Introduction
Anthropogenic habitat loss and change are rapidly expanding worldwide (Venter et al. Reference Vázquez-Pérez, Enríquez-Rocha and Rangel-Salazar2016, Watson et al. Reference Venter, Sanderson, Magrach, Allan, Beher, Jones, Fekete, Levy, Possingham, Laurance, Wood and Watson2016), causing the loss, fragmentation and structural modification of natural habitats of many forest species. Species tolerance to habitat changes depends on their ecological flexibility to deal with the new conditions, such as alterations to the microclimate and vegetation structure, and this flexibility will strongly determine their ability to survive in human-modified habitats (Swihart et al. Reference Suscke, Verderane, de Oliveira, Delval, Fernández-Bolaños and Izar2003, Tabarelli et al. Reference Swihart, Gehring and Kolozsvary2012).
Among birds, raptors are expected to be highly affected by habitat disturbance due to their high trophic position in food webs, large home ranges, and low reproductive rates (Carrete et al. Reference Carrete, Tella, Blanco and Bertellotti2009). For example, raptors have shown to be underrepresented in agricultural lands where high inputs of pesticides are applied, since bioaccumulation and magnification of such substances impact on top predators (Espín et al. Reference Espín, García-Fernández, Herzke, Shore, van Hattum, Martínez-López, Coeurdassier, Eulaers, Fritsch, Gómez-Ramírez, Jaspers, Krone, Duke, Helander, Mateo, Movalli, Sonne and van den Brink2016). Consequently, this group is often used to indicate the biodiversity status of biological communities (Sergio et al. Reference Sergio, Caro, Brown, Clucas, Hunter, Ketchum, McHugh and Hiraldo2006, Reference Schroth, Faria, Araujo, Bede, Van Bael, Cassano, Oliveira and Delabie2008) and the quality of the surrounding environment (Rodríguez-Estrella et al. Reference Ribeiro, Metzger, Martensen, Ponzoni and Hirota1998).
Three orders of diurnal raptors (Cathartiformes, Accipitriformes, and Falconiformes), encompassing 102 species, are found in the Neotropics (Remsen et al. 2017). Seventy-five species occur in Brazilian territory (Piacentini et al. Reference Philpott, Arendt, Armbrecht, Bichier, Diestch, Gordon, Greenberg, Perfecto, Reynoso-Santos, Soto-Pinto, Tejeda-Cruz, Williams-Linera, Valenzuela and Zolotoff2015) and about 76% of these inhabit the Atlantic Forest (Ferguson-Lees and Christie Reference Ferguson-Lees and Christie2006), one of the most threatened biomes in the world which is currently reduced to about 12% of its original extent (Ribeiro et al. Reference Remsen, Areta, Cadena, Claramunt, Jaramillo, Pacheco, Pérez-Emán, Robbins, Stiles, Stotz and Zimmer2009). The Corredor Central da Mata Atlântica (Central Atlantic Forest Ecological Corridor) is a region within this biome that combines a large extent of forest remnants with a high level of biodiversity (Galindo-Leal and Câmara Reference Galindo-Leal, Câmara, Galindo-Leal and Câmara2003). Within this corridor, there are some Important Bird and Biodiversity Areas (IBAs), such as the Southern Bahia region (Bencke et al. Reference Bencke, Maurício, Develey and Goerck2006), where three of the six species of Accipitriformes that are threatened in Brazil can be found: the Crested Eagle Morphnus guianensis, the Harpy Eagle Harpia harpyja and the White-necked Hawk Buteogallus lacernulatus (MMA/ICMBio 2016). In Southern Bahia, much of the original forest was converted into cocoa Theobroma cacao plantations, mostly cultivated under the “cabruca” system (Landau et al. Reference Landau, Hirsch, Musinsky and Thomas2008). In cabrucas, the understorey is replaced by cocoa trees that grow under the canopy of predominantly native forest trees partly retained when cocoa is planted, plus trees that regenerated or have been planted later (Alves Reference Alves1990).
Cabruca has been considered a wildlife-friendly system (Schroth et al. Reference Schroth, Bede, Paiva, Cassano, Amorim, Faria, Mariano-Neto, Martini, Sambuichi and Lôbo2011) because it supports a significant portion of the native fauna (Cassano et al. Reference Cassano, Schroth, Faria, Delabie and Bede2009). Despite the vertical structural simplification compared to forests, cabrucas are much more complex than other farmlands such as annual crops (Alves Reference Alves1990), thus acting as a suboptimal habitat for species from different taxonomic groups (Cassano et al. Reference Cassano, Schroth, Faria, Delabie and Bede2009). To our knowledge, there is no study specifically developed to investigate the use of cabrucas by raptors. Understanding how raptors are being affected in agricultural lands - particularly in conservation priority areas such as Southern Bahia - is a research priority in Brazil’s National Action Plan for the Conservation of Birds of Prey (Soares et al. Reference Silva2008). Also, the specific response of raptor species to environmental degradation gradients is still poorly understood in the Neotropics (Carrete et al. Reference Carrete, Tella, Blanco and Bertellotti2009), calling for new field data.
Since the carnivorous mammal fauna is very depauperate in Southern Bahia region (Cassano et al. Reference Cassano, Barlow and Pardini2012), raptors end up being the major (and sometimes the only) predators regulating populations of many species, including some native endangered species such as the Golden-headed Lion Tamarin, Leontopithecus chrysomelas (Oliveira and Dietz Reference Oliveira-Filho and Fontes2011). Predator-prey relationships can be unbalanced and sometimes lead to the local extinctions of prey in human-modified habitats (Irwin et al. Reference Irwin, Raharison and Wright2009). Therefore, understanding the abundance and diversity of raptors using cabrucas is important to evaluate the trophic structure and the stability of biological communities in this agrosystem. This study aimed to describe the diurnal raptor assemblages of cabrucas from Southern Bahia and to identify features of this system that could determine occupancy by raptors. We expected a positive relationship between raptor occupancy and habitat structural complexity (Thiollay Reference Terraube, Arroyo, Madders and Mougeot1985, Jullien and Thiollay Reference Jullien and Thiollay1996), specifically: cabrucas with better-connected canopy, high-density, taller and large-diameter shade trees, as well as a lower density of cocoa trees and a low management intensity, would have a higher chance of being occupied by raptors. We also expected the percentage of vegetation cover in the landscape to positively affect raptor occupancy in cabrucas (Jullien and Thiollay Reference Jullien and Thiollay1996).
