Management Implications
Conifers, notably Pinus contorta (lodgepole pine), are among the most widespread and invasive plants in the world, including New Zealand, where herbicides remain an important tool for management. Broadcast aerial application of an herbicide mix containing triclopyr (applied at 18 kg ha−1), dicamba (applied at 5 kg ha−1), picloram (applied at 2 kg ha−1), and aminopyralid (applied at 0.28 kg ha−1) is used operationally in New Zealand in a national program (National Wilding Conifer Management Programme) to kill dense infestations (>80% canopy cover) of mature invasive conifers. While the treatment is effective, given the large amounts of active ingredient used (∼20 kg ha−1), practitioners have concerns about the persistence of the herbicides in the environment and the potential impact on future restoration efforts. The objective of this study was to determine the persistence of triclopyr, dicamba, and picloram in cast needles, forest floor, mineral soil, and stream water following aerial spraying of P. contorta with the operationally used herbicide mix at three geographically distinct locations in New Zealand. A lack of laboratory capacity for testing aminopyralid in New Zealand precluded its inclusion in this study.
Key results of this study were:
-
All three herbicides (triclopyr, picloram, and dicamba) were still present in the forest floor layer 2 yr after spraying; that is, herbicides were retained in a heavy lignin-rich layer of dead/cast needles overlaying the soil.
-
Only triclopyr was detected in the soil for the first year after spraying.
-
Where a no-spray buffer zone (30 m) was used on the edge of streams intersecting the aerially sprayed area, herbicides in water did not exceed environmental exposure limits when rainfall occurred shortly after spraying.
The persistence of the active ingredients in the forest floor litter layer is unlikely to pose a risk to terrestrial organisms but could persist at levels that affect revegetation efforts that commence within 18 mo after aerial spraying.
Introduction
Herbicides are an important tool to control and eradicate nonnative, invasive plants to protect biodiversity and a range of ecosystem services (Clout and Williams Reference Clout and Williams2009; Radosevich et al. Reference Radosevich, Holt and Ghersa2007; Wagner et al. Reference Wagner, Antunes, Irvine and Nelson2017). Herbicides continue to be used, because they provide a cost-effective approach for removing invasive plants over large areas, particularly in terrain where manual or mechanical removal is not practical. However, their use may also result in unintended non-target impacts to native species, water quality, and restoration efforts, especially where soil-active and persistent herbicides are used (Ranft et al. Reference Ranft, Seefeldt, Zhang and Barnes2010; Rinella et al. Reference Rinella, Maxwell, Fay, Weaver and Sheley2009; Wagner and Nelson Reference Wagner and Nelson2014). From a practical standpoint, the persistence of herbicides in the soil environment determines the length of time invasive plant seedlings can be controlled but also influences how long a land manager must wait before a treated site is ready for restoration, planting, or other management actions (Douglass et al. Reference Douglass, Nissen, Meiman and Kniss2016; McManamen et al. Reference McManamen, Nelson and Wagner2018; Ranft et al. Reference Ranft, Seefeldt, Zhang and Barnes2010). The purpose of this study was to measure the persistance of aerially applied triclopyr, dicamba, and picloram in the forest soil and water environment following their aerial application to control dense infestations of invasive conifers in New Zealand. The study also determined the potential for non-target impacts to the broader environment as well estimated the period herbicide residues could impact restoration efforts.
In New Zealand, the unwanted spread of introduced conifers, notably those from the Pinaceae family [lodgepole pine (Pinus contorta Douglas ex Loudon), Corsican pine (Pinus nigra Arnold), mountain pine (Pinus mugo Turra), and Douglas fir (Pseudotsuga menziesii (Mirb.) Franco)], is a major threat to biological conservation (Dickie et al. Reference Dickie, Sprague, Green, Peltzer, ORwin and Sapsford2022; Ledgard Reference Ledgard2001) and carries enormous social and economic costs (Greenaway et al. Reference Greenaway, Bayne, Velarde, Heaphy, Kravchenko and Paul2015; Wyatt Reference Wyatt2018). Unwanted spread of conifers planted outside their native ranges for a range of purposes is not unique to New Zealand, with South Africa, Sweden, Chile, and Brazil facing similar challenges with respect to management and containment of these invasive tree weeds (Cazetta and Zenni Reference Cazetta and Zenni2020; Essl et al. Reference Essl, Moser, Dullinger, Mang and Hulme2010; McGregor et al. Reference McGregor, Watt, Hulme and Duncan2012; Richardson Reference Richardson1998; Rouget et al. Reference Rouget, Richardson, Milton and Polakow2004). In New Zealand, application of herbicides continues to be the primary means of intensively managing nonnative conifers (Dickie et al. Reference Dickie, Sprague, Green, Peltzer, ORwin and Sapsford2022; Litmus 2020; NWCCP 2019a, 2019b) and, as part of a national program to prevent unwanted spread, has been carried out over large areas (Litmus 2020; NZMPI 2014).
Broadcast aerial spraying of herbicides is currently the main method used in New Zealand to kill dense conifer infestations, defined as areas where canopy cover exceeds 80% ground cover, often with a stem density exceeding 5,000 stems ha−1 (Gous et al. Reference Gous, Raal and Watt2015; NWCCP 2019b). Herbicides are applied using helicopters fit with a boom and nozzles that produce coarse droplets (volume mean diameter of about 350 µm), selected to minimize the risk of offsite spray drift (NWCCP, 2019b; Richardson et al. Reference Richardson, Rolando, Hewitt and Kimberley2020). Given the coarse droplet spectrum, the high number of target trees per hectare and their size, which often exceeds 10 m in height, large doses of herbicides are required (∼20 kg ha−1) to kill the trees. While known to be effective (Gous et al. Reference Gous, Raal and Watt2015), the doses are high in comparison to other agricultural and forestry applications, constitute “off-label” application, and also encompass complex blends (more than three) of active ingredients (Gous et al. Reference Gous, Raal and Watt2015; Rolando et al. Reference Rolando, Gaskin, Horgan and Richardson2020).The mix used, known locally as TDPA for triclopyr, dicamba, picloram, and aminopyralid, includes 18 kg ai triclopyr (3,5,6-trichloro-2-pyridyloxyacetic acid), 5 kg ai dicamba (3,6-dichloro-2-methoxybenzoic acid), 2 kg ai picloram (4-amino-3,5,6-trichloropicolinic acid), 0.28 kg ai aminopyralid (4-amino-3,6-dichloropyridine-2-carboxylic acid), 20 L of a methylated seed oil, 0.5 L of a lecithin blend, and 2.3 kg of ammonium sulfate, all applied aerially in 400- to 600-L total volume (water) ha−1 (Gous et al. Reference Gous, Raal and Watt2015; Richardson et al. Reference Richardson, Rolando, Hewitt and Kimberley2020). While it is accepted that large amounts of herbicide are required to kill mature, invasive trees, the magnitude of the area potentially subject to broadcast aerial control (up to 50,000 ha; Wyatt Reference Wyatt2018) and large doses of herbicide used have raised concerns from stakeholders and end-users pertaining to potential longer-term impacts to the environment and restoration efforts following treatment. Understanding the fate and persistence of herbicides in the environment is critical for assessing the contamination risks to surface and groundwater (Andreu and Picó Reference Andreu and Picó2004) and impacts to biodiversity (Guynn et al. Reference Guynn, Guynn, Wigley and Miller2004) and other ecosystem services (Sing et al. Reference Sing, Metzger, Paterson and Ray2017).
