Introduction
The coastal wetlands of the Yellow Sea provide important stop-over habitat for migratory shorebirds in the East Asian-Australasian Flyway (EAAF) (Barter Reference Barter2002, Bamford et al. Reference Bamford, Watkins, Bancroft, Tischler and Wahl2008). With 58 shorebird species, this flyway supports the highest diversity of shorebirds in the world (Stroud et al. Reference Stroud, Baker, Blanco, Davidson, Delany, Ganter, Gill, González, Haanstra, Morrison, Piersma, Scott, Thorup, West, Wilson, Zöckler, Boere, Galbraith and Stroud2006, Bamford et al. Reference Bamford, Watkins, Bancroft, Tischler and Wahl2008). Rather than using many different areas along the flyway, hopping from one to another, or migrating from the southern non-breeding grounds to the Arctic breeding grounds in one flight, many shorebird species make just one or two refuelling stops, especially during northward migration (Piersma and Baker Reference Piersma, Baker, Gosling and Sutherland2000, Ma et al. Reference Ma, Hua, Peng, Choi, Battley, Zhou, Chen, Ma, Jia and Xue2013). Within the EAAF, the coastal wetlands around the Yellow Sea support approximately three million shorebirds during northward and southward migration (Barter Reference Barter2002, Hua et al. Reference Hua, Tan, Chen and Ma2015).
Unfortunately, in recent decades the availability of coastal habitats used by migratory shorebirds has declined rapidly, mainly due to land claim (Ma et al. Reference Ma, Melville, Liu, Chen, Yang, Ren, Zhang, Piersma and Li2014, Melville et al. Reference Melville, Peng, Chan, Bai, He, Tan, Chen, Zhang and Ma2016b, Moores et al. Reference Moores, Rogers, Rogers and Hansbro2016). It is estimated that up to two-thirds of the Yellow Sea intertidal flats have been lost over the past five decades, with 28% of intertidal flats present in the 1980s having disappeared by the 2010s (average annual rate of decline 1.2%) (Murray et al. Reference Murray, Clemens, Phinn, Possingham and Fuller2014, Chen et al. Reference Chen, Dong, Xiao, Zhang, Tian, Zhou, Li and Ma2016, Piersma et al. Reference Piersma, Lok, Chen, Hassell, Yang, Boyle, Slaymaker, Chan, Melville, Zhang and Ma2016). Along the EAAF, there have been significant population declines for several shorebird species and subspecies relying on the Yellow Sea region (Conklin et al. Reference Conklin, Lok, Melville, Riegen, Schuckard, Piersma and Battley2016, Piersma et al. Reference Piersma, Lok, Chen, Hassell, Yang, Boyle, Slaymaker, Chan, Melville, Zhang and Ma2016). Many studies have indicated that loss of coastal habitats around the Yellow Sea is the main cause of the decline (e.g. Moores et al. Reference Moores, Rogers, Kim, Hassell, Gosbell, Kim and Park2008, Reference Moores, Rogers, Rogers and Hansbro2016, Amano et al. Reference Amano, Székely, Koyama, Amano and Sutherland2010, Studds et al. Reference Studds, Kendall, Murray, Wilson, Rogers, Clemens, Gosbell, Hassell, Jessop, Melville, Milton, Minton, Possingham, Riegen, Straw, Woehler and Fuller2017).
The coastal wetland of the Yalu Jiang estuary (39°40’–39°58’N, 123°34’–124°07’E, hereafter Yalu Jiang) (Figure 1) is a critical staging site in the northern Yellow Sea, especially for Bar-tailed Godwits Limosa lapponica of the subspecies menzbieri and baueri and Great Knots Calidris tenuirostris (Barter Reference Barter2002, Riegen et al. Reference Riegen, Vaughan and Rogers2014, Choi et al. Reference Choi, Battley, Potter, Rogers and Ma2015, Hua et al. Reference Hua, Åkesson, Zhou and Ma2017). More than 250,000 shorebirds, including 100,000 Bar-tailed Godwits and 55,000 Great Knots (Barter Reference Barter2002), use the coastal wetland for replenishing fuel during northward migration (Ma et al. Reference Ma, Hua, Peng, Choi, Battley, Zhou, Chen, Ma, Jia and Xue2013, Riegen et al. Reference Riegen, Vaughan and Rogers2014), with 17 species meeting the 1% Ramsar criterion for wetlands of international importance (Bai et al. Reference Bai, Chen, Chen, Dong, Dong, Dong, Fu, Han, Lu, Li, Liu, Lin, Meng, Martinez, Ni, Shan, Sun, Tian, Wang, Xu, Yu, Yang, Yang, Zhang, Zhang and Zeng2015). To protect migrating shorebirds and their wetland habitats, the Dandong Yalu Jiang Estuarine Wetland National Nature Reserve was established in 1987 (Riegen et al. Reference Riegen, Vaughan and Rogers2014). The protected area covers a large area of intertidal flats, which provides foraging sites for shorebirds. Except for a port construction in the east of the protected area in 2009 (Melville et al. Reference Melville, Chen and Ma2016a), there has been very little loss of intertidal habitat.