We added some biological traits such as body mass, dietary requirements, and use of vertical strata, to predict raptor occupancy in cabruca sites. We expected a lower occupancy of (1) species with higher dependence on intermediate forest strata, since these are replaced by cocoa trees in cabrucas (Johns Reference Johns1998); (2) larger species, since they usually occur at low population densities and have large home ranges, being more sensitive to habitat disturbance (Gaston and Blackburn Reference Gaston and Blackburn1995); and (3) species with higher energetic requirements and dietary specialisation, since they are expected to be less tolerant to changes in the availability of food resources (Terraube et al. Reference Tabarelli, Peres and Melo2011).
Methods
Study area
The surveys occurred between August 2014 and May 2015, a period that includes the breeding season for most raptor species in Atlantic Forest (Mañosa et al. Reference Mañosa, Mateos and Pedrocchi2003, Zorzin Reference White and Burnham2011), and when these predators are expected to behave more conspicuously, increasing their detectability (Seymour et al. Reference Sergio, Newton, Marchesi and Pedrini2010, Monsalvo Reference Monsalvo2012). Also, according to field records, migratory species such as the Swallow-tailed Kite Elanoides forficatus and the Plumbeous Kite Ictinia plumbea can be found in the study region during the major part of this period (https://en.wikiaves.com/).
Sixteen cabruca sites separated by at least 11 km (mean 52 km; range 11–114 km) were surveyed, covering 11 municipalities (∼ 4,000 km2) in the cocoa-growing region of Southern Bahia, Brazil (Figure 1). The dominant vegetation is tropical lowland rainforest (Oliveira-Filho and Fontes 2000), the mean annual temperature is 24°C and rainfall averages 2,500 mm/yr, with no marked seasonality (Mori et al. Reference Mori, Boom, de Carvalho and dos Santos1983).
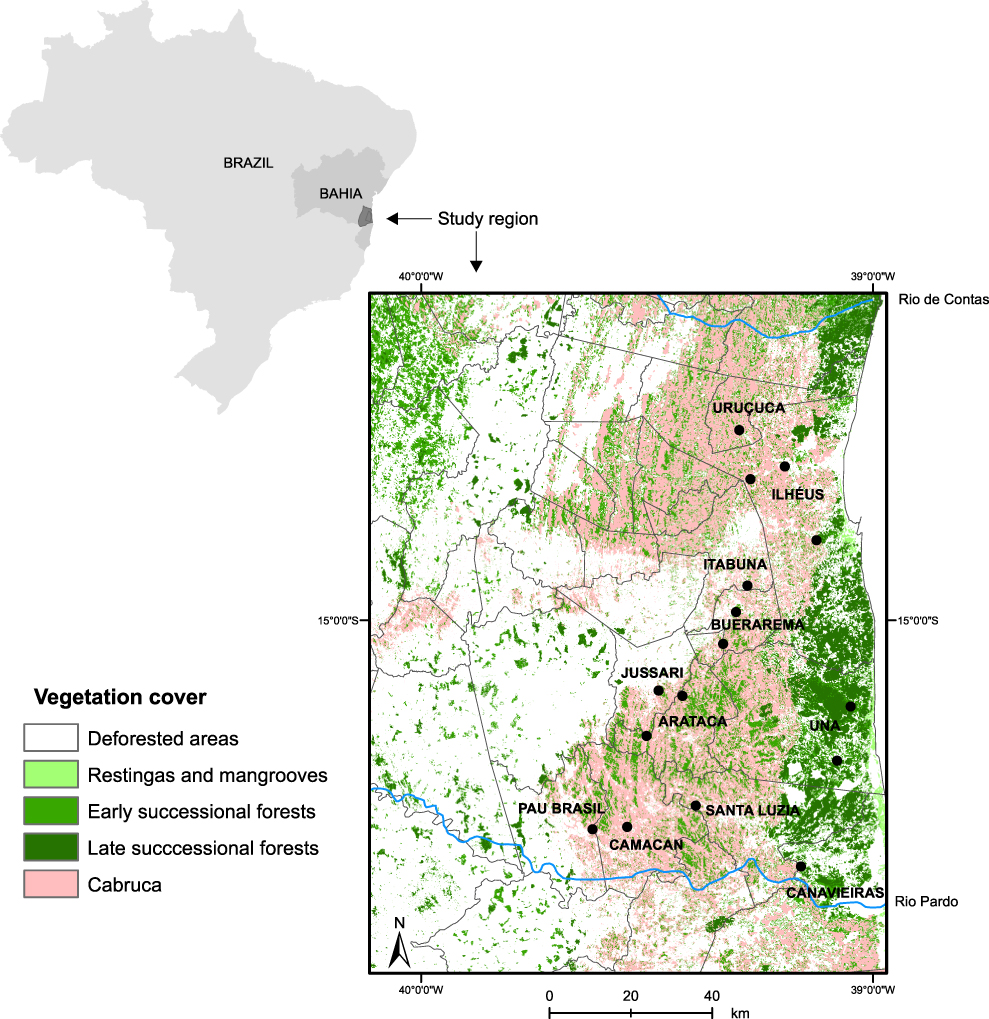
Figure 1. Vegetation cover in the study region and the location of the 16 cabruca sites surveyed in this study (black circles). Vegetation classification followed Landau et al. (Reference Landau, Hirsch, Musinsky and Thomas2008).
