Introduction
Edge effects threaten biodiversity in many landscapes fragmented as a result of human activities (Harper et al., Reference Harper, MacDonald, Burton, Chen, Brosofske and Saunders2005). Edges between different habitats were once considered beneficial for biodiversity (Leopold, Reference Leopold1933; Lay, Reference Lay1938) but as studies focused on anthropogenic edges (Chasko & Gates, Reference Chasko and Gates1982; Harris, Reference Harris1988) their detrimental effects became apparent. Edges are abrupt transitions between habitats or ecosystems (Yahner, Reference Yahner1988; Ries et al., Reference Ries, Fletcher, Battin and Sisk2004) and their effects include any changes that occur as a result of that transition (Murcia, Reference Murcia1995). Abrupt transitions in vegetation at edges are usually associated with similarly abrupt changes in climate, with consequent impacts on plants and animals (Chasko & Gates, Reference Chasko and Gates1982; Sork, Reference Sork1983; Palik & Murphy, Reference Palik and Murphy1990; Matlack, Reference Matlack1994; Oliveira et al., Reference Oliveira, Grillo and Tabarelli2004) as a result of changing resource distribution (Mills et al., Reference Mills, Dunning and Bates1991; Berg & Pärt, Reference Berg and Pärt1994; Ries & Sisk, Reference Ries and Sisk2004) and biotic interactions (Berg & Pärt, Reference Berg and Pärt1994; Fagan et al., Reference Fagan, Cantrell and Cosner1999).
Diversity often increases near edges (especially in forest fragments) as a consequence of invasion by organisms from areas adjacent to the edge, both the forest interior and the newly created open area causing the edge (Yahner, Reference Yahner1988; Laurance, Reference Laurance1994; Gascon et al., Reference Gascon, Lovejoy, Bierregaard, Malcom, Stouffer and Vasconcelos1999; Pardini, Reference Pardini2004). Conditions near edges are often similar to those of disturbed habitats (Chen et al., Reference Chen, Franklin and Spies1993; Cadenasso et al., Reference Cadenasso, Traynor and Pickett1997) and may therefore favour invasion by species typical of disturbances (Laurance, Reference Laurance1994; Pardini, Reference Pardini2004). Unique resources may be available near edges (Mills et al., Reference Mills, Dunning and Bates1991; Berg & Pärt, Reference Berg and Pärt1994; Ries & Sisk, Reference Ries and Sisk2004), also favouring invasion by species that prefer those resources.
Edge effects are well known in the context of habitat loss and fragmentation (Yahner, Reference Yahner1988; Murcia, Reference Murcia1995; Ries et al., Reference Ries, Fletcher, Battin and Sisk2004). However, linear clearings for rights of way (e.g. power lines, roads, railways) also produce edges, often in otherwise well-preserved landscapes (Laurance et al., Reference Laurance, Goosem and Laurance2009). Studies of the effects of roads and highways on wildlife have shown that these edges affect the diversity of understorey birds (Laurance, Reference Laurance2004) and small mammals (Goosem, Reference Goosem2000; Ruiz-Capillas et al., Reference Ruiz-Capillas, Mata and Malo2013). However, most studies have addressed these effects where edges are extensive and obvious. More subtle settings, such as smaller roads and rights of way, remain understudied and may have unexpected effects as a result of inputs of new resources that may, in turn, increase diversity (Carthew et al., Reference Carthew, Jones and Lawes2013). For example, agricultural products may fall from trains while in transit, introducing plants or providing food for animals.
Anthropogenic edges in tropical forests are of particular concern because of the contrast between the more constant forest interior and the variable temperatures and greater extremes of the adjacent open areas, which may be agricultural, urban or rights of way for road, rail or power lines (Matlack, Reference Matlack1993; Pohlman et al., Reference Pohlman, Turton and Goosem2007; Laurance et al., Reference Laurance, Goosem and Laurance2009). As natural areas become increasingly fragmented, understanding the effects of fragmentation and edges is important for conservation. An estimated 73% of the Atlantic Forest is < 250 m from an edge (Ribeiro et al., Reference Ribeiro, Metzger, Martensen, Ponzoni and Hirota2009). In the future, these kinds of edges may be pervasive, and understanding their impact on wildlife will be important for appropriate management of biodiversity coexisting with small-scale edges.