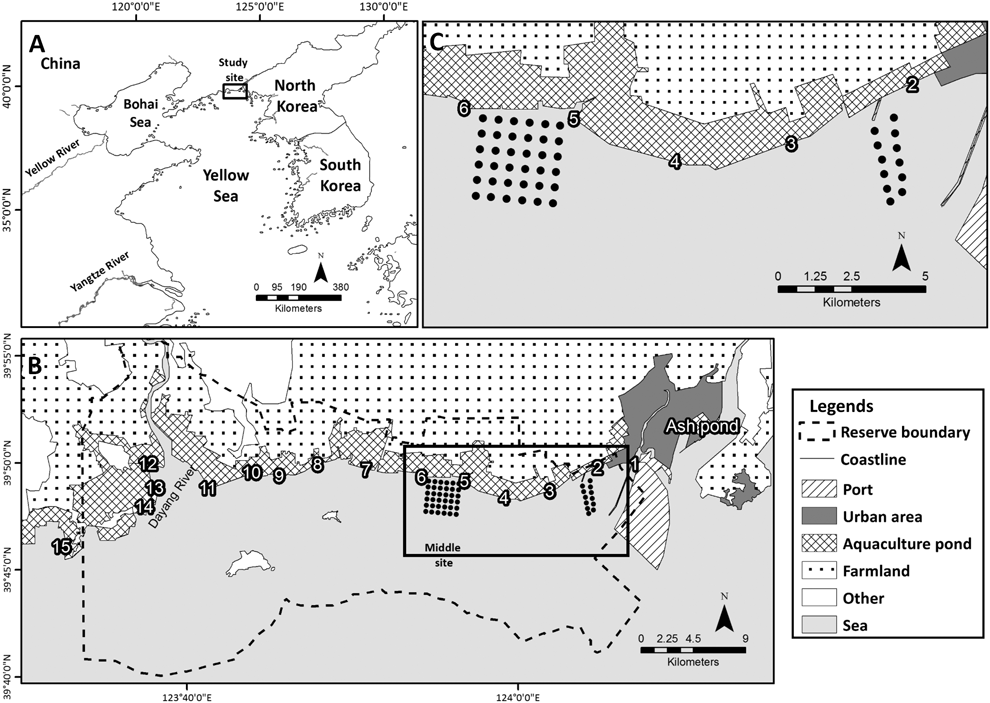
Figure 1. Locations of (A) Yalu Jiang estuarine wetland nature reserve in the Yellow Sea, (B) the 16 bird counting sites (15 numbered sites and Ash pond) across and adjacent to the reserve, and (C) the intertidal macrozoobenthos sampling sites in the eastern part of the reserve (also shown within the box in panel B).
Although many studies focused on the impact of habitat loss on shorebirds, few studies have examined the effects of food decline on shorebirds at stop-over sites. The macrozoobenthos (the marine invertebrates retained on a 0.5 mm sieve) of the intertidal mudflats is the main food resource for shorebirds. At Yalu Jiang, Potamocorbula laevis, a small bivalve, is the single most important prey for shorebirds (Choi et al. Reference Choi, Battley, Potter, Ma, Melville and Sukkaewmanee2017). This prey species accounted for 82% of the biomass consumed by Bar-tailed Godwits and 95% by Great Knots in 2010–2012 (Choi et al. Reference Choi, Battley, Potter, Ma, Melville and Sukkaewmanee2017). In this study, we monitored the dynamics of intertidal macrozoobenthos at Yalu Jiang from 2011 to 2016. We also surveyed the numbers of Bar-tailed Godwits and Great Knots during northward migration and further checked the relationship between the dynamics of shorebird numbers and their macrozoobenthic food resources. We put forward the hypothesis that the change of food resources at Yalu Jiang could affect the number of Bar-tailed Godwits and Great Knots at the staging site.
Methods
Study area
The Dandong Yalu Jiang Estuarine Wetland National Nature Reserve is located in the west of the Yalu Jiang estuary, close to the national boundary between China and North Korea (Figure 1) (Ma et al. Reference Ma, Hua, Peng, Choi, Battley, Zhou, Chen, Ma, Jia and Xue2013, Choi et al. Reference Choi, Battley, Potter, Ma and Liu2014). It is a typical coastal wetland in the northern Yellow Sea, with a large area of aquaculture ponds along the coastline (Barter Reference Barter2002, Choi et al. Reference Choi, Battley, Potter, Ma and Liu2014, He et al. Reference He, Melville, Peng, Tan, Chen and Ma2016) and wide intertidal flats (extending 3–5 km at low spring tides). Tides are semi-diurnal in this area, with an average tidal amplitude of 4.5 m and a maximum of 6.9 m (Wang et al. Reference Wang, Ren and Zhu1986). At spring tides higher than 6.4 m, all intertidal flats are submerged. At high neap tides, the upper intertidal flats in some areas in the east and west (Figure 1) are not inundated (Riegen et al. Reference Riegen, Vaughan and Rogers2014, Choi et al. Reference Choi, Battley, Potter, Rogers and Ma2015). Shorebird activities are strongly affected by the tide; they forage on the intertidal flats at low tide and rest in the high-tide roosts at high tide.
Aquaculture ponds adjacent to the high-tide roosts are mainly used for farming shellfish, sea cucumbers, and jellyfish. Many shorebirds roost in aquaculture ponds during high-tide when intertidal flats are submerged by tidewater (He et al. Reference He, Melville, Peng, Tan, Chen and Ma2016). Shorebirds rarely forage in the aquaculture ponds due to the deep water.
More than 90 species of macrozoobenthos, mainly belonging to six classes (Anthozoa, Polychaeta, Malacostraca, Bivalvia, Gastropoda, and Inarticulata) have been recorded at Yalu Jiang. P. laevis is the dominant mollusc species (Choi et al. Reference Choi, Battley, Potter, Ma, Melville and Sukkaewmanee2017) and tends to occur at high densities in the upper intertidal flats (Choi et al. Reference Choi, Battley, Potter, Ma and Liu2014, Yang et al. Reference Yang, Chen, Piersma, Zhang and Ding2016). Additionally, cultivated shellfish species such as the bivalves Mactra veneriformis and Sinonovacula constricta provide a supplementary prey resource for Bar-tailed Godwits and Great Knots. Local fishermen release cultivated spats (young shellfish) and collect adult shellfish (over 40 mm in length) 1–3 years later.