Data collection
Species dataset
A subset of 46 species potentially occur in the study region (http://www.listavermelhabahia.org.br/; http://www.iucnredlist.org/; Del Hoyo et al. Reference Del Hoyo, Elliott, Sargatal, Christie and De Juana2017). Taxonomy follows Remsen et al. (2017). For all of them, we compiled information in the literature about (1) diet preferences (proportional consumption of each food category: vertebrates, invertebrates, and fruits); (2) body mass (in grams); (3) percentage of foraging time in each stratum (ground, understorey, mid-high, canopy, and air); (4) degree of sensitivity to habitat disturbance (low, medium or high); (5) relative abundance throughout the range (uncommon, fairly common, common, or patchily distributed); and (6) conservation status according to IUCN (‘Vulnerable’, ‘Near Threatened’, ‘Endangered’, or ‘Critically Endangered’). We derived two indexes based on diet preferences: (1) trophic level – the sum of the proportional consumption of each food category weighted by its energetic value, assuming a decreasing energy content from vertebrates to invertebrates to fruits; and (2) dietary specialisation – the number of different food categories in species dietary. Species list, ecological information, details about indexes calculation, and literature references are available in the online supplementary material (Tables S1 and S2).
Field survey
The field methodology was adapted from Granzinolli and Motta-junior (Reference Granzinolli, Motta-junior, Matter, Straube, Accordi, Piacentini and Cândido2008). A combination of active search, playback and point-counts were performed in each cabruca site by the same observer with the help of a field assistant. All sampling was carried out in two visits between 06h00 and 12h00, a period during which most species of diurnal raptors are active (Thiollay Reference Thiollay1989, Mañosa et al. Reference Mañosa, Mateos and Pedrocchi2003), avoiding days with rain and strong wind due to a possible decrease in species detectability (Jones Reference Jones, Jones and Marsden2000).
Using Landsat images with 30 m resolution obtained from Google Earth (Google Inc. 2016), we designed two parallel linear transects of 800 m separated by 400 m in each cabruca site (Figure 2). Between 06h00 and 09h00, the active searches and playbacks were carried out in these transects, focusing on species which predominantly occur in forested habitat, such as the Crested and Harpy Eagles and the Forest-falcons Micrastur spp., plus soaring species that have not started their flight activities yet, such as the Grey-headed Kite Leptodon cayanensis and the Hawk-eagles Spizaetus spp. (Thiollay Reference Thiollay1989). The active search was performed by walking through these transects at a constant velocity while identifying species using 10 x 50 binoculars and a digital voice recorder to record vocalisations whenever possible.
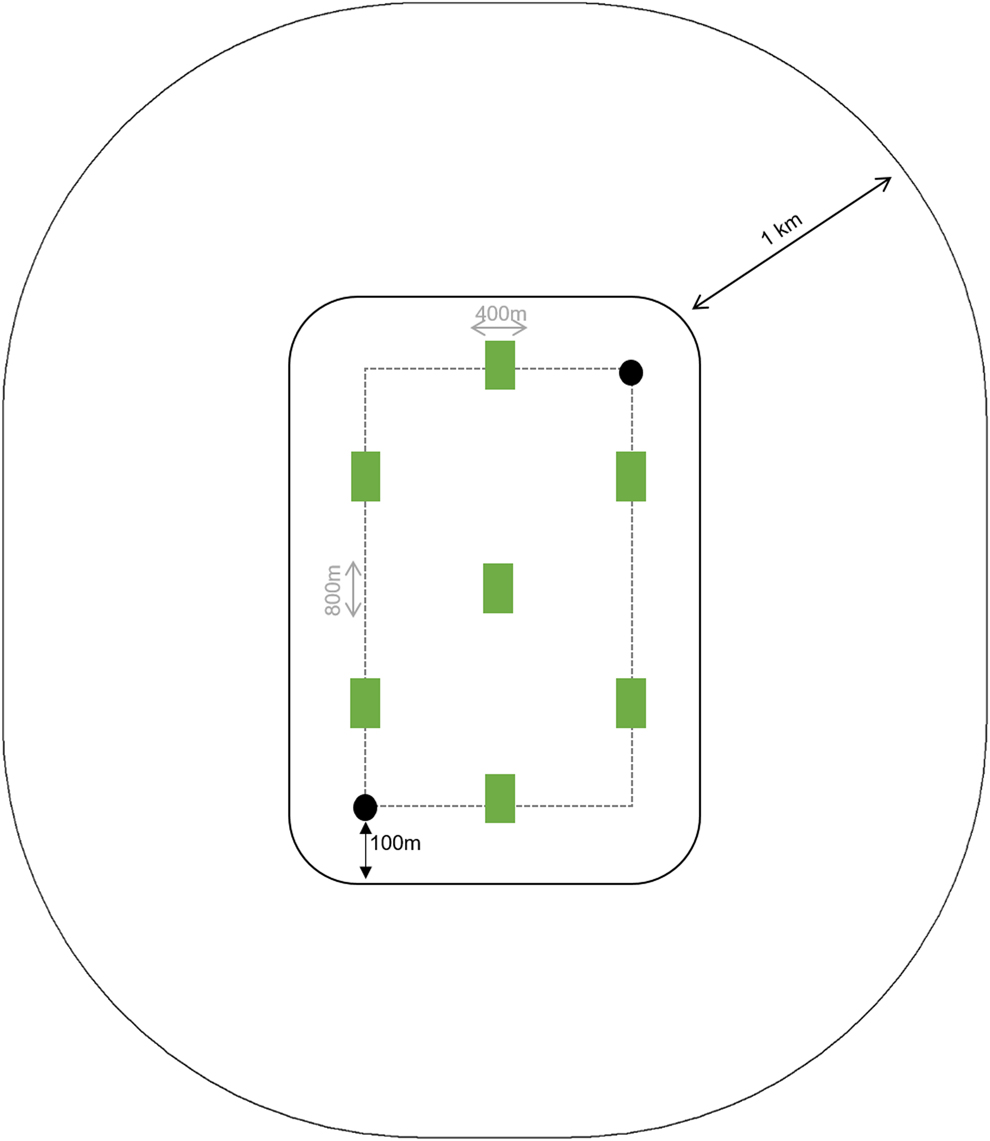
Figure 2. Scheme of the sampling grid designed in each cabruca site showing the linear transects (dashed gray line) where the active searches were performed, the location of the playback points (black circles) and the vegetation plots (gray rectangles). The transects are located at least 100m distant from fragment’s edge and the vegetation cover was estimated within the area delimited by the 1-km radius.