Here we measured the response of small mammals to a railway in an otherwise well-preserved natural landscape. This setting is useful because it minimizes the effects of overdispersion of animals from an anthropogenic matrix (i.e. farms, rural and urban areas). We tested three hypotheses: the railway creates an environmental gradient in the forest (i.e. an edge effect exists), this edge has consequences for the small mammals inhabiting the forest, and the new resources introduced by trains hauling agricultural produce (usually soy and other grains) favour small mammals near the edge. By elucidating the impact of this edge within a well-preserved forest we hope to separate edge effects from the larger scale of the surrounding matrix.
Study site
We studied edge effects in Marumbi State Park (Parque Estadual Pico do Marumbi). The Park is in the central portion of the Coastal Mountain Range (Serra do Mar) in the state of Paraná, southern Brazil, and lies within the largest remnant of Atlantic Forest (Ribeiro et al., Reference Ribeiro, Metzger, Martensen, Ponzoni and Hirota2009; Fig. 1). The climate is humid year-round and without a marked dry season (mean annual rainfall c. 2,000 mm and mean annual temperature c. 20°C; Vanhoni & Mendonça, Reference Vanhoni and Mendonça2008). The study area is at 350–450 m altitude; vegetation is evergreen (Veloso et al., Reference Veloso, Rangel-Filho and Lima1991), with emergent trees > 30 m tall and canopy at 20–25 m. The most common plant families are Bromeliaceae, Myrtaceae, Rubiaceae, Melastomataceae and Fabaceae (Silva, Reference Silva1989; Kaehler et al., Reference Kaehler, Varassin and Goldenberg2005). The Paranaguá–Curitiba railway, established in 1885, passes through the Atlantic Forest of the Serra do Mar and connects the state capital, Curitiba, with the port of Paranaguá (110 km). It is used for commercial transportation of several products, such as grains (mainly soy, wheat and corn), fuel and paper, and also for tourism. In the study area the right of way is c. 10 m wide.

Fig. 1 The study site, where the Paranaguá–Curitiba railway passes through Marumbi State Park, in the Atlantic Forest of southern Brazil. The rectangle on the inset shows the location of the main map in Paraná state. Data on distribution of Atlantic forest patches are from SOS Mata Atlântica & INPE (2011).
Methods
Capture of small mammals
We established four groups of transects parallel to the railway, with groups separated by ≥ 50 m along the railway. Each group comprised four transects, one of which was at the edge (0 m), with c. 50 m between each transect (thus the last was c.150 m from the edge). This sampling design was based on the fact that edge effects usually penetrate up to 100 m in fragments of tropical forest (Kapos, Reference Kapos1989; Matlack, Reference Matlack1993; Stevens & Husband, Reference Stevens and Husband1998; Laurance et al., Reference Laurance, Lovejoy, Vasconcelos, Bruna, Didham and Stouffer2002; Oliveira et al., Reference Oliveira, Grillo and Tabarelli2004). Sampling points were established at 5 m intervals along the 45 m transects. Sampling (8 days, 7 nights) was carried out monthly during February–July 2011; storms prevented sampling in March.
One Sherman trap (H.B. Sherman Traps, Tallahasse, USA) was placed at each sampling point. We alternated six small (231 × 87 × 76 mm) and four large (371 × 120 × 103 mm) traps on and above the ground (1.5–2 m) on each transect. Captured animals were handled without anaesthesia, and marked with a numbered ear tag. All individuals were measured (head-and-body length) and weighed. Voucher specimens collected (licenses Instituto Ambiental do Paraná n°275/10 and Instituto Chico Mendes de Conservação da Biodiversidade n°26769-1) are in the mammal collection of the Department of Zoology, Federal University of Paraná. Field procedures followed guidelines of the American Society of Mammalogists (Sikes et al., Reference Sikes and Gannon2011). Information on the species of small mammals captured is in Cerboncini et al. (Reference Cerboncini, Rubio, Bernardi, Braga, Roper and Passos2014).
Microclimate and environmental conditions
We measured air temperature (°C), relative humidity (%, thermo-hygrometer, Instrutemp ITHT-2210), light intensity (lux, Instrutemp ITLD-260) and ambient noise (dB, Instrutemp ITDEC-4000) at five randomly chosen points on all transects once during each sampling period. Noise was measured on each transect when a train passed by, which occurred at 1–3 hour intervals, day and night. The mean of each variable (except noise from trains) on each transect during each sampling period was used in analyses.
