The first ever successful Amplatzer Septal Occluder (AGA Medical, Golden Valley, MN, USA which has been eventually incorporated into Abbott, Plymouth, MN, USA) implantation was performed at our centre in September 1995. Reference Masura, Gavora, Formanek and Hijazi1 As the first experiences with the use of this type of occluder proved exceptional short-term rate of complete closure and no severe complications, self-centring Amplatzer Septal Occluder became one of the most frequently used devices for transcatheter closure of secundum-type atrial septal defect. Reference Masura, Gavora, Formanek and Hijazi1 All procedures were performed after fulfilling strict indication criteria. Reference Masura, Gavora, Formanek and Hijazi1 In 2005, an excellent long-term outcome of transcatheter secundum-type atrial septal defect closure using Amplatzer Septal Occluder was demonstrated on the group of 151 patients with median 78-month follow-up by our centre too. Reference Masura, Gavora and Podnar2 The purpose of the current study was to evaluate long-term outcomes of transcatheter closure of secundum-type atrial septal defect using Amplatzer Septal Occluder in all patients who underwent this type of procedure at our centre between September 1995 and October 2012.
Materials and methods
A retrospective analysis of patients who underwent percutaneous closure of secundum-type atrial septal defect with Amplatzer Septal Occluder between September 1995 and October 2012 in our institution was performed. The selection of patients suitable for a transcatheter closure using Amplatzer Septal Occluder had been reported in detail previously. Reference Masura, Gavora, Formanek and Hijazi1 It was generally based on the measurement of the maximum defect diameter and morphological characteristics of the defect. Pre-implantation protocol, technical characteristics of used Amplatzer Septal Occluder and delivery system, implantation procedure, as well as immediate follow-up protocol had also been presented in detail previously. Reference Masura, Gavora, Formanek and Hijazi1 Except for the transesophageal echocardiography examination performed immediately after the occluder release, transthoracic echocardiography, electrocardiogram, and chest X-ray were performed 24 hours after each procedure. Thereafter, electrocardiogram and transthoracic echocardiography were intended to be performed at 1 month, 3 months, 12 months, and then annually after the implantation. Acetylsalicylic acid, 5 mg/kg/daily, and infective endocarditis prophylaxis were recommended for 6 months after the procedure. The parameters that were evaluated during the follow-up included maximal defect diameter measured by transesophageal echocardiography, stretched defect diameter, median device size, residual atrial septal defect rate, rate of early reinterventions due to moderate or severe residual atrial septal defect, long-term moderate or severe residual atrial septal defect, long-term complete closure rate, rate of transthoracic echocardiography M-mode right ventricular end-diastolic diameter post-procedural improvement and normalisation, rate of transient and major periprocedural, short post-procedural and late complications as well as mortality rate. Consecutive possible complications were sought by transthoracic echocardiography and electrocardiogram: occluder embolisation, aortic/atrial erosion by occluder, thrombus formation on occluder, infective endocarditis on occluder, moderate or severe AV valves regurgitation developed by occluder discs interference with atrioventricular valves leaflets, flow obstruction in the caval veins, right pulmonary veins or coronary sinus developed by occluder discs interference with these structures, continuous wave doppler gradient > 30 mmHg on tricuspid regurgitation jet, and arrhythmias. The data were analysed throughout the period of 2019–2021. T-test was used for continuous data comparison. The continuous data are expressed as: mean ± SD, mean (ranges), median (ranges), or median ± SD, as appropriate. The data were analysed using the statistical program JMP 5.0.1. The echocardiographical values from the study of Kampmann et al. were considered as reference normal right ventricular end-diastolic diameter values measured in the parasternal long-axis projection. Reference Kampmann, Wiethoff, Wenzel and etal3 The study was approved by authorised institutional ethical committee. Informed written consents were obtained from all patients or their parents.
