. a,bMean values with unlike letters were significantly different (P< 0·001; two-way ANOVA).
Table 2 Effects of perinatal undernutrition (40 % food restricted) on weight and catecholamine content in the brown adipose tissue (BAT) obtained from the male offspring (postnatal day 140) before and after being exposed to cold (4±1°C for 24 h) (Mean values with their standard errors)
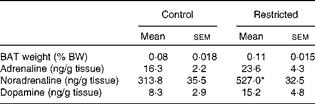
% BW, percentage of body weight.
* Mean value was significantly different from that of the control group (P< 0·01; Student's t test).
Discussion
In order to maintain a dynamic energy balance, food intake, thermogenesis and activities need to be regulated by systems that defend the stability of the energy reserves and body weight(Reference Remmers and Delemarre-van de Waal27). Previous studies(Reference Léonhardt, Lesage and Croix28, Reference Howie, Sloboda and Vickers29) have shown that maternal undernutrition during both gestation and lactation produces the most drastic alteration in the body weight of pups. In the present study, a 40 % maternal food restriction during gestation and lactation in rats induced perinatal growth retardation with lower birth weight and smaller body size and lower body weight until adulthood, confirming the results obtained under similar conditions by Holemans et al. (Reference Holemans, Van Bree and Verhaeghe30, Reference Holemans, Gerber and Meurrens31) and Miñana-Solis & Escobar(Reference Miñana-Solis and Escobar32). Undernourished rats fed ad libitum after weaning ingested less food, accumulated fewer lipids and maintained a lower RMR as adults. When we correlated food intake with these factors, only body weight exhibited a significant association, whereas fat deposits and RMR remained as lowered altered variables. The absence of catch-up growth has been described previously by Garofano et al. (Reference Garofano, Czernichow and Breant33) and Holemans et al. (Reference Holemans, Gerber and Meurrens31), who also showed that perinatal food restriction is accompanied by normal values of heart rate, blood pressure, plasma TAG, cholesterol and glycaemia, suggesting that delayed catch-up growth has protective effects on metabolism and prevents adult obesity(Reference Howie, Sloboda and Vickers29).
All our findings support the idea that these neonatal periods are crucial windows in the development of adipose depots(Reference Muhlhausler and Smith34) and predetermine RMR limits. Recent publications have addressed sex-related differences observed in neonatal nutrient restriction studies and their results are widely discordant, showing the highest sensitivity in females(Reference Zambrano, Bautista and Deás35) than in males(Reference Sugden and Holness36) or a similar sensitivity in both sexes. Nevertheless, in all these studies, it is clear that protein restriction during development results in a global metabolic readjustment. This readjustment is called metabolic programming and has been implicated in the predisposition to developing metabolic illnesses such as obesity, non-insulin-dependent diabetes and hypertension(Reference Reynolds, Walker and Phillips3). Although a limitation of the present study is that we did not perform all the measurements in male and female rats, we found that the persistent increase in TSH levels and the programming of the thyroid axis occur in both the sexes.
Extensive literature has demonstrated the crucial role of TH in the development and function of several tissues, besides in those of the brain. In metabolic terms, TH are crucial for maintaining RMR and obligatory thermogenesis and play an important role in facultative thermogenesis by exerting a synergic effect with catecholamine in BAT, promoting heat production in a cold environment(Reference Silva9) and decreasing metabolic efficiency to control body weight during overfeeding(Reference Cannon and Nedergaard10). Moreover, TH are critical for stimulating lipogenesis and maintaining fat depots in adulthood(Reference Oppenheimer, Schwartz and Lane37). In addition, a significant reduction in fat mass has been observed in perinatal hypothyroid animals, which tend to be leaner with reduced retroperitoneal fat depot and adipocyte volume(Reference Curcio, Lopes and Ribeiro38). Although there are studies that have demonstrated that energy balance can be programmed by prenatal and neonatal food restriction(Reference Remmers, Fodor and Delemarre-van de Waal39), the effect of this disruption upon the thyroid system has not been explored completely. In the present experiment, the offspring of undernourished dams exhibited lower T3 levels with normal plasma T4 and TSH levels at weaning, but on postnatal day 90 and maybe for the rest of their lives (the remaining 140 d), apparent normal T3 and T4 levels were accompanied by significantly lower levels of free T4 and persistently higher levels of TSH, thus providing evidence that perinatal undernutrition might induce functional changes in the thyroid gland.