The synthetic auxin herbicides triclopyr, dicamba, picloram, and aminopyralid have selective activity and are systemic, being rapidly absorbed by both foliage and roots (McBean Reference McBean2012). Triclopyr is commonly used for perennial broad-leaved and woody weed control in uncultivated areas and for conifer release (Bovey et al. Reference Bovey, Hein and Meyer1983; Rolando et al. Reference Rolando, Richardson, Paul and Somchit2021; Thompson et al. Reference Thompson, Pitt, Buscarini, Staznik and Thomas2000; Weatherford et al. Reference Weatherford, Tatum and Wigley2015); dicamba is a selective, systemic herbicide used for general weed control, often in agricultural systems (McBean Reference McBean2012); picloram is a persistent herbicide that controls broad-leaved weeds; and aminopyralid is used in the long-term control of noxious and invasive broad-leaved weeds (McBean Reference McBean2012). Based on the water solubility and soil adsorption coefficient (Koc) values (Table 1), all four active ingredients are considered to be mobile in soils, with potential to leach into groundwater. The estimated half-lives in soil (DT50) for the active ingredients range from very low (dicamba at <14 d) to moderately persistent for triclopyr, picloram, and aminopyralid, depending on site characteristics, with a wide variation reported in the literature (Table 1). The behavior of herbicides in soil and water varies, depending on chemical structure and mechanism of action (Tu et al. Reference Tu, Hurd and Randall2001) and is regulated by soil properties (soil type and soil organic matter), meteorological factors (temperature and rainfall), and microbial activity (Van Acker Reference Van Acker2005). Weak acid herbicides (such as picloram, dicamba, and triclopyr) are often less sorbant than non-ionic and weak base herbicides (such as metolachlor) on the same soils, with sorption also regulated by microbial activity and the quantity and quality of organic litter (Garrett et al. Reference Garrett, Watt, Rolando and Pearce2015; Tu et al. Reference Tu, Hurd and Randall2001). None of the herbicides investigated here are considered particularly toxic to terrestrial and aquatic vertebrates and invertebrates when applied at label recommendations (Table 2).
Table 1. Toxicology and ecotoxicological parameters for key herbicides used in the operationally used TDPA (triclopyr, dicamba, picloram, and aminopyralid) mix (McBean Reference McBean2012; Shaner Reference Shaner2014).

a Soil organic carbon (OC) affinity coefficient (Koc) represents the soil distribution coefficient (Kd) normalized for soil OC content.
b Solubility in water.
c Acute LC50 (hours) is the concentration that kills half (50%) of the animals tested. LC, lethal concentration.
d EC50 (effective concentration) refers to the concentration of a toxicant required to induce 50% effect.
e LD50 is the dose that kills (50%) of the animals tested. LD, lethal dose.
Table 2. Exposure limits for active ingredients aminopyralid, dicamba, picloram, and triclopyr. a

a MAV, Mean Acceptable Value for drinking water; EEL, Environmental Exposure Limit (the limit on the concentration of a substance (or any element or compound making up the substance) with ecotoxic properties in an environmental medium as set in accordance with Environmental Protection Authority notices (Water Services Regulations 2022; NZ Environmental Protection Authority 2022).
Over the past 40 yr, much has been published on the degradation, fate, and persistence of triclopyr (Bentson and Norris Reference Bentson and Norris1991; Neary et al. Reference Neary, Bush and Michael1993; Newton et al. Reference Newton, Roberts, Allen, Kelpsas, White and Boyd1990; Petty et al. Reference Petty, Getsinger and Woodburn2003; Stephenson et al. Reference Stephenson, Solomon, Bowhey and Liber1990; Thompson et al. Reference Thompson, Pitt, Buscarini, Staznik, Thomas and Kettela1994, Reference Thompson, Kreutzweiser, Capell, Thomas, Staznik and Viinikka1995), dicamba (Caux et al. Reference Caux, Kent, Taché, Grande, Fan, MacDonald and Ware1993; Helbert Reference Helbert1990; Soltani et al. Reference Soltani, Oliveira, Alves, Werle, Norsworthy and Sprague2020; Tu et al. Reference Tu, Hurd and Randall2001), and picloram (Altom Reference Altom1973; Helbert Reference Helbert1990; Newton et al. Reference Newton, Roberts, Allen, Kelpsas, White and Boyd1990; Tu et al. Reference Tu, Hurd and Randall2001) in foliage, mineral soils, and water of forested/agricultural environments, with substantially less published for aminopyralid (Gurvich Reference Gurvich2020; Tomco et al. Reference Tomco, Duddleston, Schultz, Hagedorn, Stevenson and Seefeldt2016). It is not the intent of this study to review this body of literature, except where results prove insightful to the patterns observed in the current study. Few of the published studies had environmental conditions and treatments comparable to that typical for invasive conifer management in New Zealand, resulting in questions as to the levels at which herbicides might persist when used as prescribed for operational management (NWCCP 2019b). A preliminary study by Howell (Reference Howell2014) on persistence of herbicides used in broadcast aerial control of dense infestations of conifers in New Zealand indicated levels of herbicide in soil were below 0.1 ppm after 12 mo and unlikely to impact native species regeneration (although not measured). Howell (Reference Howell2014) attributed low levels of soil residues to canopy interception of the majority of spray; however, herbicide levels in cast needles and forest floor litter were not determined. Wilcock et al. (Reference Wilcock, Costley, Cowles, Wilson and Southgate1991) studied the persistence of triclopyr in soil following aerial spraying of gorse (Ulex europaeus L.) and pasture at 3.6 kg ha−1 in New Zealand. These authors determined a half-life of triclopyr of approximately 100 d in soil when residues of the acid were less than 0.6 ppm. Not only was the rate of triclopyr used by Wilcock et al. (Reference Wilcock, Costley, Cowles, Wilson and Southgate1991) substantially lower than that used for dense conifer control in New Zealand, but the vegetation structure (a shrub and grass) was also different, meaning their results were not directly comparable to the situation pertinent to management of dense infestation of conifers.