Macrozoobenthos sampling
Macrozoobenthos sampling was conducted once a month from March to May in 2011, 2012, 2015 and 2016, and April–May in 2013 and 2014. We set up grids in the eastern (12 stations) and the middle part (36 stations) of the Yalu Jiang (Figure 1). This region is the main foraging area for Bar-tailed Godwits and Great Knots (Riegen et al. Reference Riegen, Vaughan and Rogers2014, Choi et al. Reference Choi, Battley, Potter, Ma, Melville and Sukkaewmanee2017). Macrozoobenthos samples were collected from the middle part in 2011 and 2014 and from both parts in 2012, 2013, 2015, and 2016. At each station, one core sample (diameter 15.5 cm, depth 30 cm) was collected and then separated into the upper 5 cm and the lower 25 cm (in 2013 only the top 5 cm was collected) and then washed through a 0.5 mm sieve.
All soft-bodied organisms, small-sized (< 10 mm) bivalve spat, and small-sized (< 10 mm) crabs were soaked in 5% formalin for at least 72 hours before being placed into 70% ethanol. Hard-bodied organisms such as large bivalves, gastropods, and crabs were kept frozen for further analysis. We identified all organisms to the finest practicable taxonomic level (Table S1 in the online supplementary material) and measured their length (to 0.01 mm) using a measurable dissecting microscope. In general, polychaetes were identified to family level (nearly half of them were identified to species or genus), while molluscs and crustaceans were identified to species or genus.
We selected complete individuals of different length for each taxonomic group to calculate the ash-free dry mass (hereafter AFDM). Flesh was separated from the shell before drying for all bivalves, Gastropods, and Inarticulata. Dry mass of flesh of these three classes and the whole body of the Anthozoa, Polychaeta, and Malacostraca was obtained by drying the samples at 60°C for 60 hours; ash mass was obtained by incinerating at 560°C for five hours. All masses were weighed to the nearest 0.1 mg and the difference between dry mass and ash mass was the AFDM.
Bird counts
Bird counts were carried out over periods of 2–3 days during each spring tide from early March to late May in 2011–2016. Six surveys were conducted in each year except for 2013 when four surveys were conducted. The same approach was used during bird surveys in each year (see details in Riegen et al. Reference Riegen, Vaughan and Rogers2014, Choi et al. Reference Choi, Battley, Potter, Rogers and Ma2015). Briefly, as the tide approaches the seawall, birds are forced to concentrate at the upper tidal flats at 16 fixed pre-roost areas. During incoming tide but before high tide, we counted birds at the 16 fixed pre-roosts before they flew to high-tide roosts (Riegen et al. Reference Riegen, Vaughan and Rogers2014, Choi et al. Reference Choi, Battley, Potter, Rogers and Ma2015; Figure 1).
Photos of Bar-tailed Godwits in flight were taken during bird surveys and then were used to estimate the proportions of L. l. baueri and L. l. menzbieri in late April from 2011 to 2016. We distinguished the two subspecies, based on their plumage characteristics: individuals with whitish rump and lower back were assigned to L. l. menzbieri and darker individuals to L. l. baueri (Choi Reference Choi2015). A total of 3,339 individuals were identified, including 2,670 L. l. baueri (392, 758, 105, 155, 613, and 644 from 2011 to 2016, respectively) and 669 L. l. menzbieri (269, 228, 37, 26, 48, and 61 from 2011 to 2016, respectively). Two observers (C. Y. Choi and Q. Q. Bai) identified the two subspecies in the photos and cross validation between the observers revealed no detectable differences.
Data analyses
The peak numbers of the two Bar-tailed Godwit subspecies historically overlap and remain stable in late April at Yalu Jiang (Battley et al. Reference Battley, Warnock, Tibbitts, Gill, Piersma, Hassell, Douglas, Mulcahy, Gartrell and Schuckard2012, Choi et al. Reference Choi, Battley, Potter, Ma and Liu2014, Riegen et al. Reference Riegen, Vaughan and Rogers2014). Therefore, the number of Bar-tailed Godwits recorded in late April was used as the sum of the peak number of both subspecies. The peak numbers of L. l. baueri and L. l. menzbieri in each year were estimated according to the proportions of two subspecies in the photos and the actual peak number of all the Bar-tailed Godwits recorded in the field.
Arrivals of shorebirds at Yalu Jiang extend over one month (Ma et al. Reference Ma, Hua, Peng, Choi, Battley, Zhou, Chen, Ma, Jia and Xue2013, Riegen et al. Reference Riegen, Vaughan and Rogers2014, Peng et al. Reference Peng, Hua, Choi, Melville, Gao, Zhou, Chen, Xue, Ma and Wu2015). Thus, the peak number of shorebirds does not represent the actual number of individual birds present on each day during the study periods. The daily number of shorebirds was estimated using an extended version of the Thompson model (Thompson Reference Thompson1993). The model assumes that the arrival and departure number of birds is normally distributed during the study periods (Rogers et al. Reference Rogers, Yang, Hassell, Boyle, Rogers, Chen, Zhang and Piersma2010, Choi et al. Reference Choi, Rogers, Gan, Clemens, Bai, Lilleyman, Lindsey, Milton, Straw, Yu, Battley, Fuller and Rogers2016). We used this model to estimate the daily cumulative number of Bar-tailed Godwits and Great Knots staying at Yalu Jiang during the northward migration in each year on the basis of four to six surveys.
We calculated the biomass of total macrozoobenthos at each sampling point and the taxonomic-group-specific biomass densities using the regression formula of each taxonomic group during the study period. The relationships between AFDM and body length were established using regression analysis (polynomial, e-exponential, logarithmic, and power regression) conducted for each taxonomic group. Models with the largest correlation coefficient were selected as the most suitable models. With the large sample sizes of P. laevis and M. veneriformis (Bivalvia), regression models could be created for each year, while sample sizes of other groups were relatively small and regression models were created for the study period as a whole by selecting individuals of different length from all years. We estimated AFDM for macrozoobenthos using regression models (R2 > 0.87, P < 0.05 for all) for each taxonomic group.