Playback was performed to a set of species that are known to respond well to this technique: the Grey-headed Kite, Barred Forest-falcon Micrastur ruficollis, Collared Forest-falcon M. semitorquatus, Bicolored Hawk Accipiter bicolor and Black Hawk-eagle Spizaetus tyrannus (Zorzin Reference White and Burnham2011, JABM pers. obs.). This method consists of playing a recording of the species’ vocalisation to attract individuals and stimulate intra-specific responses. Often playback resulted in inter-specific responses as well. Playback was performed at two opposite points of the sampling grid (Figure 2), separated by a mean linear distance of 665 m ± 160 SD, a placement that is consistent with those used by previous studies (Carvalho Filho et al. Reference Carvalho Filho, Zorzin, Canuto, Carvalho and Carvalho2008/2009, Vázquez-Pérez et al. Reference Thiollay2009). We used recordings available on Wiki Aves (http://www.wikiaves.com/), preferentially those made in the study region and avoiding aggressive vocalisations and pair duets. Using the aforementioned recorder coupled with a portable speaker, the recordings of all focal species were played in a pre-established order that took into account both body size and aggressive behavior, considering that large and fierce species could repel smaller ones (Mosher et al. Reference Mosher, Fuller and Kopeny1990). Each vocalisation was played continuously for 3 min, holding the speaker at approximately 2 m above the ground and rotating it over 360° at a constant speed, followed by a 3-min on-site wait.
Most raptors, such as the hawks Buteogallus spp. and the Mantled Hawk Pseudastur polionotus, often start soaring when thermals are well-formed, so the best period to perform the point-count methodology is between 09h00 and 12h00 (Thiollay Reference Thiollay1989, Mañosa et al. Reference Mañosa, Mateos and Pedrocchi2003). During this period, we recorded all individuals visually or through their vocalisations from a fixed location. Point-counts for raptors are typically located in high places that offer a wide view of the canopy, such as tops of hills adjacent to the study area (Mañosa et al. Reference Mañosa, Mateos and Pedrocchi2003). Such an approach was used whenever possible, but in six cabruca sites the relief was very flat so we located two complementary point-counts at the edge of the cabruca (∼100 m from the edge and 690 ± 170 m apart from each other) and remained for 1h30 min at each point.
Except for single point-counts, the starting sampling point of each method was alternated on the second visit to ensure that species with different peaks of activity could be detected at all points (Jones Reference Jones, Jones and Marsden2000). Since most Atlantic Forest fragments are smaller than the mean territory sizes of Neotropical raptors (Thiollay Reference Thiollay1989, Zorzin Reference White and Burnham2011), repeated detections of the same species at the same site were attributed to the same individual unless when more than one individual was observed simultaneously.
Habitat characterisation
In each cabruca site, seven 200-m2 plots were placed at interspersed points of the sampling grid (Figure 2). Six features related to vegetation structure and cabruca management were collected in these plots, resulting in eight explanatory variables to be used in data analysis: (1) shade tree density; (2) mean shade tree diameter; (3) mean canopy height; (4) mean canopy connectivity; (5) heterogeneity of the vertical strata; (6) cocoa tree density; (7) shading level and (8) management intensity (Table S3). For the statistical analysis, we created an abundance index for each variable so that each of them was represented by a unique value per area. This index is the sum of the values obtained for each variable per plot.
Responses of diurnal neotropical raptors to forest fragmentation usually occur between the scales of 500 m and 1 km (Zorzin Reference White and Burnham2011). Based on the Landsat images, we created a buffer defined by a 1-km radius from the extreme points of the sampling grid (Figure 2) where we estimated the percentage of vegetation. Clearings within the buffers were visually identified and their area was subtracted from the total area of the buffer. This estimate included both cabruca and native forest due to the difficulty of accurately differentiating it in the images. Spatial analyses were performed on Quantum GIS 2.18.2 (www.qgis.org) using its interface with Google Earth (Google Inc. 2016). The distance measurements were made with the Raster Package (Hijmans et al. Reference Hijmans, van Etten, Cheng, Mattiuzzi, Sumner, Greenberg, Lamigueiro, Bevan, Racine and Shortridge2016) in R 3.3.1 (R Core Team Reference Piacentini, Aleixo, Agne, Maurício, Pacheco, Bravo, Brito, Naka, Olmos, Posso, Silveira, Betini, Carrano, Franz, Lees, Lima, Pioli, Schunck, Amaral, Bencke, Cohn-Haft, Figueiredo, Straube and Cesari2016).
Data analysis
Occupancy modeling was performed in the program Mark version 8.x (White and Burnham Reference Watson, Shanahan, Marco, Sanderson and Mackey1999) to evaluate the influence of several variables on raptor occupancy. We used the single-season multi-species method to model the occupancy (Ψ) and detectability (p) of all species simultaneously, assuming an imperfect detection (Mackenzie et al. Reference Mackenzie, Nichols, Lachman, Droege, Andrew and Langtimm2002). In this approach, ‘species’ and ‘visits’ represent the sample units and occasions, respectively (see chapter 9 of Mackenzie et al. [Reference Mackenzie, Nichols, Royle, Pollock, Bailey and Hines2006] for more details). For each species, a detection history was created based on the two independent visits per sampling method, which resulted in six occasions per species/site. This analysis included only the subset of species detected at least once at one of the sampling areas.