The aim of this research was to determine the persistence of triclopyr, dicamba, and picloram in foliage, forest floor litter, mineral soil, and water following aerial spraying of dense infestations of P. contorta with the TDPA mixture. A lack of laboratory capacity for testing aminopyralid in New Zealand precluded its inclusion in this study. This study will determine post-spray herbicide levels in the environment following the application of the TDPA mixture to evaluate whether environmental limits set for soil and freshwater ecosystems are likely to be exceeded.
Materials and Methods
Study Areas and Plots
Three sites—Kaweka Forest (KF), Glen Eyrie Downs (GE) and Mid Dome (MD)—representing three geographic, physiographic and climatic regions across New Zealand were selected for herbicide persistence experiments (Figure 1; Table 3). The KF site (780 m above sea level [m asl]) is characterized by steep, erodible hillslopes of well-draining shallow Tephric soils (Typic Orthic Allophanic Soils) over sandstone, and annual rainfall is generally more than 1,200 mm. The GE site (620 m asl) is part of a large glaciated intermontane drylands basin with annual rainfall less than 700 mm. Soils are a mantle of silty loess over densely packed well-draining glacial till (Allophanic Brown). The MD site (1,113 m asl), situated near the natural treeline, is steep, exposed mountain landscape. Soils are shallow silty loam loess (Allophanic Brown) over angular sandstone colluvium. Annual rainfall is near 1,000 mm, much of which is precipitated as fog or snow (Mager et al. Reference Mager, Trevelyan, Wilson and Kingston2016). The three sites each exhibit generally wide seasonal temperature fluctuations with warm summers (36 C maximum recorded at MD) and cold winters (−21 C minimum recorded near GE). The soil physical and chemical characteristics of the three sites differed (Table 3). Total carbon (%) in soil was lower at the KF and GE sites compared with the MD site, whereas the KF site had the lowest total nitrogen (%) and bulk density (Table 3). All three sites were dominated by P. contorta infestations forming dense stands. Each site had an estimated P. contorta stem density exceeding 5,000 stems ha−1 of P. contorta and a canopy cover exceeding 70% (Table 4). At ∼31 yr, the KF site had the oldest and tallest stands with the lowest stem density, while the stand at GE was the youngest of the three (∼17 yr) with the highest stem density and the lowest canopy coverage. With an average maximum crown height of 6.4 m, the stand at MD was the shortest.

Figure 1. Locations of three study sites in New Zealand selected to determine herbicide persistence following aerial application of herbicides for control of dense infestations of Pinus contorta. KF, Kaweka Forest; GE, Glen Eyrie Downs; MD, Mid Dome.
Table 3. Location, physiography, and soil (chemical and physical) characteristics for study sites. a

a MAP, mean annual precipitation; MAT, mean annual temperature; LFH, forest floor comprising principally litter (L), a fermented (F) layer, and a well-developed humified (H) layer.
b Site temperature and rainfall data were retrieved from the National Institute of Water and Atmospheric Research (NIWA/Taihoro Nukurangi), Virtual Climate Station Network (VCSN). The nearest VCSN station was used for each study site. The geographic location (WGS84) for each is: MD: 45.575°S,168.525°N; GE: 44.325°S, 169.875°N; KF: 39.475°S, 176.275°N.
c Soil types from S-Map Online, https://smap.landcareresearch.co.nz, accessed: December 2022.
Table 4. Stand characters at each site (mean ± SE).

a Maximum age recorded using tree cores to estimate age.
n = 25 (5 trees in each of 5 plots).
b n = 5 (plots).
Herbicide Application
Operational aerial boom-spraying at the three sites was carried out between January 2018 and February 2019. At each location, operational spraying was undertaken in summer according to best practice guidelines for dense infestations of invasive conifers (NWCCP 2019b). The herbicide formulation contained the recommended herbicide rates and mixtures for operational conifer spray applications (Table 5) applied in total spray volumes of 400 L ha−1 for trees less than 10-m tall and 600 L ha−1 for trees more than 10-m tall. Spray was applied 3 to 4 m above the tree canopy using the half-overlap, opposite-pass technique. At KF, a minimum 30-m no-spray margin was retained along the stream boundary, except for the southeast corner where spraying came to within 30 m of the stream edge (Supplementary Figure S1a). At GE, a no-spray margin was retained along either side of the stream ranging from 15 to 45 m in width (Supplementary Figure S1b). At MD, at least half the stream length within the spray area was over-sprayed with the herbicide application. No-spray margins were retained along the remaining stream length at MD, varying in width from ca. 10 to 150 m (Supplementary Figure S1c).
Table 5. Products and active ingredients used in the operational TDPA (triclopyr, dicamba, picloram, and aminopyralid) mix used to spray infestations of invasive conifers at three study sites.

a New Plymouth 4343, New Zealand; https://www.corteva.co.nz/contact-us.html.
b Auckland 0892, New Zealand; https://adria.nz.
c Auckland 1051, New Zealand; https://www.upl-ltd.com/nz/contact.
Sampling
Soil, Forest Floor Litter, and Cast Needles
At each site, five 10 by 10 m vegetation plots were established across the sprayed area such that they were more than 20 m from the nearest spray boundary. Within each plot, samples were taken from the needle fall (dead cast needles), forest floor litter, and soil before and up to 24 mo after spraying and analyzed for the presence of triclopyr, picloram, and dicamba (Table 6). The forest floor litter material included a thick distinct litter layer (L), comprising principally needles, a fermented (F) layer, and well-developed humified (H) layer. The combined LFH (litter, fermented humic) layer was sampled separately from mineral soil.
Table 6. Sampling regime expressed as the number of days before and after spray date for the three sites.

a Pre-spray sampling included soils and litter, fermented humic layer (LFH).
b Spraying was delayed for 1 yr at the KF site due to bad weather, resulting in an extended period between pre-spray sampling and the time spray was applied. Because no operations occurred at the site during this period, no further sampling was conducted until after the application of herbicide.