We assumed that the biomass available to Great Knots occurred only in the upper 5 cm of the sediment, whereas we treated all biomass sampled as being available to Bar-tailed Godwits (Choi Reference Choi2015). We analysed the macrozoobenthos in the upper 5 cm and 0–30 cm (except in 2013 when we only sampled at the top 5 cm). Inter-annual macrozoobenthos mean (across months) data for total biomass for the six Classes and individual density for P. laevis from the 36 sampling stations in 2011 and 2014 and 48 sampling stations in 2012, 2013, 2015 and 2016 were compared using Analysis of Variance (ANOVA) followed by Fisher’s least significance difference (LSD).
At Yalu Jiang, Bar-tailed Godwits mainly consume P. laevis, ghost shrimp, polychaetes, Anthozoa and other bivalves, and Great Knots mainly consume P. laevis as well as other bivalves and gastropods, most of which were found within the top 5 cm of the sediment surface (Choi et al. Reference Choi, Battley, Potter, Ma, Melville and Sukkaewmanee2017, authors’ pers. obs.). Because we only collected macrozoobenthos from the upper 5 cm in 2013, the ghost shrimp, polychaetes and Anthozoa in the potential food of Bar-tailed Godwits in 2013 were estimated based on the average of the other five years for depths up to 30 cm. We calculated the food density (g AFDM m-2) of the potential food of Bar-tailed Godwits and Great Knots separately based on their diets. Least Squares Linear regressions were used to test the correlation between peak numbers of birds and year, and between peak numbers of birds and food density. Data were logarithmically transformed before analysis. The significance level was set at 0.05. All statistical analyses were carried out in SPSS 20.0.
Results
In total 8,835 macrozoobenthic individuals (from 11 classes, 65 families, and 94 genera or species) were collected at Yalu Jiang in 2011–2016. More than 98% of the individuals came from six classes (Anthozoa, Polychaeta, Malacostraca, Bivalvia, Gastropoda, and Inarticulata). The most common taxa were Potamocorbula laevis (45.6% of the total individuals), Capitellidae (Polychaeta, 17.7%), Nephtys ciliate (Polychaeta, 5.7%), Bullacta exarata (Gastropoda, 3.6%), Glycera chirori (Polychaeta, 2.6%), Mactra veneriformis (Bivalvia, 2.6%), Stenothyra glabar (Gastropoda, 2.1%), and Moerella iridescens (Bivalvia, 1.5%). The macrozoobenthos taxa found were similar among years, while the inter-annual variation in biomass was significant (F = 5.23, P < 0.01, n = 264).
The macrozoobenthos biomass (mean per month from March to May, mean ± SE) declined by 83% from 2011 and 2012 (means of 20.55 ± 6.19 g AFDM m-2 from the entire core and 16.34 ± 4.54 g AFDM m-2 from the upper 5 cm) to 2013–2016 (means of 3.35 ± 0.91 g AFDM m-2 from the entire core and 2.74 ± 0.66 g AFDM m-2 from the upper 5 cm) (Figures 2 and 3). This was a consequence of a huge decline in the abundance and biomass of P. laevis. Numbers declined by 99.7% from 2011 (708.06 ± 175.66 ind m-2) to 2016 (2.21 ± 2.21 ind m-2) (Figure 4) and biomass declined by 99.9% from 2011 (14.00 ± 4.76 g AFDM m-2) to 2016 (0.01 ± 0.01 g AFDM m-2) (Figure 2). A dramatic decrease of P. laevis was the main cause to the decline of the macrozoobenthos: P. laevis accounted for 94% of the total macrozoobenthos biomass (across months) in 2011, but only 0.3% in 2016 (Figure 4). The biomass of other groups did not exhibit a significant decline (P > 0.1 for all).
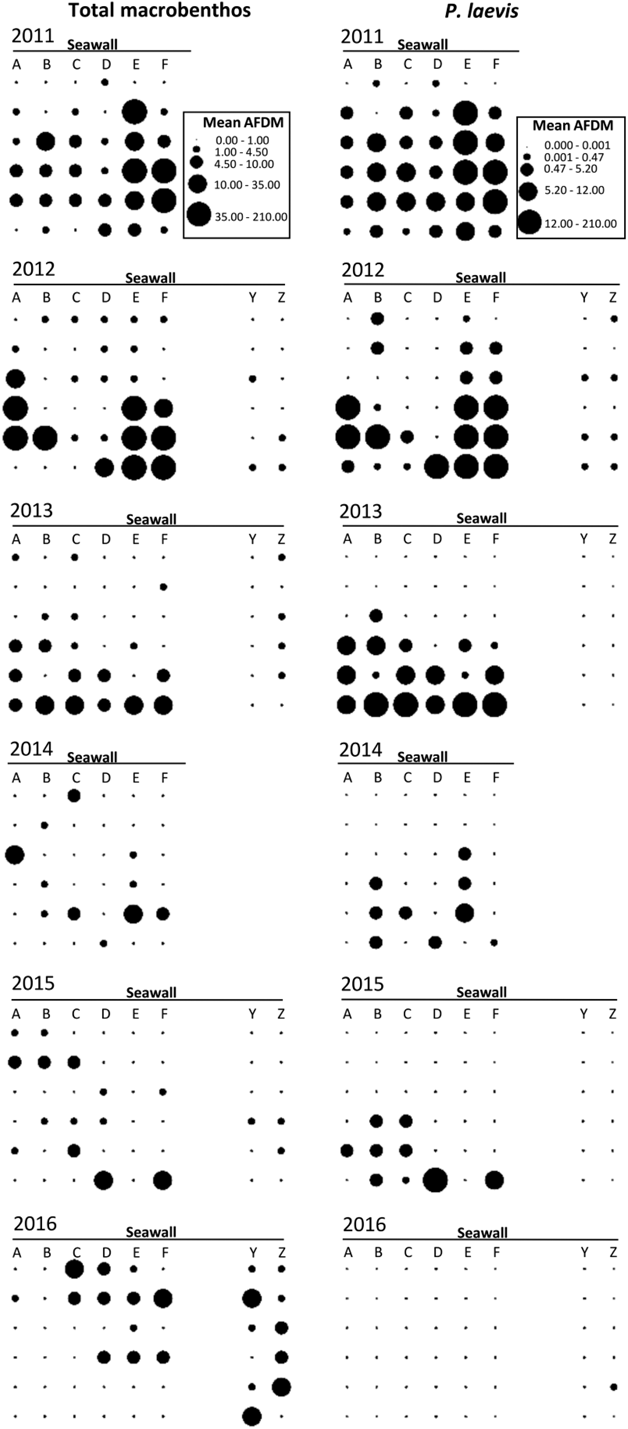
Figure 2. Distribution of benthos biomass across the intertidal flats from 2011–2016. Circles show the mean biomass (g AFDM m-2) for March–May for the total macrozoobenthos (left-hand plots) and P. laevis (right-hand plots). Data represent the benthos contained in the upper 5 cm sampled from 36 sampling stations in 2011 and 2014 and 48 sampling stations in 2012, 2013, 2015, and 2016.