Single-season modeling assumes that the population is closed to changes in occupancy during the season (Mackenzie et al. Reference Mackenzie, Nichols, Royle, Pollock, Bailey and Hines2006). Given that raptor home ranges are probably larger than all of our sampling areas (Thiollay Reference Thiollay1989), this assumption may not have been achieved. To deal with this problem, we interpreted the estimated occupancy as the proportion of the area ‘used’ by the species rather than the true occupancy (Mackenzie et al. Reference Mackenzie, Nichols, Royle, Pollock, Bailey and Hines2006). The detectability was interpreted as the probability of detecting the species when it is present in the area and using the sampling unit during the survey, assuming that movement of species through their home range is random (see similar interpretations in Keane et al. Reference Keane, Hobinjatovo, Razafimanahaka, Jenkins and Jones2012 and Kalan et al. Reference Kalan, Mundry, Wagner, Heinicke, Boesch and Kühl2015).
Habitat features, landscape metrics, and biological traits were used as covariates to model Ψ and p in a series of competing models. Prior to analyses, we assessed their pairwise correlations through a Spearman correlation test using the R Stats package (R Core Team Reference Piacentini, Aleixo, Agne, Maurício, Pacheco, Bravo, Brito, Naka, Olmos, Posso, Silveira, Betini, Carrano, Franz, Lees, Lima, Pioli, Schunck, Amaral, Bencke, Cohn-Haft, Figueiredo, Straube and Cesari2016), and excluded those with correlation ≥ 0.60, that is: percentage of foraging in intermediate strata (negatively correlated with body mass; r = -0.63); percentage of foraging in air (positively correlated with percentage of foraging in the canopy; r = 0.67); percentage of shade (positively correlated with canopy connectivity; r = 0.60 and vertical stratification; r = 0.66); and canopy height (positively correlated with canopy connectivity; r = 0.84) and diameter of shade trees (r = 0.64)]. We assessed the multicollinearity among the remaining variables through the Variance Inflation Factor (VIF) using the CAR package of R (R Core Team Reference Piacentini, Aleixo, Agne, Maurício, Pacheco, Bravo, Brito, Naka, Olmos, Posso, Silveira, Betini, Carrano, Franz, Lees, Lima, Pioli, Schunck, Amaral, Bencke, Cohn-Haft, Figueiredo, Straube and Cesari2016). Since no variable had VIF > 4, we kept all of them for the analyses.
We finished with seven covariates to model p: (1) sampling method; (2) body mass; (3) percentage of foraging in the canopy; (4) canopy connectivity; (5) vertical strata heterogeneity; (6) shade tree density; and (7) cocoa tree density. We expected that: sampling methods would differ in species detectability; larger species would be more easily detected than smaller ones; species could be more or less easily detected depending on its preferred foraging strata; canopy connectivity, vertical heterogeneity, shade tree density, and cocoa density could interfere with visual obstruction and sound propagation, thus affecting species detectability during active searches and playbacks. We modeled Ψ as a function of 10 covariates: (1) shade trees density; (2) shade trees diameter; (3) canopy connectivity; (4) vertical strata heterogeneity; (5) management intensity; (6) cocoa tree density; (7) vegetation cover; (8) body mass; (9) trophic level; and (10) specialisation degree. We also included models where p and Ψ were held constant.
Since we were more interested in determining the importance of covariates rather than in the final estimates of Ψ and p, we built a model set based on all possible additive covariate combinations (Doherty et al. Reference Doherty, White and Burnham2012), totalling 3,215 competing models. Then we calculated the cumulative AICc weight (w +) for each covariate to interpret their relative importance, and the final estimate of Ψ was averaged among all competing models (Burnham and Anderson Reference Burnham and Anderson2002). We investigated the fit of the most general model (the model with the greatest number of parameters) by estimating the overdispersion parameter c-hat using 10,000 bootstrap samples (Mackenzie and Bailey Reference Mackenzie and Bailey2004) in the program PRESENCE 11.7 (http://www.mbr-pwrc.usgs.gov/software/presence.shtml).
Results
Raptor assemblages in cabrucas
A total sampling effort of 64 playback points (four per area), 91h11min of active search (mean effort: 5h40 per area; range: 4h48–6h45) and 96h of point-count (6h per area), resulted in the record of 18 species plus unconfirmed records of three species (Table S4). Some species for which we did not perform playbacks, as Buteo hawks, responded to the vocalisations of other species, therefore increasing their detectability (Table S5). Although we did not systematically survey for the smaller New World vultures – the Turkey Vulture Cathartes aura, Lesser Yellow-headed Vulture C. burrovianus, and Black Vulture Coragyps atratus - they were observed in all cabruca sites with all survey methods. Considering their constant presence in almost all sampling units and the difficulty in estimating their abundance, we decided not to include them in the analyses.
Among all recorded species, 44% (11 spp.), 48% (12 spp.), and 8% (2 spp.) are considered to be slightly, moderately and highly sensitive to habitat disturbance, respectively (Parker III et al. Reference Oliveira and Dietz1996). The most common species in our survey - ordered from the most to the less abundant - were the Southern Caracara Caracara plancus, the Zone-tailed Hawk Buteo albonotatus, the Laughing Falcon Herpetotheres cachinnans, and the Roadside Hawk Rupornis magnirostris. With the exception of the Zone-tailed Hawk - which is classified as uncommon, patchily distributed, and moderately sensitive to habitat disturbances - the aforementioned species are all considered to be common and slightly sensitive to habitat disturbance. The highly sensitive species detected in this study were the Black-and-white Hawk-eagle Spizaetus melanoleucus (recorded in four sites), and the Mantled Hawk Pseudastur polionotus (recorded in 10 sites).
The recorded species vary a lot in their dependence on forested habitats. The Collared Forest-falcon Micrastur semitorquatus, a forest-dependent species that uses mainly the low forest strata to forage, was recorded in two cabruca sites. Both records were made near regenerating forest plots, in places with a high density of shade trees. At the opposite extreme, the Savanna Hawk, Buteogallus meridionalis, a species that is typical of open areas, was observed during point counts in pastures near cabruca sites.