Soil sampling was conducted using a Hoffer sampler (25-mm diameter). Ten 0- to 10-cm soil samples were collected randomly from across each plot at each site, then bulked into one sample per plot for submission to the laboratory. The sampling plan was to collect three 10- to 50-cm soil samples from each plot at each site; however, the shallow rocky nature of the steep montane soils precluded consistent sampling deeper than 20 cm at the KF site and 10 cm at MD. Therefore, three bulked soil samples were taken from 10- to 20-cm depth across each plot at KF and 10- to 50-cm depth at GE, with no deep core samples taken at MD. To characterize the soil structure, bulk density samples were collected from each plot (n = 5) at each site using 10-cm-diameter stainless steel rings.
Forest floor litter, or LFH, was collected at three points across the plot using a 0.1-m2 sampling square and bulked per plot. Fresh needle fall (cast needles) was collected using funnel traps (60-cm diameter) installed on the perimeter of each plot immediately after spray treatment, except at the MD site, where the remote location and weather precluded installment until the first sampling event 1 mo after spraying (i.e., first collection was at 201 d; Table 6). All samples were kept chilled (5 C) before processing in the laboratory.
Stream Sampling
Stream sampling was undertaken at the KF and MD sites. At KF, stream water was monitored for herbicide residues downstream from the treated area (Supplementary Figure S1a). Water samples were taken before spraying, on the day of spraying, and 1 d after spraying. Further water samples were taken 26 d later following the first rainfall event after herbicide application (a total of 37.5 mm for days 24 to 26) and 29 d later (1 mo after herbicide application), with a final sample taken 119 d after spray application. Except for the day of spraying, 1-L water samples were collected for herbicide analysis on each sample date. On the day of spraying, four 1-L samples were collected at 15-min intervals in the first hour following spray application. Thereafter, composite water samples (four 0.25-L samples taken at 15-min intervals and combined to make a 1-L composite sample) were collected for a further 3 h. Flow measurements were taken on each sample date using a using a Hach FH950 portable velocity meter (Hach Lange NZ, Auckland, NZ). The lack of fine sediment in the streambed precluded sediment sampling at KF.
At MD, water samples were collected downstream from the sprayed area at Tank Creek (refer to water sampling position on Supplementary Figure S1c). One-liter water samples were collected 1 day before spraying and 1, 3, 14, 82, and 167 d post-spraying. Samples taken at 3 d after spraying were within 2 h of the site receiving approximately 10 mm of rainfall, and samples taken at 13 d after spraying were within 1 d of the site receiving a total of ∼80 mm of rainfall. All samples were kept chilled (5 C) after sampling and during transport to the testing laboratory (Hill Laboratories, Hamilton, NZ) where they were refrigerated before analysis.
Sample Processing
Soil, Forest Floor Litter, and Cast Needles
Cast needles, forest floor litter (LFH), and soil samples were returned to Scion, Rotorua, for processing. Soil samples were transferred fresh into labeled glass jars, chilled, and couriered to the Hills Laboratories, New Zealand, for acid herbicide testing. Forest litter and cast needle samples were sieved to remove any large woody debris, stones, and gravel. The total field moisture content of the forest litter (three bulked 0.1 m2 per plot) and cast needles was determined by drying the samples in a forced-air oven at 30 C and then reweighing until no further weight loss occurred. Samples were then mixed thoroughly to form a uniform and representative sample. An ∼100-g subsample from each sample was then finely ground using a blender, transferred to a labeled jar, and sent for acid herbicide testing (Hills Laboratories, Hamilton, NZ).
Soil, forest litter (LFH), and cast needle samples were analyzed for the presence of triclopyr, dicamba, and picloram using solvent extraction with sonication, dilution, analysis by liquid chromatography coupled with tandem mass spectrometry. Detection limits were 0.2 mg kg−1 dry weight for the three active ingredients. The analytical laboratory used in the study did not screen for the presence of aminopyralid, so no testing for the presence of this herbicide could be undertaken.
Water samples collected from MD and KF were submitted to Hills Laboratories and analyzed for the presence of triclopyr, picloram, and dicamba. The detection limits for triclopyr, picloram, and dicamba in water were 0.04, 0.06 and 0.04 mg m−3, respectively.
Analyses
The data were analyzed to determine the decline in herbicide residues (mg kg−1) in each layer over time and the total amount of residues within the system reflecting persistence in the environment (kg ha−1).
The decline of herbicide residues (mg kg−1) over time within individual pools (cast needles, forest floor litter, and mineral soil) was analyzed separately at each site using localized regression models (loess) with weighted least squares that used all the data in the weighted smoothing (Cleveland et al. Reference Cleveland, Gross, Shyu, Chambers and Hastie1992). Estimates of mean herbicide residues were supplemented with 95% confidence intervals that indicated whether differences were significant among sites (i.e., no overlapping intervals) or not significantly different (i.e., overlapping).
Peak herbicide residue levels (mg kg−1) were assumed to occur between the range of days (inclusive) for which samples were collected; that is, if the maximum was detected on the first day of collection, then it was assumed that residue levels on all days prior were not higher than the value detected on the first day of collection.
Peak residue concentrations were calculated from the maximum of the localized regression, and half-peak residues were subsequently determined by dividing the peak value by 2. The time difference between the days coinciding with the residue peak (Dmax) and half-peak (DT50), was used to estimate the average number of days required for the residues to halve (DT50). The same approach was applied at the upper confidence level to determine an upper bound on DT50 and the time difference (in days) between that upper bound and DT50 divided by 2 to provide an estimate of the standard error (because 95% confidence level is approximately 2 SE above the mean).
All analyses were undertaken using R v. 4.2.1 (R Core Team 2022) supplemented by packages dplyr (Wickham et al. Reference Wickham, Francois, Henry and Muller2022) and ggplot (Wickham Reference Wickham2016).
Results and Discussion
Input of the active ingredients into the environment during spraying was either (1) direct through needle uptake or deposition onto ground/stream water and/or (2) indirect through runoff from the canopy where rainfall occurred shortly after spraying (which occurred at MD and GE). Subsequently, further input occurred from needle cast that continued for up to 1 yr after spraying (data not shown).
Herbicide Degradation in Cast Needles
Degradation of triclopyr in cast needles was undifferentiated at sites across time, as indicated by the overlap in confidence intervals (Figure 2A). Peak residue levels for triclopyr were detected on the first sample date for all sites. At GE, peak triclopyr residue levels (41.6 mg kg−1) were detected at 27 d post-spray, and DT50 was estimated to be 330 (±29) d later (Table 7). At KF and MD, peak residues of triclopyr were also detected in needle samples from the first data-collection date, while DT50 values were estimated at 196 (±7) and 156 (±87) d, respectively. At KF, no needles had been cast at the first post-spray sampling made at 29 d, hence estimation of the peak residue level occurring at the second sampling date at 119 d after application of herbicide (Table 7). With the restricted sample numbers taken across time and the delay in deploying the litter traps at MD, it is possible that the actual peak in residue content of triclopyr was not sampled.