An additional 150 m transect perpendicular to the railway was used to measure a temperature gradient. For a fine-scale assessment of the microclimate, air temperature was measured along this transect using 16 Thermochron data loggers (Maxim Integrated, San Jose, USA). Data loggers were placed at 5 m intervals for the first 25 m from the edge, and subsequently at 10 m intervals to 75 m and then 15 m intervals to 150 m. All data loggers recorded temperature simultaneously every hour for 71 hours during each sampling period. Based on natural temperature divisions, we categorized the sampling periods as warm (February and April), mild (May and July), and cold (June), to compare how temperature varied with distance from the edge, controlling for seasonality.
Resources and edges
We estimated resource availability at the railway tracks by collecting grains that had fallen from trains. We used seven seed-collectors (70 × 70 cm) set in place on the first day of each sampling period, at 5 m intervals on both sides of the track (three on one side, four on the other). After 6 days we counted the number of grains collected as our relative estimate of grain availability. We focused on comparing sampling periods rather than estimating grain abundance on the railway.
Data analysis
We tested for correlations between total number of captures, abundance and number of species of small mammals, and distance from the edge. We also tested for relationships between distance from the edge and environmental variables, using linear regression analysis. Species abundance estimates were categorized according to distance from the edge (for the same sampling effort): < 10 m, 30–80 m, 81–120 m, and > 120 m. We calculated the Jaccard dissimilarity index between all sampling transects and tested if species composition depended on these edge distance categories, using ANOSIM (analysis of similarity) with 999 permutations.
To estimate body condition we used the residuals of a linear regression of body weight against length (Schulte-Hostedde et al., Reference Schulte-Hostedde, Zinner, Millar and Hickling2005). For species with > 10 individuals captured on more than three transects within an interval of at least 100 m from the edge we tested for a relationship between condition, sex and distance from edge, using analysis of covariance. To avoid pseudoreplication we included only the first capture of any individual in the analysis.
We tested for a correlation between small mammal abundance near the edge (0 m) and number of grains collected, firstly using overall abundance of all species, and secondly focusing on granivorous species, following the dietary classification of Paglia et al. (Reference Paglia, Fonseca, Rylands, Herrmann, Aguiar and Chiarello2012). All statistical analyses were carried out with R v. 3.0.2 (R Development Core Team, 2013); vegan (Oksanen et al., Reference Oksanen, Guillaume Blanchet, Kindt, Legendre, Minchin and O'Hara2013) was used for ANOSIM. For statistical significance we assumed a 5% error rate.
Results
Edge and climate
Air temperature (F1,78 = 0.005, P = 0.94), relative humidity (F1,78 = 0.59, P = 0.44) and environmental noise intensity (F1,78 = 0.32, P = 0.58) were independent of distance from the edge. Light intensity (F1,78 = 39.84, r2 = 0.33, P < 0.001) and train noise intensity (F1,80 = 190.5, r2 = 0.7, P < 0.001) decreased with increasing distance from the edge (Fig. 2). There was no temperature gradient beginning at the edge during any season; only at the edge itself was temperature different from all other points. In general, higher maximum temperatures (but similar minimum temperatures) and thus greater variability were found at the edge (Fig. 3).