Some evidence of reproductive activity were observed during surveys, such as breeding pairs from six species (Black-and-white Hawk-eagle, and Black Hawk-eagle, Great Black-hawk Buteogallus urubitinga, Mantled Hawk, Southern Caracara, and Zone-tailed Hawk), juveniles of three species (Gray-lined Hawk, Roadside Hawk and King Vulture), and a family group of the Zone-tailed Hawk (two adults and one juvenile) foraging in the canopy near the edge of a cabruca.
Habitat characterisation
The structure of the cabrucas varied significantly among sites, with a mean ± SD density of 623 ± 182 cacao trees/ha, and 182 ± 60 shade trees/ha (Table S6). Mean ± SD diameter of shade trees is 37.2 ± 30.7 cm, mean ± SD canopy height is 15.6 ± 2.6 m and mean ± SD shading level is 73 ± 10%. Hunting signs (traps, hunters and/or firearm blows) and logging signs (chainsaw noises and stumps) were recorded in 10 and nine of the 16 areas, respectively. The vegetation cover ranged between 73% and 96%.
Occupancy modeling
The most parsimonious model had a low AICc weight (w+ = 0.08), reflecting the high number of competing models and a high degree of uncertainty about the best-ranked models (Table 1). There is no evidence of overdispersion (χ2 = 73.37; P = 0.12; c-hat = 1.28). Although the detectability of diurnal raptors was higher with the point-count method (P = 0.24; 95% CI: 0.19, 0.32) compared to the others (P both = 0.13; 95% CI: 0.09, 0.18), the sampling method did not significantly affect detectability (Table 2). Detectability decreases as both the percentage of foraging in the canopy (w+ = 0.97) and the density of shade trees (w+ = 0.82) increase (Table 2; Figures 3 and 4), and there is a significantly negative correlation between occupancy and dietary specialisation (w+ = 0.60; Table 2; Figure 5). The model-averaged estimate of Ψ was 0.66 (95% CI: 0.42; 0.84).
Table 1. Results for the 10 top-ranked models of occupancy (Ψ) and detection (p) probabilities of diurnal raptors in 16 cabrucas of Southern Bahia, Brazil. ‘AICc’ is the Akaike information criterion corrected for small samples, ‘∆AICc’ is the difference between the AICc value of each model and the top-ranked model, ‘AICcW’ is the Akaike weight, and ‘Dev’ is the deviance (model adjustment).
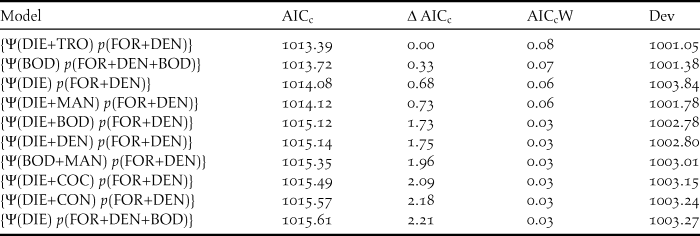
DIE = dietary specialisation; DEN = shade tree density; FOR = percentage of foraging in the canopy; BOD = body mass; MAN = management intensity; COC = cocoa tree density; and TRO = trophic level.
Table 2. Cumulative AICc weight for covariates used to model occupancy (Ψ) and detection (p) probabilities of diurnal raptors in 16 cabrucas of Southern Bahia, Brazil. The covariate effects (ß parameters) were derived from the most parsimonious model including each covariate. LL and UL represent the lower and upper limits of the confidence interval (95%), respectively.
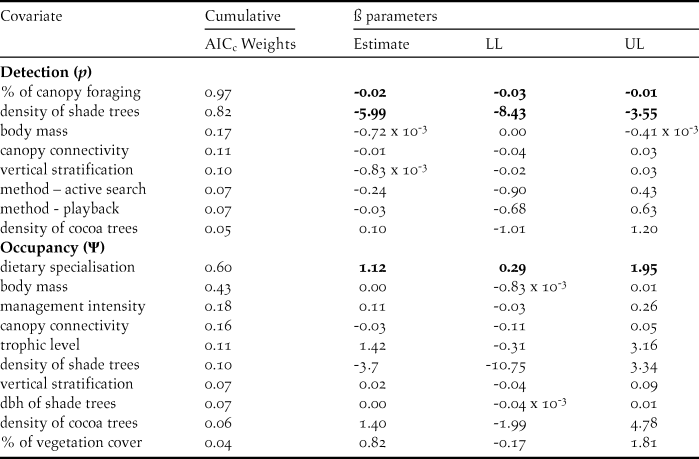
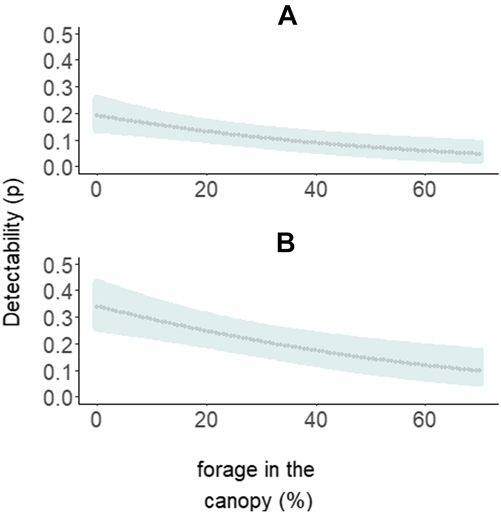
Figure 3. Relationship between diurnal raptor detectability and species’ percentage forage in the canopy using three sampling methods: active search and playback (both represented in graph A), and point-count (B). Dotted lines and shaded areas represent the predicted values and the 95% confidence intervals, respectively.