Figure 2. Estimated degradation of (A) triclopyr, (B) dicamba, and (C) picloram residues in cast needles at three study sites in New Zealand. The solid lines represent localized regressions, and shaded areas represent 95% confidence intervals.
Table 7. Estimated peak herbicide residues, days to peak levels (Dmax), and days from peak to half-life values (DT50) for residues in cast needles at Kaweka Forest (KF), Glen Eyrie Downs (GE), and Mid Dome (MD).

Dicamba residues in cast needles were less than triclopyr residues, reflecting the lower levels of active ingredient applied (Figure 2B; Table 7). Peak dicamba residues in cast needles at KF were, on average, 19.2 mg kg−1, coinciding with the first collection of cast needles (day 119); at the GE and MD sites peak dicamba residues were detected on days 227 (19.8 mg kg−1) and 305 (24.0 mg kg−1), respectively. Dicamba DT50 values in cast needles at KF, GE, and MD were 172 (±3), 218 (±55), and 209 (±47) d later (Table 7).
Picloram residues in cast needles were considerably lower than either triclopyr or dicamba residues, with peak residue levels of 3.2–4.8 mg kg−1 (Figure 2C; Table 7). As for dicamba, peak picloram residue levels were detected at the first sampling event at KF, and later at the other two sites. DT50 values were similar at GE and KF sites (210 ± 100 and 196 ± 8 d, respectively) but higher on average at the MD site (288 ± 25 d); however, given the large variation, differences were not significant.
Herbicide Degradation in Forest Floor Litter (LFH)
Peak triclopyr levels in the LFH at KF and MD sites were detected on the first sample date. Degradation in LFH did not differ significantly at the MD and KF sites, as indicated by the overlap in confidence intervals, reducing most rapidly over the first 200 d (Figure 3A). Triclopyr residues in LFH at GE were consistently low, reaching a maximum level of just 7.8 mg kg−1 in comparison to KF (peak = 54.5 mg kg−1) and MD (peak = 43.8 mg kg−1) (Table 8). The low value of triclopyr residues in LFH collected from GE may be attributed to a rainfall event shortly after spraying, resulting in most of the triclopyr moving into the soil layer at the first assessment (see Table 9). DT50 for triclopyr was 97 (±6) d at KF and 112 (15) d at MD (Table 8).

Figure 3. Estimated degradation of (A) triclopyr, (B) dicamba, and (C) picloram residues in the litter, fermented humic layer (LFH) at three study sites in New Zealand. The solid lines represent localized regressions, and shaded areas represent 95% confidence intervals.
Table 8. Estimated peak herbicide residues, days to peak levels (Dmax), and days from peak to half-life values (DT50) values for residues in the litter, fermented humic layer (LFH) at Kaweka Forest (KF), Glen Eyrie Downs (GE), and Mid Dome (MD).

a ND, not determined.
Table 9. Estimated peak triclopyr residues, days to peak levels (Dmax), and days from peak to half-life values (DT50) values for soil by depth at Kaweka Forest (KF), Glen Eyrie Downs (GE), and Mid Dome (MD).

Peak dicamba residue in LFH coincided with the first measurement at KF (15.0 mg kg−1 on day 29), whereas at GE and MD, consistently low residue levels were detected, reaching a maximum on days 217 (3.2 mg kg−1) and 209 (3.4 mg kg−1), respectively (Figure 3B; Table 8). DT50 for dicamba was 110 (±8) d for KF and considerably longer for GE (173 ± 125) and MD (271 ± 67), where no real peak was detected but where residues appeared to persist at low levels (Table 8).
Peak picloram residue concentrations were detected in samples taken from KF on the first sampling occasion on day 29 (6.1 mg kg−1). However, peak concentrations were detected in later samples from the other two sites (Figure 3C; Table 8). DT50 at KF (68 ± 6 d) was lower on average than at the GE and MD sites (170 ± 126 and 335 d respectively). Note that a standard error could not be calculated at MD due to DT50 of the upper confidence interval being undefined. This was a feature of the loess method for which the residue concentrations beyond 634 d after spraying were approximately constant (at about 0.56 mg kg−1), while the confidence intervals increased slightly.
Herbicide Degradation in Soil
Triclopyr was detected in soil at all three sites, declining to below detection levels within 1 yr (Table 9). Dicamba and picloram were not detected in any soil samples. At GE, triclopyr residues were highest in the topsoil (0 to 10 cm) and lower soil (10 to 50 cm) at the first assessment at 27 d (Table 9). Peak triclopyr residue concentrations were detected in topsoil (0 to 10 cm) sampled from KF at 29 d after spraying (1.3 mg kg−1) whereas at 10- to 20-cm soil depth, peak concentrations (0.52 mg kg−1) were detected at 229 d after spraying (Table 9). At MD, residues in the topsoil (0 to 10 cm) peaked (0.89 mg kg−1) at 33 d after spraying (the first post-spraying measurement date). Overall, herbicide residues in the topsoil (0 to 10 cm) did not differ significantly across sites. Given the amount of active ingredient applied and the duration the herbicide persisted in the forest floor layer (still detectable in forest floor samples from all sites at final assessment), it is notable that almost no dicamba or picloram, and only low levels of triclopyr, were detected in the soil samples taken from the three study sites.
Herbicide Persistence in Cast Needles, Forest Floor, and Soil
Patterns of herbicide residues in the terrestrial samples varied across the three sites, with triclopyr comprising the bulk of the residue detected across all sample material (Figure 4). Except for the first assessment made at GE, the bulk of herbicide across all sites was present in the forest floor layer (LFH), accounting for ∼55% of total residues (kg ha−1) in samples taken at the start of the study and increasing to >85% by the end of the study (Figure 5).

Figure 4. Concentrations (mean ± SE) of triclopyr, dicamba, and picloram (kg ha−1) estimated in cast needles, litter, fermented humic layer (LFH), and soil layers (10–20 cm; 10–50 cm) sampled at Glen Eyrie (GE), Kaweka Forest (KF), and Mid Dome (MD) in New Zealand from the first post-spray assessment. The y axis varies between herbicide panels.

Figure 5. Total herbicide residue (kg ha−1) detected in the litter, fermented humic layer (LFH), cast needles, and soil layers (0–10 cm; 10–20 cm) sampled from the (A) Kaweka, (B) Glen Eyrie, and (C) Mid Dome sites in New Zealand.