Normally, binding of TSH to the TSH receptor (TSHR) stimulates both growth and function of thyroid cells, promoting cell proliferation(Reference Medina and Santisteban40, Reference Kimura, Dumont and Fusco41) and TH production(Reference De Felice, Postiglione and Di Lauro42). With regard to metabolic disorders, in recent years, TSHR have been identified in a number of tissues including the brain, testes, kidney, heart, bone, thymus, lymphocytes, adipose tissue and fibroblasts, suggesting that TSH may have a wider functional role than is traditionally recognised(Reference Davies, Marians and Latif43). In normal and hypothyroid conditions, TSH exhibits lipolytic and thermogenic effects in white adipose tissue and BAT, respectively, during the postnatal period(Reference de Lloyd, Bursell and Gregory44–Reference Endo and Kobayashi46). Studies showing whether these adipose tissue TSH sensitivities are modified by a neonatal food-restricted regimen have not been documented, but could explain in part the present results, showing that although TH (T4 and T3) levels are normal, the white adipose depot is scarce, whereas BAT sympathetic tone is exacerbated (BAT Dio2).
Another objective of the present study was to determine the functional integrity of the HPT axis of food-restricted animals on postnatal day 140. The moderate pituitary response to release TSH in a cold environment with a preferred formation/secretion of T3 over T4, as well as discrete increases in HD1 activity, is accompanied by the maintenance of an exacerbated sympathetic BAT response (a significant increase in NA concentrations) and suggests the existence of a state resembling subclinical hypothyroidism in these animals(Reference Ross, Braverman and Utiger47). Thermogenesis is mainly controlled in a synergistic way by sympathetic NA release and local T3 generation (Dio2 activity) in the BAT of small mammals(Reference Silva9). The BAT response to both signals is due to an increase in the synthesis of uncoupling protein 1, which allows protons to leak into the mitochondrial matrix, reducing ATP synthesis, and, as a consequence, energy is lost as heat (for a review, see Harper & Seifert(Reference Harper and Seifert48)). It is well known that thyroid dysfunctions are accompanied by opposite alterations in sympathetic nervous system responses. Cvijic et al. (Reference Cvijic, Petrovic and Djordjevic49) have reported increased serum dopamine-β-hydroxylase and BAT monoamine oxidase levels in thyroidectomised rats in response to a cold environment. Higher plasma NA levels have been reported in hypothyroid subjects(Reference Coulombe, Dussalult and Walker50). Moreover, it has been shown that in rats under intrauterine food restriction, thermoregulation during cold acclimatisation is altered in the offspring on postnatal day 90(Reference Luz, Griggio and Vieira51). On the other hand, recent studies have reported that TSHR-deficient hyt/hyt mice become hypothermic in cold conditions despite T4 administration. Transfection of TSHR into the BAT of these mice resulted in a marked improvement in core temperature, leading to the conclusion that both functional TSHR and adequate free TH are required for normal temperature regulation(Reference Endo and Kobayashi45). Association of TSHR with sympathetic activation in BAT has not been documented, but studies on THSR in thermogenesis have demonstrated that TSH/TSHR activation is accompanied by an increase in cyclic AMP content in BAT, suggesting that the stimulated uncoupling protein 1 expression is mediated via cyclic AMP response element (CRE)(Reference Endo and Kobayashi45).