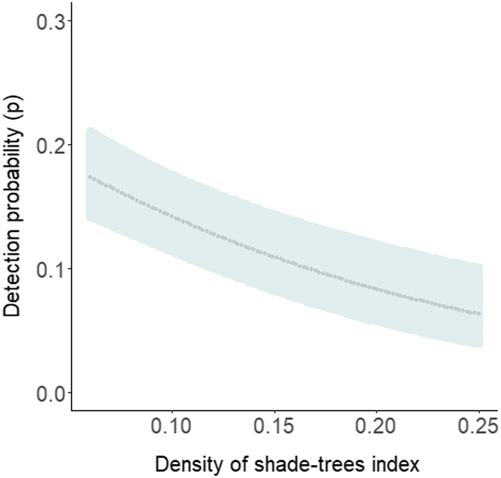
Figure 4. Diurnal raptor occupancy probability as a function of the density of shade trees in cabruca sites. The dotted line represents predicted values and the shaded areas the 95% confidence intervals.
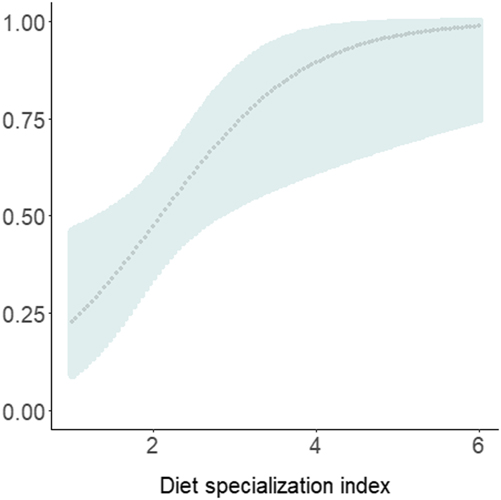
Figure 5. Diurnal raptor occupancy probability as a function of the diet specialisation index (= the number of food items in species diet). A high index value means a low degree of dietary specificity. The dotted line represents predicted values and the shaded areas the 95% confidence intervals.
Discussion
In this study we described the diurnal raptor assemblages found in cabrucas of Southern Bahia, Brazil. We used occupancy modeling to investigate the role of vegetation structure, management intensity, landscape context, and biological traits in determining raptor occupancy in this agroecosystem. A significant percentage of the species expected to occur in the study region (∼40%) was recorded in our study sites, and they have a high probability of occurrence in cabruca sites. It does not necessarily mean that cabrucas can assure the conservation of these species, but considering the high degree of deforestation and habitat loss in this region, cabrucas can act as a suboptimal habitat and assist in the persistence of diurnal raptor populations. Despite this promising perspective, there is a tendency towards impoverishment of communities in this system from the decrease of specialists.
Raptor assemblages in cabrucas
At least 18 (possibly 21) diurnal raptor species occur in the cabrucas of Southern Bahia. Most of these are considered to be naturally common, but species such as the Grey-headed Kite, Mantled Hawk, and Zone-tailed Hawk, which are classified as ‘uncommon’ by Parker III et al. (Reference Oliveira and Dietz1996), were actually very frequent in our surveys. It is important to emphasise that such a classification is being used to allow comparison with other studies since we believe this is the only classification available for all Neotropical raptors to date. We recognise that it may suffer some limitations since it does not consider regional variations in factors that strongly determine species abundance. Anyway, since no systematic surveys of raptor assemblages have been made in forests from this region, we cannot conclude if the aforementioned species are particularly common in cabruca, or in the study region as a whole. However, as they are frequently sighted throughout this region (even foraging in urban areas, in the case of the Zone-tailed Hawk; JABM pers. obs.), we believe that they can indeed be fairly common in Southern Bahia.
More than half of the recorded species are classified as moderately or highly sensitive to habitat disturbance, such as the Black-and-white Hawk-eagle and the Mantled Hawk. In fact, some authors have been questioning this classification by claiming that species-specific responses to habitat alterations depend on the disturbance context (Sergio et al. Reference Schroth, Faria, Araujo, Bede, Van Bael, Cassano, Oliveira and Delabie2008; Alexandrino et al. Reference Alexandrino, Buechley, Piratelli, Ferraz, Moral, Sekercioglu, Silva and Couto2016). As the classification proposed by Parker III et al. (Reference Oliveira and Dietz1996) did not specify the type and intensity of disturbance under consideration, conclusions may have been based on habitats with levels of disturbance more intense than those observed in cabrucas.
We did not record any globally threatened species, and only one ‘Near Threatened’ species, the Mantled Hawk, in the cabruca sites. The commonness of this species in cabruca is supported by unpublished reports from the Serra Bonita reserves complex in Camacan, located within the study region (JABM pers. obs.). An earlier study has found the Mantled Hawk to be extremely affected by forest fragmentation (Zorzin Reference White and Burnham2011), thus a high tolerance to cabrucas may reveal an important role of this agroecosystem on mitigating the detrimental effects of forest fragmentation.
The observation of juveniles and breeding pairs from many species in cabruca sites was a very interesting and promising finding because it suggests they may be breeding there. However, specific studies on this topic are needed to confirm if these species are actually nesting in cabrucas since it would contradict the high-quality requirements proposed for raptor breeding sites (Joenck et al. Reference Joenck, Zilio and Mendonça-Lima2011, Canuto et al. Reference Canuto, Zorzin, Carvalho-Filho, Carvalho, Carvalho, Benfic, Sudarshana, Nageswara-Rao and Soneji2012). There are some nesting records of Neotropical accipitrids such as the Short-tailed Hawk and the Harpy Eagle in areas that do not match such high-quality demands (Silva Reference Seymour, Hatherley, Contreras, Aldred and Beeley2007, Monsalvo Reference Monsalvo2012), suggesting that a re-evaluation of this topic is necessary at least for some hawks and eagles (Monsalvo et al. Reference Monsalvo, Heming and Marini2018).