Results
Between September 1995 and October 2012, 803 patients with isolated secundum-type atrial septal defect underwent transcatheter defect closure using Amplatzer Septal Occluder. At the time of implantation, the mean patient age was 9,6 (range: 2–19) years and weight 33,5 ± 18,4 kg. Gender ratio (female/male) was 1,35/1,0 (462 versus 341). The mean maximal defect diameter measured by transesophageal echocardiography was 9,5 ± 4,6 mm. The mean stretched defect diameter was 14,0 ± 5,2 mm. The median device size was 16 (4–40) mm. Two occluders were implanted in two patients having two widely separated defects. Although in older children and adults atrial septal defect closure can be performed as an outpatient procedure, all patients from our study were hospitalised for a minimum of 48 hours. Ninety-five per cent of all procedures were performed under general anaesthesia. Local anaesthesia was used in adult patients only. All procedures were performed under transesophageal echocardiography guidance. The length of hospital stay was 48 hours in 794/803 patients. Longer hospitalisations (3–9 days) were necessary only in patients with associated periprocedural complications. A complete follow-up was not reached in all patients. We encountered long-term > 5-year follow-up in 651/803 patients (81%), 10-year follow-up in 508/730 patients (70%), and 20-year follow-up in 85/151 patients (56%). The early and also late loss to follow-up was most likely caused by the following reasons: 1. early transmission from paediatric to adult cardiologist, 2. patient non-compliance, 3. continuous change of patient names or addresses, and 4. foreign patients. The median long-term > 5-year follow-up time was 12 (5–25) years. Moderate residual shunt with the diameter 2–4 mm was present in 0,2% (2/803 patients) and severe residual shunt with the diameter > 4 mm was present in 0,1% (1/803 patients). All three moderate or severe residual shunts were completely closed by the next Amplatzer Septal Occluder during 12 months after the primary intervention. Long-term complete closure rate at 5-year, 10-year, and 20-year of follow-up was 98,8% (643 / 651 patients), 98,5% (500/508 patients), and 100% (85/85 patients), respectively, as the rest of residual shunts were evaluated as trivial (diameter < 2 mm).
Pre-procedural median right ventricular end-diastolic diameter Z-score 1,97 (0–3) improved to post-procedural median Z-score 0,53 (−0,4–2,0) (p < 0,05). The rate of M-mode right ventricular end-diastolic diameter post-procedural normalisation was 100%, as documented in Figure 1.
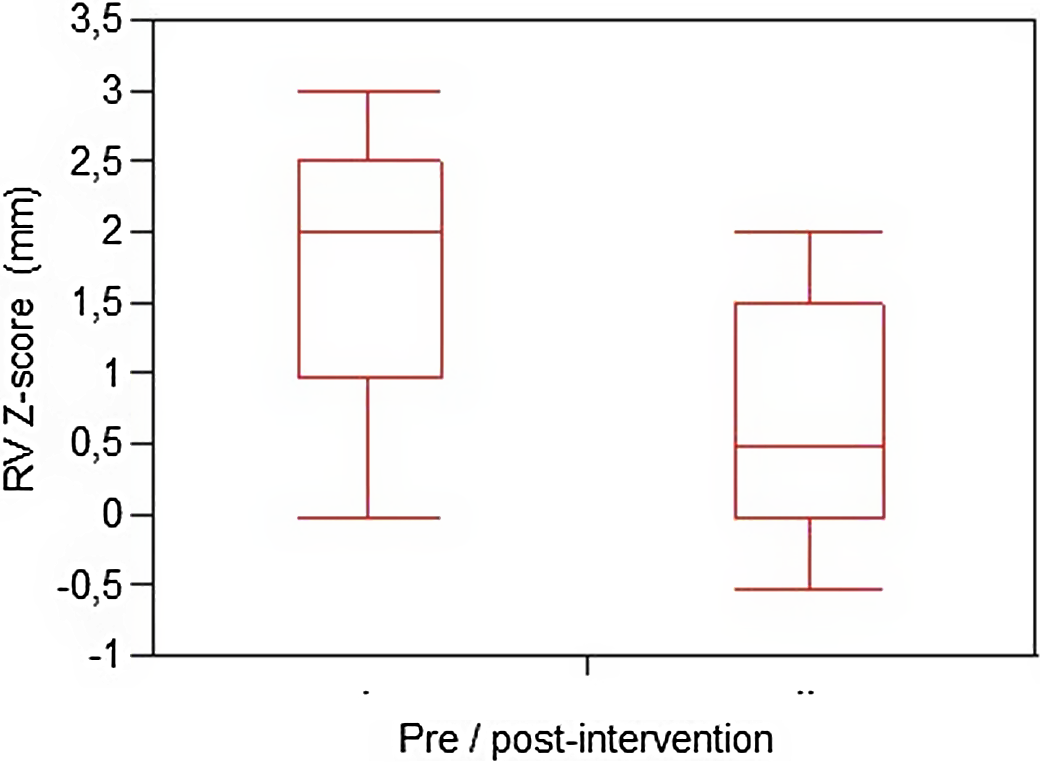
Figure 1. The comparison of pre- and post-procedural RVEDd (right ventricular end-diastolic diameter) values expressed as median ± 2 SD.