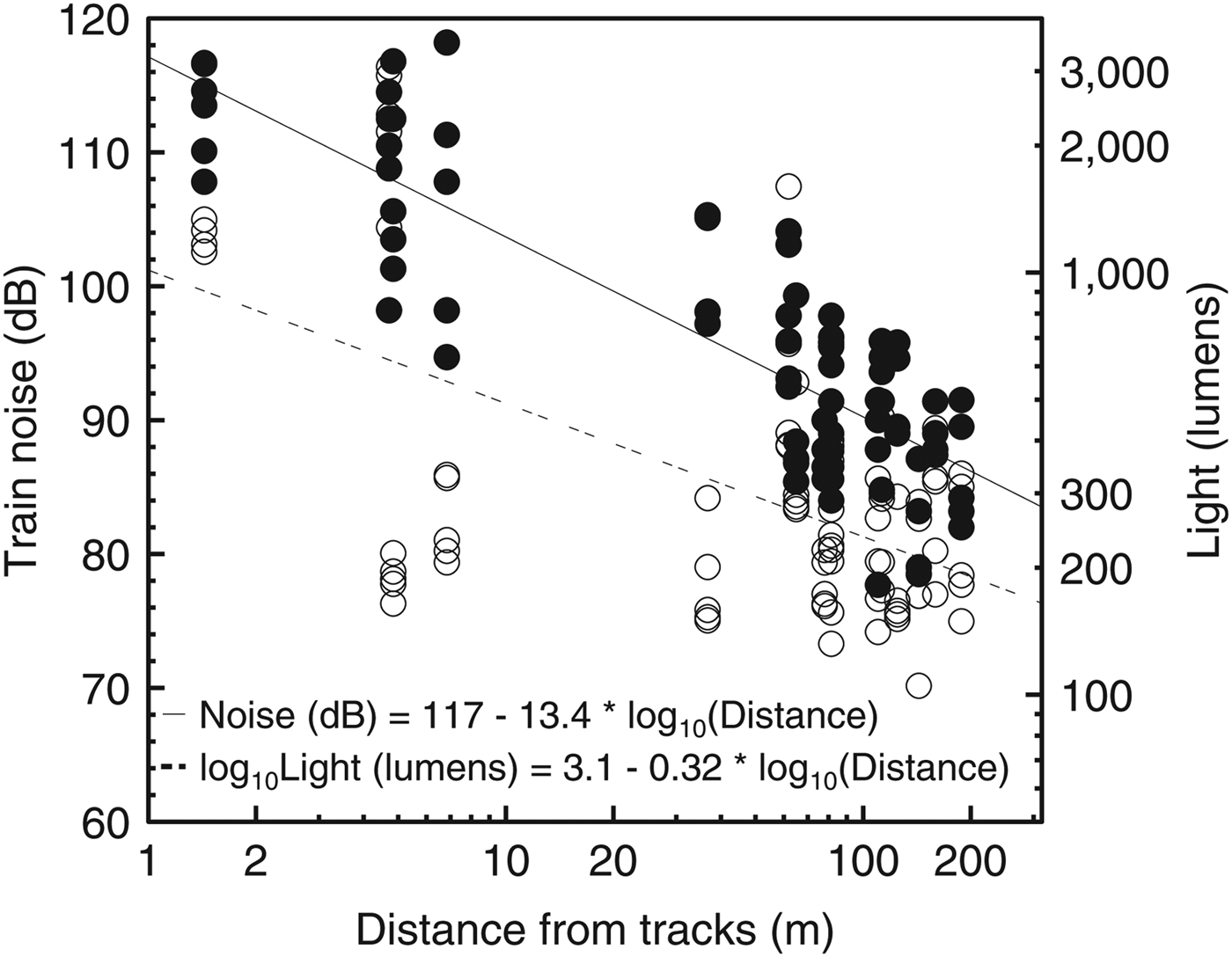
Fig. 2 The relationship of distance from the Paranaguá–Curitiba railway to light intensity (unfilled circles) and train noise (filled circles) in the Atlantic Forest in southern Brazil (Fig. 1). Axes are log10 scaled.

Fig. 3 Variation in temperature with distance from the Paranaguá–Curitiba railway towards the interior of the Atlantic Forest in southern Brazil (Fig. 1) during warm (February and April), mild (May and July) and cold (June) seasons.
Small mammals
We captured 12 small mammal species, four of which are granivorous (Table 1). Total capture (S = 887.3, P = 0.25, transect median = 22.5) and abundance (S = 907.7, P = 0.2, transect median = 13) were independent of distance from the edge, whereas the number of species captured declined towards the forest interior (S = 1049, rs = −0.54, P = 0.03, median for transects < 10 m from the edge = 7, 30–70 m from the edge = 5, and > 70 m from the edge = 3; Fig. 4). The most common species were approximately equally abundant at various distances from the edge, whereas the most uncommon species were captured only near the edge (Fig. 5). Species composition was similar at all distances from the edge (ANOSIM, R = 0.09, P = 0.18). Body condition of all animals was independent of sex, and therefore sex was not included in the analysis of body condition and distance from edge. Body condition was independent of distance from the edge in all species (P > 0.1).