The failure to detect some species from the potential dataset may be partly explained by their high dependence on specific habitat types. The Snail Kite Rostrhamus sociabilis for example, is most commonly associated with wetlands, and the Long-winged Harrier Circus buffoni prefers open areas (Thiollay Reference Thiollay2007). An adult pale-morph male of the latter species was occasionally observed while flying above a restinga (vegetation on the sandbanks) confirming their presence in the region despite the absence of records in the cabruca sites. A group of Swallow-tailed Kites, a migratory species that reproduces in the Atlantic Forest, was also occasionally recorded foraging under a pasture in the proximities of a cabruca site. To our knowledge, this is the first published record of this species in this region, and although we did not detect any individual within cabruca sites, such record suggests an ability to forage in landscapes totally dominated by this system. The same conclusion applies to our records of the Savanna Hawk, a common species in altered landscapes (Thiollay Reference Thiollay2007).
The largest species from our potential dataset (the Crested and Harpy Eagles) are among the rarest Neotropical raptors (Thiollay Reference Thiollay2007) and were also not recorded in our surveys. However, there are recent records of both species near and within our study region (Sánchez-Lalinde et al. Reference Rodríguez-Estrella, Donázar and Hiraldo2011, Araújo et al. Reference Araújo, Silveira and Luz2015, Suscke et al. Reference Soares, Amaral, Granzinolli, Albuquerque, Lisboa, Azevedo, Moraes, Sanaiotti and Guimarães2016), including the observation of Harpy Eagles foraging in cabrucas from the Serra Bonita reserves complex between 2012 and 2013, and the recent record of nesting in that same area (JABM unpublished. data). Considering the rarity of such species, an increased survey effort would be necessary to best estimate their occupancy in cabruca sites.
Determinants for raptor occupancy in cabrucas
Our analysis suggests that the availability of food resources may play a key role in determining the assembly of raptor communities in cabrucas. Despite the certain degree of habitat complexity and heterogeneity that is preserved in cabrucas, this system is indeed simplified compared to forests, so the overall prey availability is expected to be lower (August Reference August1983). Consequently, specialists may be less likely to occupy cabrucas than species with more diversified diet. Previous studies have already shown that specialist species are strongly affected by habitat disturbance and may be excluded from altered habitats, which tend to be dominated by generalists (Ferrer-Sánchez and Rodríguez-Estrella Reference Ferrer-Sánchez and Rodríguez-Estrella2014). Thus, although cabrucas may represent a suboptimal habitat for a significant number of diurnal raptor species, this system alone may not support complete assemblages of raptors.
Finally, although we failed to find any significant effect of habitat and landscape features on raptor occupancy, our results should be interpreted under the regional context. The cabruca sites surveyed in this study preserve a high density of shade trees, which is typical of traditional cabrucas in this region (Schroth et al. Reference Sánchez-Lalinde, Vélez-García, Cornélio, Silveira and Alvarez2015). There may be a critical density of trees, below which significant impacts on raptor occupancy may be observed, and it is therefore possible that the study did not include cabrucas with such a low density of trees. Assuming this possibility is particularly important due to the current scenario in Southern Bahia of encouragement of cabruca management intensification. A state decree published by the Bahia Government in 2014 (N° 15.180; article 19) has sanctioned the management intensification of cabrucas by allowing the reduction in shade tree density to very low levels (40 trees per ha). If implemented, it will promote a huge change and will likely decrease habitat heterogeneity in cabruca (Benton et al. Reference Benton, Vickery and Wilson2003). Unfortunately, we cannot predict how raptors will respond to the changes that will occur in habitat features such as canopy connectivity or the diameter of shade trees. However, since a minimum level of habitat complexity and heterogeneity is necessary to support species-rich bird communities in altered habitats (Abrahamczyk et al. Reference Abrahamczyk, Kessler, Dwi Putra, Waltert and Tscharntke2008, Philpott et al. Reference Parker, Stotz, Fitzpatrick, Stotz, Fitzpatrick, Parker and Moskovits2008), raptor assemblages may not be sustained in very intensified cabrucas, as already shown in other types of farmland (Donald et al. Reference Donald, Green and Heath2001, Carrete et al. Reference Carrete, Tella, Blanco and Bertellotti2009).
Conservation implications and recommendations
This study is a first attempt to understand the role of cabrucas for diurnal raptor conservation in Southern Bahia. We found a significant number of diurnal raptor species are able to use cabrucas, and evidence that some species may even be breeding in these areas. However, there is a tendency towards impoverishment of communities through the reduction in dietary specialists’ occupancy. These findings emphasise the importance of considering human-modified habitats in conservation planning but highlight the need for the maintenance of forest remnants in the landscape to increase the regional species diversity.
Finally, a complete understanding of the role of cabrucas in assisting raptor conservation depends on a better knowledge about raptor assemblages in the forests of this region. More surveys using appropriate sampling methods, as well as ecological and behavioural studies in both forests and cabruca sites, are crucial to better understand how raptors are dealing with habitat changes. Such knowledge would clarify the extent to which cabrucas can contribute to raptor conservation at a regional scale, and how different species can be specifically affected by cabruca intensification. Ultimately, since raptors play a key role as top predators, understanding such issues would allow a better understanding of how stable these communities are, and also of the long-term persistence probability of many species (including endangered species) in cabrucas of Southern Bahia.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270919000017
Acknowledgements
We are grateful to all landowners, especially Juliana Torres and Elizabeth Torres from Fazenda Almada, and to the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) for the permission to work in the Biological Reserve of Una. We also thank Gastón Giné for the help with spatial analysis; Rodrigo Massara and Ana Maria Paschoal for the help with occupancy analysis; Victor Arroyo-Rodrigues and Stephen Ferrari for helpful comments on the manuscript; and all people who helped in field work, especially Edivaldo Francisco de Jesus and Jiomário dos Santos Souza. JMAR’s doctoral studentship was funded by the Fundação de Amparo ao Pesquisador do Estado da Bahia (FAPESB) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ) through the “Ciência sem Fronteiras” Program. JABM’s master studentship was funded by CNPq. This study received funds from CNPq/CAPES through the Casadinho/PROCAD Project UESC-UFRJ (N°:552198/2011-0) and had the support of the Idea Wild (http://www.ideawild.org) through field equipment donations.