The stimulus produced by cold environment exposure is conducted by adrenergic neurons from the medulla, which projects its afferents to the hypothalamic paraventricular nucleus, where the TRH-releasing neurons are stimulated(Reference Fekete and Lechan52). It has been suggested that catecholamines released by this pathway increase the set point for negative feedback regulation of TRH gene expression by T3 through adrenaline-stimulated cyclic AMP response element binding protein (CREB) phosphorylation, which activates the TRH promoter, thus increasing TRH release in the median eminence, and subsequently activates the thyroid axis. Additionally, leptin in the arcuate nucleus inhibits the production of neuropeptide Y, which is a potent transcription inhibitor of the TRH gene in the paraventricular nucleus(Reference Fekete and Lechan52, Reference Chiamolera and Wondisford53). Leptin levels were lower in the perinatal restricted rats in adulthood during cold exposure, suggesting that neuropeptide Y production in the arcuate nucleus is less inhibited and partially antagonises the adrenergic stimulatory effect reducing the paraventricular nucleus release of TRH and, as a consequence, lowers TSH and TH production. On the other hand, the increased TSH levels that we found in the restricted rats in basal conditions are controversial. In the pituitary gland, brain and BAT, there is additional T3 generation by Dio2-catalysed T4 deiodination. In the feedback regulation of TSH, this mechanism correlates better with plasma T4 or T3 produced locally by Dio2 activity than with plasma T3. Interestingly, a lower RMR is correlated with lower free T4 and higher TSH levels and not with serum T3 levels(Reference Bianco, Salvatore and Gereben54). In recent years, Dutra et al. (Reference Dutra, Passos and Lisboa55) have found that adult rat offspring whose mothers were protein restricted during lactation show some aspects of hyperthyroidism with increased serum T3 and higher leptin levels at weaning, suggesting that leptin could contribute to higher plasma T3 levels by an enhanced HD1 activity. In the same model, they also found a lower in vitro TSH-releasing response to TRH, lower plasma TSH levels and a higher pituitary Dio2 activity, indicating that higher T3 local generation lowers TSH production and plasma levels(Reference Lisboa, Fagundes and Denolato19). We hypothesise that in the present study in the offspring of energy-restricted rat dams throughout gestation and lactation, in contrast to that observed in dams protein restricted during lactation, the lower plasma free T4 levels reduce T3 production in the pituitary and the hypothalamus and the T3 feedback mechanism causes an increase in plasma TSH levels.
Persistent increases in plasma TSH levels have been described in the so-called subclinical hypothyroidism, which exhibits a world prevalence between 3 and 8 % and is correlated with lipid abnormalities, principally dyslipidaemia and, in some cases, insulin resistance and cardiac failure(Reference De Felice, Postiglione and Di Lauro42, Reference Biondi and Cooper56, Reference Ruhla, Weickert and Arafat57). Although the origin of this syndrome has not been elucidated, an epidemiological analysis has pointed out that women who develop spontaneous hypothyroidism in adulthood are characterised by low birth weight and short length at birth, short height during early childhood and low RMR during late childhood. Moreover, many of these women exhibited previous subclinical hypothyroidism and positive thyroperoxidase antibodies, suggesting that spontaneous hypothyroidism must be included among those adult disorders whose development is initiated during early life(Reference Kajantie, Phillips and Osmond20).
Further studies are needed to clarify the role of persistently higher TSH levels in the development and function of adipose tissue and other organs, but the present data suggest that perinatal undernutrition programmes permanent alterations in both thyroid function and HPT axis, which could be implicated in the predisposition to developing metabolic illnesses associated with perinatal undernutrition.
Acknowledgements
The authors are grateful to Guadalupe Delgado for technical assistance and Marcela Sánchez for proofreading in the preparation of the article. L. Q. is a fellow of Comisión de Operación y Fomento de Actividades Académicas-IPN. The present study was supported by the Instituto Politécnico Nacional (SIP 20110605). L. Q. designed and implemented the study with assistance from R. A.-M., C. A. and R. R.; B. A. conducted the experiment to determine HD1 activity. L. Q. wrote the first draft of the manuscript with assistance from C. A., R. A.-M. and B. A. The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.