The survival rate of all followed patients was 100%. Only two major periprocedural complications were encountered. In the first case, immediately post-correct device positioning on transesophageal echocardiography, Amplatzer Septal Occluder embolised into the left ventricle due to technical issues with the screwing mechanism. When trying to recapture it to the long sheath, the device embolised. A successful transatrial cardiosurgical occluder extraction with atrial septal defect suture closure was performed immediately. In the second case, immediate post-occluder release transesophageal echocardiography examination found a significant thrombus at the left occluder disc surface, which had most likely been formed in the tip of the delivery sheath. An immediate alteplase fibrinolytic therapy with consequent 12-month oral anticoagulation and antithrombotic therapy led to complete thrombus dissolution. Haematological investigation excluded congenital thrombophilia.
Only one major short post-procedural complication occurred in a 15-year-old patient. Secundum-type atrial septal defect with 25-mm stretched defect diameter was closed by 28-mm Amplatzer Septal Occluder. Transthoracic echocardiography performed 24 hours after the device implantation showed a good result with only a trivial residual shunt at the upper side of occluder, with no pericardial effusion. Transthoracic echocardiography performed 1 month after the intervention delineated 15 mm pericardial effusion at the apex and next to the left ventricle. In spite of applied diuretic and non-steroidal anti-inflammatory therapy, pericardial effusion persisted and eventually became more significant 3 months after the intervention. As haemoglobin levels dropped at the same time, an erosion of the atrial or aortic wall by Amplatzer Septal Occluder was suspected. Three hundred and thirty millilitres of haemorrhagic content were drained within the pericardial puncture. Transesophageal echocardiography imaged close position of occluder discs to the aortic wall and dilated transverse sinus of the pericardial sack. A 5-mm perforation of the left atrial wall damaged adventitia and exposed media of the aortic wall by device was detected by a surgeon at the early phase of consequent cardiosurgical procedure. The deformed occluder was extracted, secundum-type atrial septal defect was closed by a pericardial patch, and four stitches were used to stabilise the aortic and left atrial walls. Mild periprocedural or short post-procedural complications were encountered in 8/803 patients (1,0%). Transient arrhythmias (supraventricular tachyarrhythmia in five patients, ectopic atrial rhythm in one patient, and junctional rhythm in one patient) were observed in seven patients. Casual finding of iatrogenic groin arteriovenous fistula 9 months after the intervention was noted in one patient. An effective surgical fistula transection was performed consequently. Only one major complication was observed in the long-term follow-up. An 18-year-old patient, 12 years after secundum-type atrial septal defect closure by Amplatzer Septal Occluder, clinically presented with fever, chest pain, dyspnoea, elevated C-reactive protein, and positive hemoculture (methicillin-resistant staphylococcus aureus). Shortly after targeted antibiotic therapy was applied, the patient presented with haemodynamic instability. Transthoracic echocardiography imaged significant vegetation at the right and left side of the implanted device, at the anterior mitral valve cusp, and at the region of the non-coronary aortic valve cusp, whose damage led to severe aortic valve regurgitation. Amplatzer Septal Occluder was surgically extracted, the aortic valve was excised, an artificial valve was implanted into the position of the aortic valve, the anterior cusp of the mitral valve was reconstructed, and secundum-type atrial septal defect was closed with a pericardial patch. No post-procedural moderate or severe atrioventricular valves regurgitation development, no post-procedural flow obstruction in the caval veins, right pulmonary veins or coronary sinus development, and no continuous wave doppler pressure gradient > 30 mmHg on eventual tricuspid regurgitation jet were observed in our patient cohort.