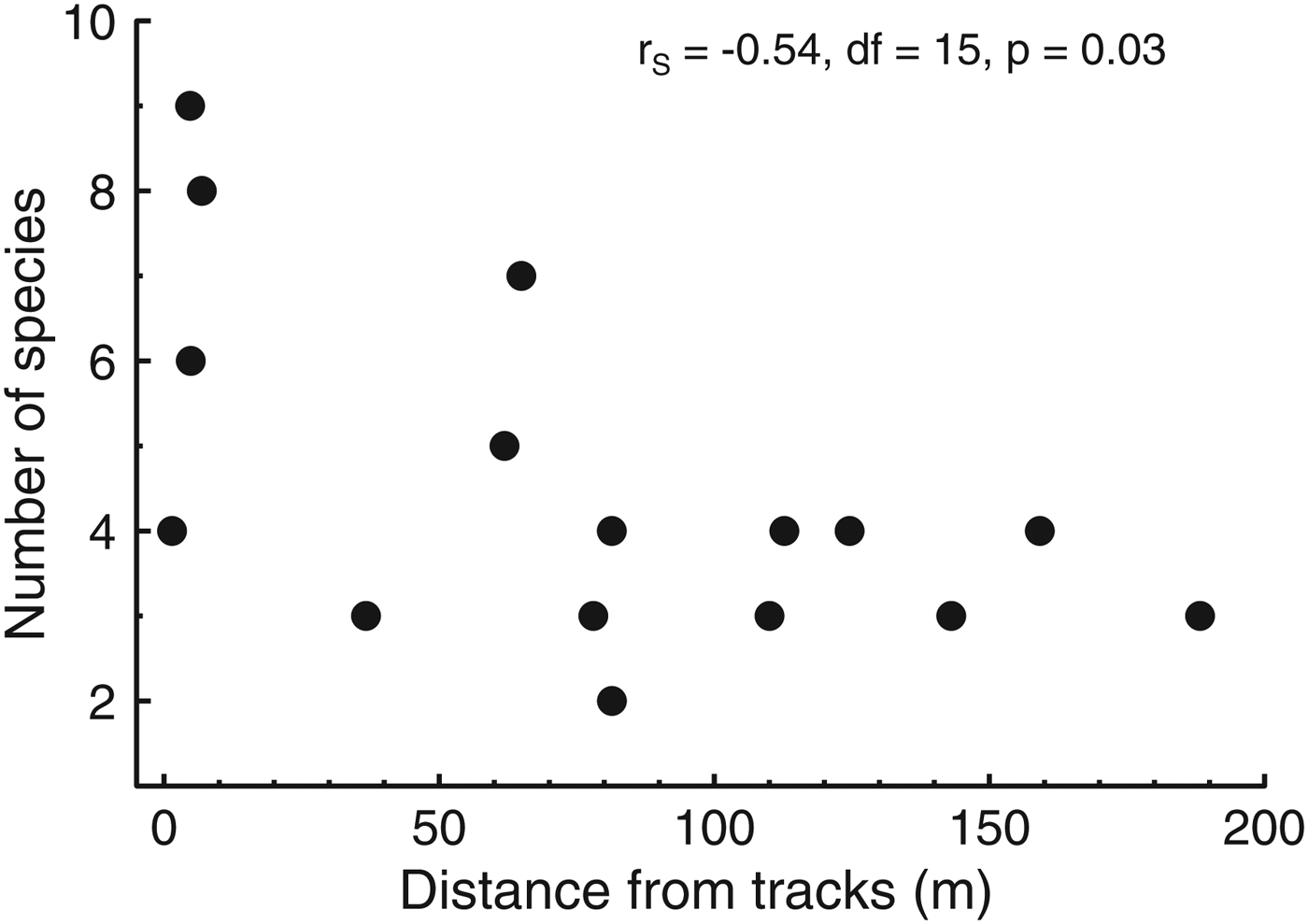
Fig. 4 The variation in number of species of small mammals captured with distance from the Paranaguá–Curitiba railway in the Atlantic Forest in southern Brazil (Fig. 1). The higher number of species near the edge is partly attributable to the capture of one or few individuals of some species (Fig. 5).