The bulk of herbicide residue detected in soil at the GE site comprised triclopyr residues in the samples taken from below 10 cm, most likely an outcome of rainfall in the week following spraying. A total of 90 mm of rainfall was recorded at the GE site in the week following spraying, with 65 mm recorded on the fifth day after spraying. While the herbicide detected in the soil collected from the GE site at the first assessment date accounted for 73% of total residues, no herbicide was detected in soil after 1 yr.
What is significant about the sites occupied by dense stands of invasive conifers is the thickness of the forest litter layer and, while the depth of this layer was not measured in this study, the estimated mass of this layer at the three study sites was 4.5 × 104, 3.1 × 104, and 6.9 × 104 kg ha−1 for the KF, GE, and MD sites, respectively (Table 3). Many studies report on the adsorption of herbicides in different agricultural soils and soil constituents; however, fewer studies have reported on herbicide adsorption to forest soil organic horizons (Garrett et al. Reference Garrett, Watt, Rolando and Pearce2015; Pinho et al. Reference Pinho, Matos, Morris and Costa2007), a layer that represented a significant component of the forest floor at all three sites. Adsorption of herbicides within the organic horizon plays an important role in limiting herbicide movement away from treated sites (Garrett et al. Reference Garrett, Watt, Rolando and Pearce2015; Helbert Reference Helbert1990; Pinho et al. Reference Pinho, Matos, Morris and Costa2007), and given the amounts of herbicide applied in dense stands of conifers, the role of this organic horizon in limiting movement of herbicide beyond the zone of application is an important consideration.
Herbicide Detection in Water
No herbicides were detected in stream water before herbicide application at either the KF or MD site (data not shown). At the KF site, concentrations of all three herbicides peaked on the day of herbicide application and were below detection limits the following day (Figure 6A). At day 26 (following the first rainfall event) and day 29, herbicide concentrations increased to just above detection limits. At the final measurement, 119 d after herbicide application, concentrations for all three herbicides were again below detection limits in the water. At no point did levels of herbicide exceed the Mean Acceptable Values (MAVs) or Environmental Exposure Limits (EELs) for water in New Zealand (New Zealand Ministry of Health 2018; Table 2).
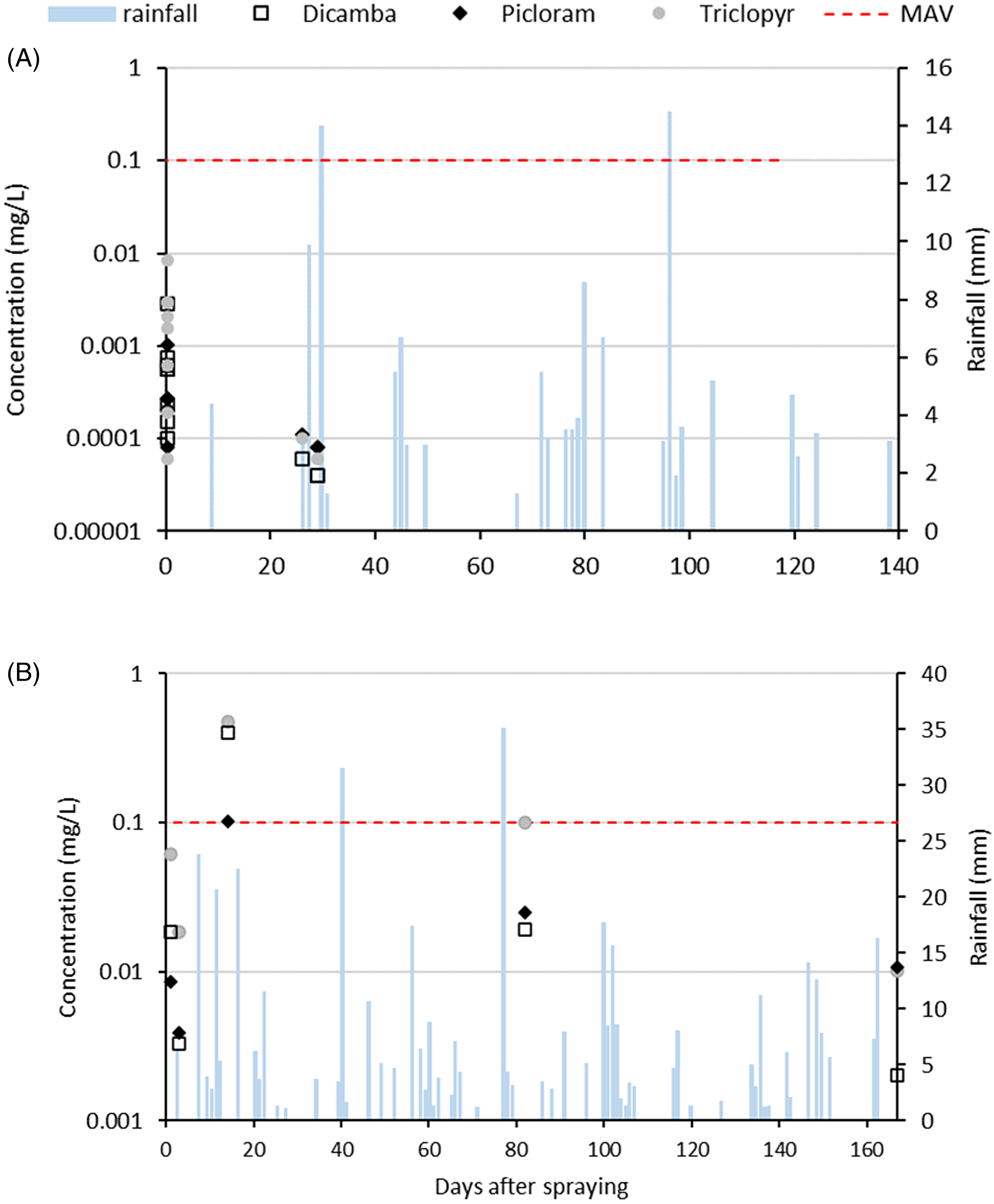
Figure 6. Rainfall and concentrations (mg L−1) of triclopyr, dicamba, and picloram detected in stream water at the (A) Kaweka and (B) Mid Dome sites in New Zealand. mean acceptable value (MAV).