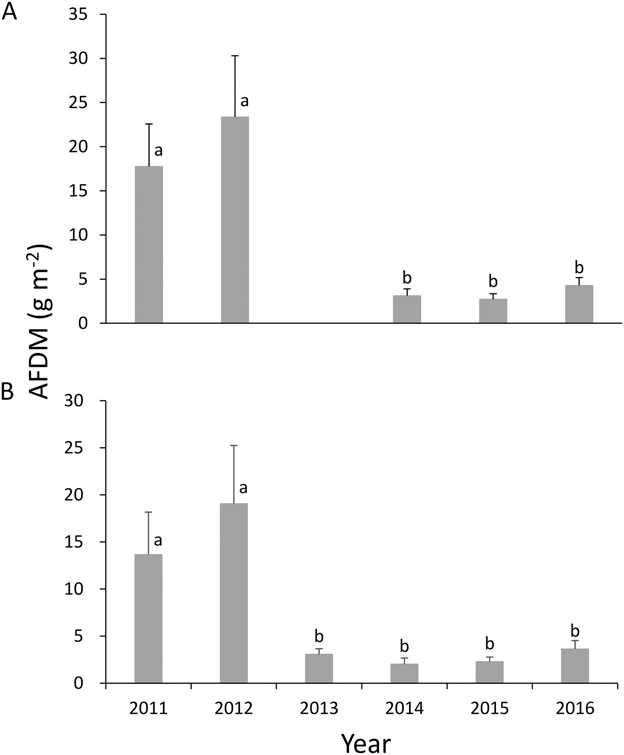
Figure 3. Annual variation from 2011–2016 in the mean macrozoobenthos biomass (total AFDM from all taxa, averaged from March–May). (A) shows total biomass in the whole 30 cm cores (except 2013); (B) shows the biomass in the upper 5 cm. Different lower-case letters represent significant differences between years (P < 0.05).
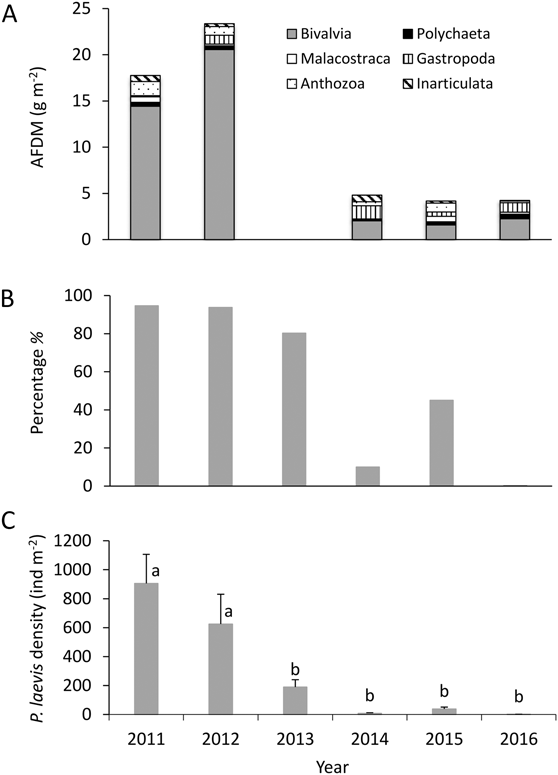
Figure 4. Macrozoobenthos by taxonomic group by year from 2011–2016. (A) AFDM of Bivalvia, Polychaeta, Malacostraca, Gastropoda, Anthozoa and Lingulata. (B) Percentage of the bivalve P. laevis AFDM in the total Bivalvia AFDM. (C) Annual variation in the mean density (March–May) of P. laevis. Different lower-case letters represent significant differences between years (P < 0.05). N equals 36 sampling stations in 2011 and 2014 and 48 in 2012, 2013, 2015 and 2016.
Numbers of Bar-tailed Godwits decreased through the study period. Peak numbers declined from more than 60,000 individuals in 2011–2013 to fewer than 30,000 in 2016 (Figure 5); the daily cumulative number also declined significantly (Table 1). This decrease was mainly due to a decline in L. l. menzbieri, which declined by 91% from 19,147 in 2011 to 1,861 in 2016 (Table 1, Figure 6). In contrast, L. l. baueri numbers declined less and there was no significant trend over time (Table 1, Figure 6). Great Knot numbers fluctuated between 74,900 in 2013 and 29,938 in 2016 but there was no significant trend over time (Table 1, Figure 5).