Discussion
This study demonstrates excellent long-term outcomes from percutaneous closure of secundum atrial septal defects with Amplatzer Septal Occluder. In comparison with our previous study concluding 100% rate of complete defect closure in 151 patients at 3-year follow-up, we encountered 98,8% (643/651 patients), 98,5% (500/508 patients), and 100% (85/85 patients) rate of complete defect closure at 5-year, 10-year, and 20-year follow-up, respectively. Reference Masura, Gavora and Podnar2 Other studies declared similar rates of complete post-procedural atrial defect closure with Amplatzer Septal Occluders 97,9% in 927 patients in 2-year follow-up to 100% in 166 patients in 5-year follow-up, respectively. Reference Turner, Owada, Sang, Khan and Lim4,Reference Bialkowski, Kusa and Szkutnik5 Hundred per cent survival rate of our patients cohort confirmed very low short- and long-term device-related mortality rate (0.01% and 0.1%, respectively) as reported in a meta-analysis of 28,142 patients from 203 studies. Reference Jalal, Hascoet and Baruteau6 The complications of percutaneous secundum-type atrial septal defect closure using Amplatzer Septal Occluder reported in the literature are rare and very early in the vast majority of patients. No deaths and a very low incidence of major complications in the present study confirm high safety of this procedure in the long-term follow-up. Erosion of the aorta or the atria is a rare but serious and potentially life-threatening complication following percutaneous secundum-type atrial septal defect closure by Amplatzer Septal Occluder. Most of the cases occur in early post-interventional period (up to 72 hours) but can occur even years after the intervention. Reference Amin, Hijazi and Bass7 The estimated incidence of the aortic or atrial wall erosion with the Amplatzer Septal Occluder is 0,1%, equal to its incidence in our study. The exact mechanisms of erosion remain unclear despite several analyses. The absent or deficient anterior–superior rim together with the device oversizing are thought to be crucial risk factors for erosion. In our patient, the anterior–superior rim was absent, and the device was slightly oversized. Following this event, we modified our approach and implemented the stop-flow technique for atrial septal defect balloon sizing in subsequent 96 patients. It is important to educate the patient as well as the physicians, especially at the emergency departments of risks and signs of erosion (pericardial effusion, chest pain, and haemodynamic instability). Periprocedural, early, or late device embolisation is a rare, avoidable complication of percutaneous secundum-type atrial septal defect closure using Amplatzer Septal Occluder, appearing in the cases of device undersizing. Reference Chessa, Carminati and Butera8,Reference Levi and Moore9 In contrast to that, only one periprocedural device embolisation encountered in our study was caused by technical issues with the screwing mechanism. Infective endocarditis on Amplatzer Septal Occluder is a potentially serious complication requiring a long-term antibiotic treatment and often surgical removal of vegetations and affected device. Prevention of infective endocarditis is mandatory for 6 months post-Amplatzer Septal Occluder implantation, but infective endocarditis can occur also later after the device endothelialisation. Reference Dong-Jun, Chi Young, Seng Chan, Seung-Hyun and Geu-Ru10 Late form of infective endocarditis was experienced and previously published only in one patient in our follow-up. Reference Toporcer, Kolesár, Ledecký and Sabol11 It is important to educate the patients of the necessity of good dental hygiene and discourage them from tattoos and piercings. In case there is a suspicion of vegetations, in children with a good echocardiographic window a transthoracic study might be sufficient, whereas in adults a transesophageal echocardiography is the method of choice for the vegetation detection.
Thrombus formation on Amplatzer Septal Occluder is a rare complication, as the Amplatzer Septal Occluder is less thrombogenic than other types of occluders. Reference Krumsdorf, Ostermayer and Billinger12,Reference Hikmet, Ugur, Ergun Baris and Kudret13 A thrombus can occur during the catheterisation procedure or later in the follow-up period, and these patients can present with a stroke or be completely asymptomatic. Reference Giraldo-Gonzalez, Olaya, Domınguez, Sanchez and Vesga14 Coagulation disorders may be a risk factor for thrombus formation, but large trombi were described even in patients without any known thrombotic predisposition. Acetylsalycilic acid should be administered for 6 months post-intervention in all patients. Similarly, as in our study, temporary arrhythmias were mostly reported only early after the Amplatzer Septal Occluder implantation. Reference Hill, Berul and Patel15
Study limitations
The complete long-term follow-up was not reached in all patients for objective reasons. We encountered 19% (152/803 patients) loss to the long-term follow-up (> 5 years). In comparison with our study, 7,3% rate of patient loss to the 2-year follow-up was registered in a prospective, non-randomised, multicentre clinical study enrolling 1000 patients. Reference Turner, Owada, Sang, Khan and Lim4
Conclusion
As far as we know, we present one of the most robust single-centre study evaluating long-term results of percutaneous closure of secundum-type atrial septal defect using Amplatzer Septal Occluder. The results of our study showed that percutaneous closure of secundum-type atrial septal defect using Amplatzer Septal Occluder is a safe and effective procedure accounting for a very low incidence of major complications in the long-term follow-up.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.