There was a total of ∼100 mm of rainfall in the month after spraying at the MD site, with four rainfall events exceeding 10 mm of rainfall. Herbicide concentrations in stream water from Tank Creek at MD increased the day following herbicide application (Figure 6B). There was a smaller increase in concentrations following the first rainfall event, but the highest concentrations occurred during the higher rainfall event, 13 d after spray application, when levels of herbicide exceeded the MAVs for dicamba (four times the MAV) and triclopyr (five times the MAV; Figure 6B). Herbicides were still detectable in the water at 3 mo after spraying but did not exceed the MAV for drinking water.
Triclopyr: Persistence and Environmental Impacts
Triclopyr (as the butoxyethyl ester) is the principal active ingredient of the TDPA mix and was the active ingredient detected at the highest level at all sites, in the cast needles, LFH, and soil. DT50 values calculated for triclopyr residues in foliage and LFH in this study are higher than half-life values calculated elsewhere (compare Table 1 with Tables 7–9). For example, Newton et al. (Reference Newton, Roberts, Allen, Kelpsas, White and Boyd1990) calculated DT50 values of 74 d for total triclopyr residues in tanoak [Notholithocarpus densiflorus (Hook. & Arn.) P.S. Manos, C.H. Cannon, & S.H. Oh.] foliage, and Thompson et al. (Reference Thompson, Pitt, Buscarini, Staznik and Thomas2000) recorded values of <50 d for dissipation from the forest floor. Several factors specific to dense stands of conifers could underpin the persistence of this active ingredient in this study:
-
1. Low uptake of triclopyr by pine needles: Rolando et al. (Reference Rolando, Gaskin, Horgan and Richardson2020) estimated only 29% uptake occurred within the first 24 h after application with <50% translocated out of the needle. Low uptake and translocation levels indicate that: (a) a large proportion of the applied triclopyr remains on the exterior of the needles, subject to wash-off if rain occurs soon after spraying, and (b) much of the active ingredient absorbed into the needles persists there until the tissues are degraded in the environment (Tu et al. Reference Tu, Hurd and Randall2001).
-
2. High affinity of the triclopyr ester for organic matter, with adsorption rates increasing with higher organic content and lower soil pH (Tu et al. Reference Tu, Hurd and Randall2001): a thick litter layer and low soil pH are two factors that predominate in the forest floor below a stand of dense invasive pines (Giddens et al. Reference Giddens, Parfitt and Percival1997; Johnson et al. Reference Johnson, Lavy and Gbur1995; Tu et al. Reference Tu, Hurd and Randall2001).
-
3. Slower microbial degradation of triclopyr acid (parent compound rapidly produced in situ) at cooler, drier sites (Tu et al. Reference Tu, Hurd and Randall2001): all study sites were relatively cool (mean annual temperature <11 C) with pronounced winter conditions, with GE and MD experiencing lower mean annual precipitation (Table 2).
The combination of low uptake of active ingredient, high organic matter in the LFH, and cool, dry conditions likely accounts for the slower breakdown and persistence of triclopyr in the LFH, particularly at the two South Island sites, GE and MD. No soil EEL is set for triclopyr in New Zealand, so it is not possible to determine whether any thresholds were exceeded. However, triclopyr butoxy ethyl ester is considered relatively nontoxic to terrestrial vertebrates and invertebrates and is unlikely to have posed any risk to these organisms at the levels detected at these sites (Table 1). The persistent low levels of triclopyr in the LFH could potentially affect regrowth of vegetation at these sites, notably via root uptake of germinating seedlings. Ranft et al. (Reference Ranft, Seefeldt, Zhang and Barnes2010) showed that triclopyr residues ranging between 0.33 and 0.85 kg ai ha−1 reduced the biomass of seedlings of four agricultural species to 50% of that of a control, with species varying in their sensitivity to residue levels. The ranges tested by Ranft et al. (Reference Ranft, Seefeldt, Zhang and Barnes2010) are within those observed in this study, particularly for the LFH layer, where residues of picloram and dicamba were also present (Figure 4).
The highest concentrations of herbicides in waterways are typically recorded on the day of application or during and after high-rainfall events shortly after application (Baillie et al. Reference Baillie, Neary, Gous and Rolando2015; McBroom et al. Reference McBroom, Louch, Beasley, Chang and Ice2013). At the MD site, shallow soils, steep slope, and rainfall shortly after spraying might have contributed to surface flow of contaminated water into the local catchment, accounting for the high levels of triclopyr detected in water at the stream below this site in the month after spraying. Further, a very large total area was sprayed at the MD site (Supplementary Figure S1c), with herbicide directly applied across some sections of the stream channel. The triclopyr concentrations measured in stream water at the MD site were below freshwater toxicity standards for a range of aquatic organisms and plants (Pesticide Properties Database, https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/659.htm; Table 1). Concentrations exceeded New Zealand drinking water standards in the second rainfall event (Figure 6B), although the duration of this exceedance is unknown, but it is likely to have decreased once flow levels declined. Stream water was not sampled on the day of application at MD. High herbicide concentrations have been recorded when applying herbicides directly across a stream channel (Neary et al. Reference Neary, Bush and Michael1993; Wan Reference Wan1986), so the potential risks to the aquatic environment and whether any acute toxicity standards were exceeded on the day of application is unknown. Currently, the Australian and New Zealand Guidelines for Fresh and Marine Water Quality (https://www.waterquality.gov.au/anz-guidelines) do not provide freshwater guidelines for triclopyr, so the impact of these concentrations on the long-term health of the aquatic environment is unknown.
The ester formulation of triclopyr is not water soluble and can be persistent in aquatic environments, where it can bind to organic particles in the water column and precipitate to sediment layers. Unfortunately, no analysis of sediment was undertaken at the stream site/catchment below the MD sprayed area, so it is unknown whether triclopyr persisted in this catchment. The triclopyr ester is highly toxic to fish and aquatic invertebrates, for example, it has an LC50 (96 h) of 0.74 mg L−1 in rainbow trout (Oncorhynchus mykiss); however, studies have shown that the ester degrades in less than a day into triclopyr acid, which is less toxic (Ganapathy Reference Ganapathy1997). Concentrations were below toxicity thresholds for aquatic organisms and the New Zealand drinking water standard (Table 2) and much lower than concentrations recorded at the MD site, indicating the effectiveness of the riparian no-spray buffer in protecting the stream from herbicide inputs, as evidenced by other studies (McBroom et al. Reference McBroom, Louch, Beasley, Chang and Ice2013; Wan Reference Wan1986). These results indicate a low risk to human health and the aquatic environment under the spraying regime applied to the KF site.