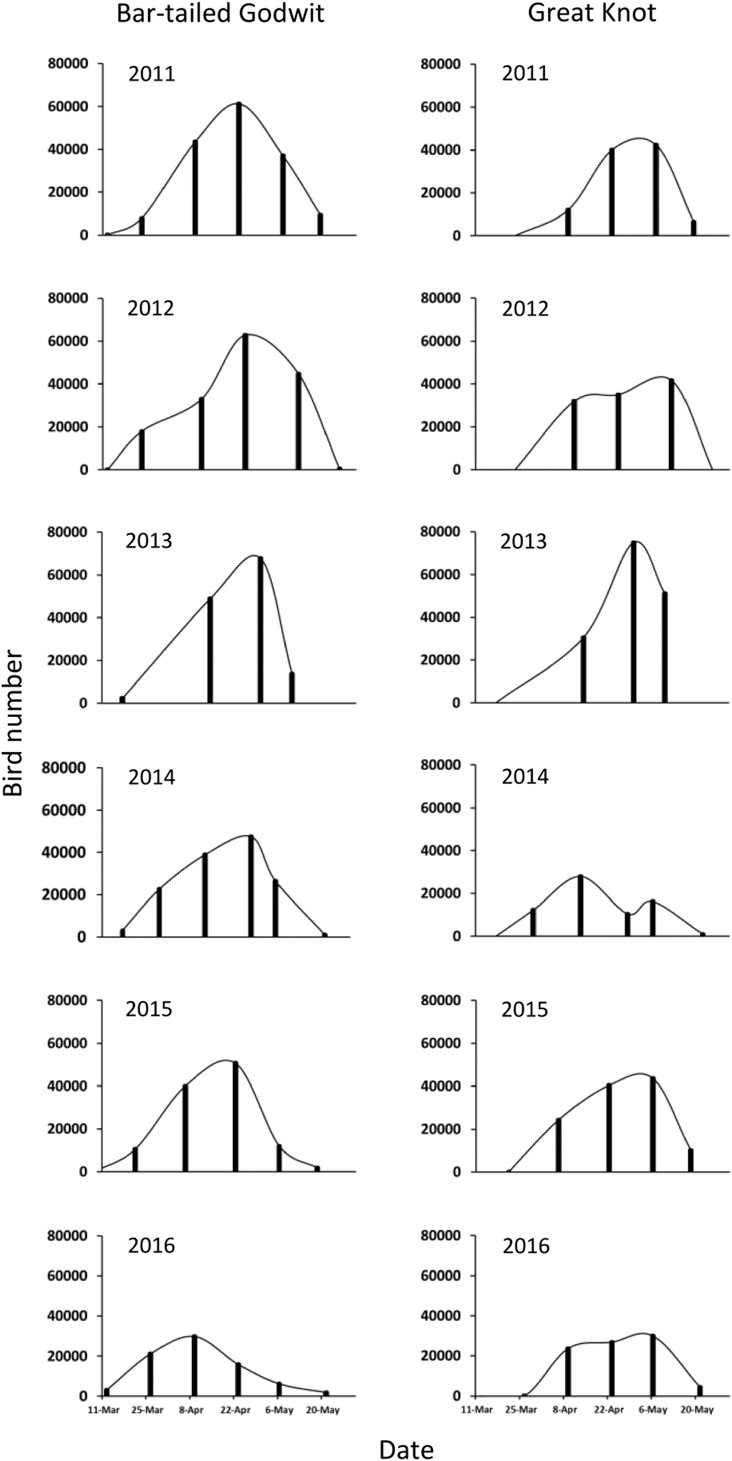
Figure 5. The numbers of Great Knots (A) and Bar-tailed Godwits (B) counted in surveys from March–May 2011–2016 at Yalu Jiang coastal wetland. Each survey is represented by the vertical bars, which are connected by a smooth curve.
Table 1. Correlations between shorebird numbers and year, and shorebird numbers and food density.

* P < 0.05, **P < 0.01.
BAGO represents Bar-tailed Godwits and GRKN Great Knots.
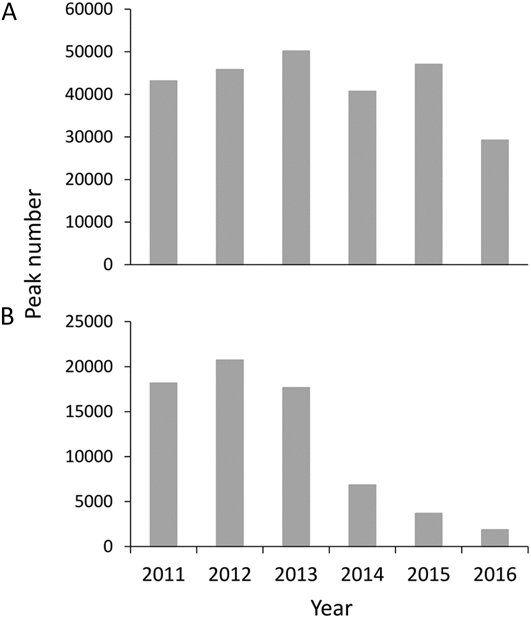
Figure 6. Peak numbers estimated of L. l. baueri (A) and L. l. menzbieri (B) from 2011–2016.
Along with the food decline, the corresponding bird density per gram of food increased by one order of magnitude, both for Bar-tailed Godwits and for Great Knots (Figure 7). However, there was no significant relationship between food density and the daily cumulative number of Bar-tailed Godwits or Great Knots (P > 0.05 for both) (Table 1, Figure 7).
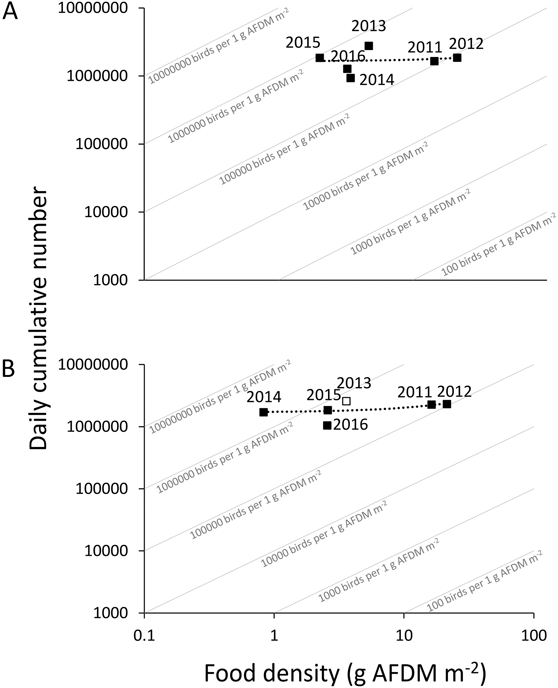
Figure 7. Daily cumulative number of Great Knots (A) and Bar-tailed Godwits (B) in relation to food density (g AFDM m-2) from 2011–2016. Empty squares indicate that the ghost shrimp, Polychaeta and Anthozoa in the potential food of Bar-tailed Godwits in 2013 were estimated based on the average of the other five years. Angled lines represent different proportions of bird numbers and food density.
Discussion
With respect to the macrozoobenthic food resources for shorebirds, this study demonstrates that (1) the biomass of P. laevis decreased dramatically at Yalu Jiang during the study period, and (2) other macrozoobenthos exhibited no significant decline during this period. What has caused the substantial declines of P. laevis?
Firstly, the changes of hydrological conditions and sediment characteristics have probably changed due to the construction of Dandong Port in the western part of the Yalu Jiang estuary (eastern part of the study region). This may have affected the distribution and growth of P. laevis. Along with port construction, a seawall extending 10 km out to sea was established (Melville et al. Reference Melville, Chen and Ma2016a). Such a large-scale structure will have likely influenced tidal flow, freshwater supplementation and sediment deposition at Yalu Jiang (Wang et al. Reference Wang, Ren and Zhu1986, Wang and Aubrey Reference Wang and Aubrey1987), especially in view of the fact that the discharge of the Yalu River previously flowed westwards (Zhang and Li Reference Zhang and Li2016). Previously, land claims in South Korea (Saemangeum), the southern North Sea in Germany, and the eastern side of Liaodong Peninsula in China have reshaped the tidal flows, freshwater supplementation and sediment deposition in those areas (Han Reference Han, Healy, Wang and Healy2002, Lee and Ryu Reference Lee and Ryu2008). The distribution of P. laevis is strongly affected by salinity (Wei Reference Wei1984) and the size of sediment particles (Choi et al. Reference Choi, Battley, Potter, Ma and Liu2014) on tidal flats. Many studies have indicated that seawall construction can result in a change in the distribution and abundance of macrozoobenthos on the remaining tidal flats (e.g. Hong et al. Reference Hong, Yamashita and Sato2007, Ryu et al. Reference Ryu, Nam, Park, Kwon, Lee, Song, Hong, Chang and Khim2014).
Secondly, run-off/discharge of agrochemicals from extensive shoreline sea cucumber farms may have led to the decrease of P. laevis. Limited agrochemicals were used in the aquaculture ponds before 2011 as razor clams Sinonovacula constricta were the main aquaculture species. The culture of sea cucumber became popular in 2011, and exhibited explosive growth in 2012 and 2013, with most aquaculture ponds then being developed for sea cucumber culture. The sea cucumber ponds were sterilized with a mass of quicklime (calcium oxide; 1,000–1,200 kg per ha). Moreover, amounts of sodium hypochlorite and medical hydrogen peroxide were used for the hatching of sea cucumber seedlings. Antibiotics such as ceftriaxone sodium and penicillin were used to prevent disease and improve the survival rate of the sea cucumber. Other agrochemicals, such as Norfloxacin, six anti-Diana, Povidone-iodine solution, polysaccharides of yeast cytoderm, and pesticide (Meothrin containing esbiothrin), were also used (Wang Reference Wang2015). The waste water with residues of these chemicals from sea cucumber ponds is discharged directly onto intertidal flats, and can potentially affect the macrozoobenthos (Boxall Reference Boxall2004, Cabello et al. Reference Cabello, Godfrey, Tomova, Ivanova, Dölz, Millanao and Buschmann2013, Chen Reference Chen2015). Although the scale of sea cucumber farming declined in 2014 due to low profitability, the consequences of pollution may persist for long periods.
Thirdly, parasite induced mortality might be another cause for dramatic decline of P. laevis after 2012. We observed large amounts of P. laevis that came out to the surface of the mudflats and died afterwards in 2012 (C. Y. Choi and D. S. Melville pers. obs.). This could be due to parasitic infection, as emerging from sediments to the surface is a main effect of some parasite infections in bivalves (Edelaar et al. Reference Edelaar, Drent and de Goeij2003, Escudero et al. Reference Escudero, Navedo, Piersma, de Goeij and Edelaar2012).
P. laevis reproduces twice a year, in early May and late September, at Yalu Jiang (Liu and She Reference Liu and She2003). Choi (Reference Choi2015) indicated there were abundant spat of P. laevis in spring 2011 while the density of spat P. laevis decreased by 94% in spring 2012, suggesting a failure of reproduction in 2011. Lack of spat recruitment in 2012 would cause a decline of adult P. laevis from 2013. The possible (combined) effects of pollution, parasitic infection, and changes in salinity and sediment characteristics on the reproduction of P. laevis remain unclear. Long-term monitoring on the population dynamics of P. laevis (e.g. Yang et al. Reference Yang, Chen, Piersma, Zhang and Ding2016) as well as the relevant environmental factors is warranted.
We did not find significant decline of other macrobenthos in this study. Beside the possible effects mainly on P. laevis mentioned above, this might be caused by two other reasons. Firstly, the sampling areas were chosen at areas with high bird density, where the densities of P. laevis were also high while the densities of other macrozoobenthos were relatively low in 2011. It is hard to detect changes in other macrozoobenthos. Secondly, some other bivalves (Mactra veneriformis and Sinonovacula constricta) are cultivated species. The natural changes of those bivalves could be masked by seeding on the mudflat.