Dicamba: Persistence and Environmental Impacts
Dicamba was applied at a lower rate than triclopyr in the operationally applied herbicide mix and was below detectable levels in soils for the duration of the study. Lack of detection of dicamba in soils was unsurprising, as the DT50 of dicamba in soils is ∼ 4 d due to high solubility and low capacity for soil adsorption (McBean Reference McBean2012; Table 1). However, as with triclopyr, residues of dicamba were detected in needle fall and LFH at all sites up to the final assessment dates. The persistence of dicamba in the LFH across sites is likely to represent adsorption of this active ingredient by the thick layer of decaying needles. Azejjel et al. (Reference Azejjel, Astouf, Draoui, Rodrigues-Cruz and Sanchez-Martin2007) determined soil organic matter content significantly affected the sorption properties of dicamba. Few other studies using dicamba make reference to a heavy lignin-rich litter layer overlaying the soil, as was encountered in this study, and, as such, comparison of these results with other studies is difficult. No soil EELs are set for dicamba in New Zealand. Dicamba is not considered to be highly toxic to terrestrial vertebrates and invertebrates and is unlikely to pose a risk to terrestrial organisms at the levels detected in this study.
While dicamba is rated very ecotoxic in the aquatic environment (Table 1), concentrations detected in stream water at the KF site were more than two orders of magnitude lower than the toxicity thresholds for freshwater organisms (Pesticide Properties Database, https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/213.htm), influenced by the retention of a no-spray riparian buffer. Although the Australian and New Zealand Guidelines for Fresh and Marine Water Quality (https://www.waterquality.gov.au/anz-guidelines) do not provide freshwater guidelines for dicamba, dicamba concentrations detected at KF were also well below the Canadian Water Quality Guidelines for dicamba for the protection of freshwater aquatic life (10 µg L−1, or 0.01 ppm) (CCME 2007), indicating a low risk to the aquatic environment. Similarly, dicamba concentrations at MD were below toxicity thresholds for aquatic organisms (Pesticide Properties Database, https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/213.htm; Table 1), although we acknowledge that concentrations on the day of application were not assessed. Dicamba concentrations exceeded Canadian guidelines for the protection of freshwater aquatic life (CCME 2007), in the second rainfall event, although the duration of this exceedance, and hence the risk to aquatic organisms, is unknown.
Picloram: Persistence and Environmental Impacts
Picloram was only detected in soils at KF up to 4 mo after spraying but was otherwise undetected in soils. Picloram, applied at 2 kg ha−1 in the TDPA mix (Table 5), was detectable at all sites in the LFH, either 1 or 2 yr after spraying, albeit at low levels. Few studies on the adsorption of picloram in forest floor layers (not including mineral soil) could be found; however, the mobility of picloram in soils is determined by the adsorption capacity of the soil, soil moisture, and post-application rainfall (Tu et al. Reference Tu, Hurd and Randall2001). As with dicamba, picloram does not bind strongly with soil particles (i.e., has low adsorption capacity); however, unlike dicamba, this active ingredient is not degraded rapidly in the environment, allowing it to be highly mobile and potentially persistent. Further, unlike many other herbicides, clay content does not affect adsorption of picloram. However, high organic content, heavy soil texture, low pH, and low soil temperature can increase adsorption capacity (Tu et al. Reference Tu, Hurd and Randall2001). In soils with high organic content that can bind the herbicide, picloram tends to remain in the top 30 cm, and results from our study support this, with picloram remaining and persisting in the LFH layer. Where picloram persists, it has a high potential to move vertically and horizontally, which can lead to contamination of water sources and non-target sites (Tu et al. Reference Tu, Hurd and Randall2001); however, such movement was not detected in this study. Levels of picloram in the LFH exceeded the New Zealand EEL set for soil (Table 2); however, even at these levels, the low toxicity of this active ingredient to terrestrial organisms mean it is unlikely to pose any risk to vertebrates and invertebrates. The persistence of this herbicide, together with dicamba and triclopyr residues, could affect germination and development of vegetation in the years following spraying. Tran et al. (Reference Tran, Harrington, Robertson and Watt2015) found a triclopyr/picloram mix (3 kg/1 kg ai ha−1) applied to soil to be the most persistent out of a range of mixes tested; it negatively affected the germination of broom [Cytisus scoparius (L.) Link] seedlings, with none germinating within the first 180 d after application.
Similar to triclopyr and dicamba, picloram was detected in stream water at KF at concentrations well below toxicity thresholds (Table 1) and the Canadian Water Quality Guidelines for picloram for the protection of freshwater aquatic life (29 μg L−1, or 0.029 ppm) (CCME 2007). Picloram concentrations were also well below the New Zealand drinking water standard (Table 2). At MD, picloram concentrations were also well below toxicity thresholds for freshwater organisms, New Zealand drinking water standards (Tables 1 and 2), and the Canadian guidelines for the protection of freshwater aquatic life (CCME 2007). These results indicate that the risk to aquatic, environmental, and human health from this active ingredient at both the KF and MD sites was likely to be low.
Thompson et al. (Reference Thompson, Pitt, Buscarini, Staznik, Thomas and Kettela1994) stated that typical residue dissipation patterns are biphasic in nature, with an initial rapid-decline phase followed by a slower phase in which low-level residues persist for lengthy periods. It is possible that the peak residue (day after spraying) was missed in this study, and therefore our estimation of degradation represents this second phase. Either way, this study has highlighted the time over which these herbicides are present in the environment following spraying. Highest concentrations of herbicides detected in stream water at KF were on the day of spray application and in the first rainfall event after application. Concentrations detected were low and not considered a risk to the aquatic environment. These results reflected the application techniques used at this site, including the retention of no-spray buffers along the stream margin along with the propensity of these herbicides to bind to organic matter in the litter layer, limiting their ability to move off-site during rainfall events and into waterways. At MD, spraying over the stream channel was the most likely reason for the higher herbicide concentrations detected in the stream water.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/inp.2023.20
Acknowledgments
The authors would like to acknowledge Liam Wright, Vanessa Cotterill, John Meredith, and Dave Henley, who assisted with the collection and processing of samples. We would also like to acknowledge Loretta Garrett, who advised on soil and litter sampling collection. The work was funded by the Ministry for Primary Industries through the Sustainable Farming Fund “Beyond Conifer Control” contract no. SFF405328 and through the MBIE Programme Vive la resistance (C04X2102), with cofunding also received from the Wilding Pine Network (Wilding Conifer Management Group). We also thank Boffa Miskel for collection of the water samples at Tank Creek at the Mid Dome site.
Competing interests
The authors declare no conflict of interest.


