P. laevis is the main food resource for Bar-tailed Godwits and Great Knots at Yalu Jiang (Choi et al. Reference Choi, Battley, Potter, Ma, Melville and Sukkaewmanee2017). However, the food declines were not followed by corresponding changes in the daily cumulative number of Bar-tailed Godwits and Great Knots (Table 1, Figure7). Part of this mismatch could be methodological, with birds counted at high tide foraging on mudflats outside our macrozoobenthos sampling region. For example, we recorded some birds flying from North Korea to site 2 and ash pond (Figure 1), i.e. two major pre-roost sites close to China-North Korea border. However, although there was abundant food in 2012, food intake rates by Bar-tailed Godwits and Great Knots halved compared to in 2011 due to a lack of small-sized spat (optimal prey) (Choi et al. Reference Choi, Battley, Potter, Ma, Melville and Sukkaewmanee2017). Moreover, along with the P. laevis decline, Bar-tailed Godwits and Great Knots shifted to alternative prey with harder and larger shells than P. laevis (S. D. Zhang and K. Tan per. obs.). This could increase the handling time and thus decrease their intake rate.
The peak numbers of L. l. baueri did not significantly decrease with the decline of food stocks at Yalu Jiang, whereas the peak numbers of L. l. menzbieri did (Table 1). This might be explained by the different migration strategies between the two subspecies. The mean arrival date of L. l. menzbieri (11 April) was about two weeks later than L. l. baueri (29 March) at Yalu Jiang (Choi et al. Reference Choi, Battley, Potter, Rogers and Ma2015). Because the two subspecies share the same food niche, the later arrivals might suffer more from the food decline (Peng et al. Reference Peng, Choi, Jia, Melville, Tan, He, Chen and Ma2014). Another explanation could be that L. l. menzbieri use the Yellow Sea on both northward and southward migration have better knowledge on habitat conditions in the wider Yellow Sea than L. l. baueri, which spend only one season in the Yellow Sea (Battley et al. Reference Battley, Warnock, Tibbitts, Gill, Piersma, Hassell, Douglas, Mulcahy, Gartrell and Schuckard2012). L. l. menzbieri may therefore be able to make better decisions in selecting high quality habitats. Indeed, some birds moved between the coasts north or south of the Yangtze River and the Yalu Jiang/North Korea region on both migrations (Battley et al. Reference Battley, Warnock, Tibbitts, Gill, Piersma, Hassell, Douglas, Mulcahy, Gartrell and Schuckard2012). Also, the majority of the L. l. menzbieri population uses staging sites other than Yalu Jiang in the Yellow Sea (Battley et al. Reference Battley, Warnock, Tibbitts, Gill, Piersma, Hassell, Douglas, Mulcahy, Gartrell and Schuckard2012). Therefore, individuals that previously used Yalu Jiang might benefit from the experience of other birds and relocate to those sites with flock mates on arrival in the Yellow Sea.
Birds could move to other feeding sites when facing food shortage. Radio-tracking the migrating Great Knots indicated that some individuals arrived at Yalu Jiang but soon moved to Gaizhou (150 km to the westward) in spring 2015, rather than staying at Yalu Jiang for the whole migration season, as they had done in 2012 when food was relatively abundant (W. J. Ke et al. in prep.). However, although 60,000 Great Knots were recorded in Gaizhou in 2015, fewer than 2,000 were recorded there in 2016 (Melville et al. Reference Melville, Peng, Chan, Bai, He, Tan, Chen, Zhang and Ma2016b). Meanwhile, our results indicated that with the exception of the population decrease of L. l. menzbieri, the numbers of L. l. baueri and Great Knots did not decline dramatically despite the severe food decline. In view of the rapid loss of mudflat area in the Yellow Sea (Murray et al. Reference Murray, Clemens, Phinn, Possingham and Fuller2014, Melville et al. Reference Melville, Chen and Ma2016a, Piersma et al. Reference Piersma, Lok, Chen, Hassell, Yang, Boyle, Slaymaker, Chan, Melville, Zhang and Ma2016), it is likely that a lack of alternative habitats where they can achieve equivalent energy intake makes them stay at Yalu Jiang. Further studies are required to clarify the factors affecting staging site use of migrating shorebirds.
The vast coastal wetlands of the Yellow Sea region provide important staging sites for millions of shorebirds along the EAAF (Barter Reference Barter2002, Rogers et al. Reference Rogers, Yang, Hassell, Boyle, Rogers, Chen, Zhang and Piersma2010). However, coastal wetlands in the Yellow Sea region face diverse threats including land claim, pollution, invasive species, and over-exploitation (Hua et al. Reference Hua, Tan, Chen and Ma2015, Melville et al. Reference Melville, Chen and Ma2016a). The dramatic decline of food for shorebirds at Yalu Jiang indicates that food declines, even in protected mudflats, could be an overlooked but important factor contributing to population declines of shorebirds along the flyway. Although protecting a certain area of coastal wetland from land claim is important, maintaining a healthy ecosystem with high quality and abundant food for migratory birds should also be the goal of conservation at staging sites. The relationships and dynamics of both shorebirds and their food resources in intertidal flats call for widespread and long-term monitoring work to target effective conservation strategies along the flyway.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270917000430
Acknowledgements
We thank the Dandong Yalu Jiang Estuary Wetland National Nature Reserve for facilitating our fieldwork. We are indebted to Xiaojing Gan, Peter Brakels, Peter Crighton, Rosanne Michielsen, Jason Loghry, Julia Melville, Dezhong Xing, Yongjun Sui, and Haoying Huang for field assistance. We also thank Jingyao You, Shaoping Zang, and Youjia Li for helping us process macrozoobenthos samples. We thank Phil F. Battley and an anonymous reviewer for comments on the manuscript. This study was financially supported by the National Natural Science Foundation (31572280 and 31071939) and the National Basic Research Program of China (2013CB430404) and the CSC scholarship (201706100078) and the KNAW China Exchange Programme grant (530-5CDP16) awarded to TP.