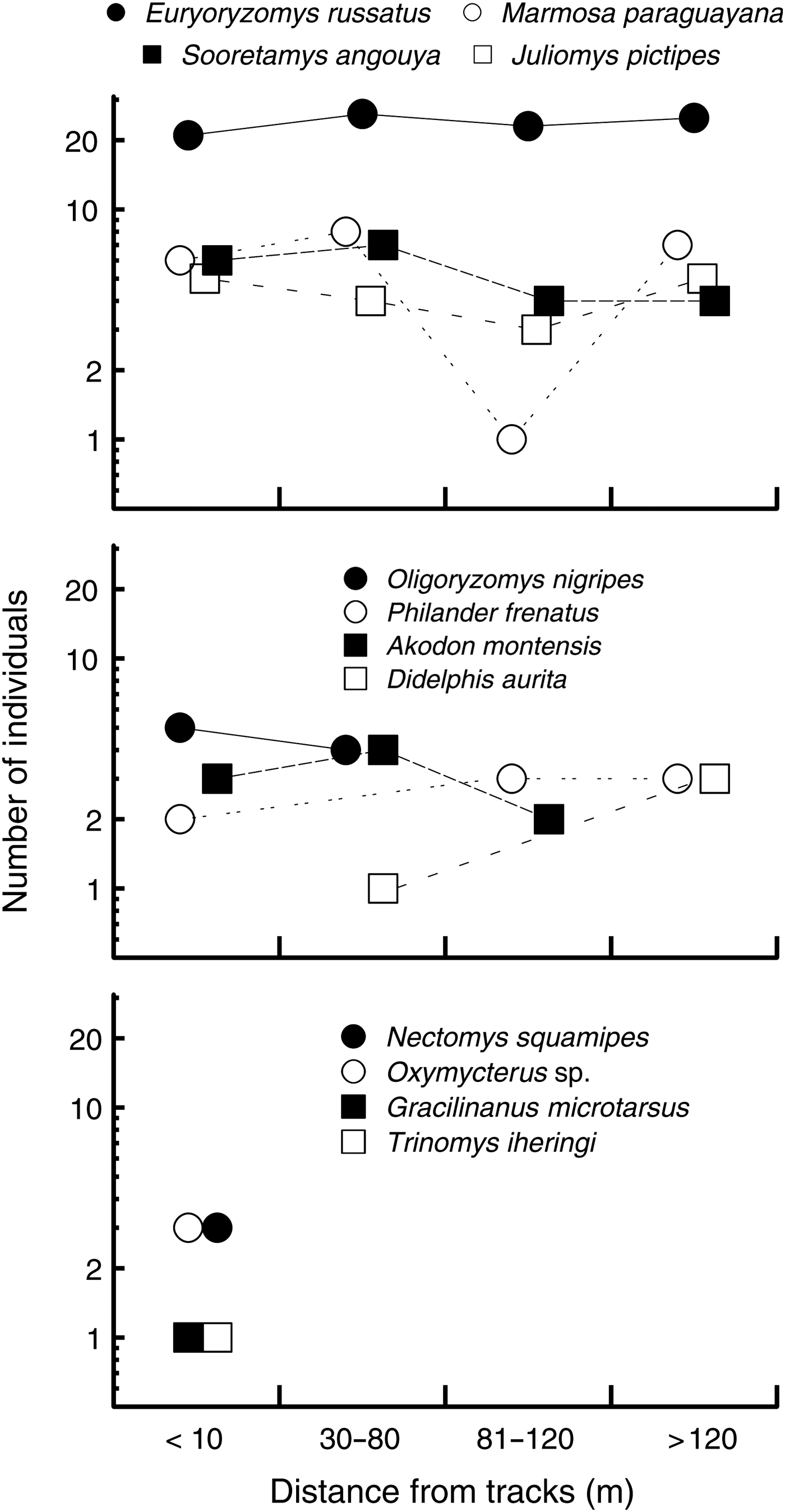
Fig. 5 Variation in abundance of small mammal species with distance from the Paranaguá–Curitiba railway in the Atlantic forest in southern Brazil (Fig. 1).
Table 1 The 12 small mammal species captured along the railway at Marumbi State Park, Paraná, Brazil (Fig. 1), with number of captures, and type of diet.

* Diet classification following Paglia et al. (Reference Paglia, Fonseca, Rylands, Herrmann, Aguiar and Chiarello2012)
Railway resources
Soy (85% of all grains), corn (10%) and wheat (5%) that fell from passing trains were collected in our seed traps. The number of grains collected varied from 0 in February to 33 in May (13 in April, 7 in June and 12 in July). Grain was available near the tracks during every period, and the number of grains collected seemed to represent true availability (few grains were seen on the tracks during February, whereas large numbers were seen in May). The number of individuals of all mammal species captured at the edge was always independent of the number of grains collected (all species: S = 872.7, n = 20, P = 0.14; granivorous species only: S = 998.4, n = 20, P = 0.28).
Discussion
Microclimate and edge
Despite the clear and abrupt transition between the open space and the forest, the edge had no apparent influence on microclimate. There was a marked gradient in both light and noise, both of which diminished with distance from the train tracks, yet there was only a marked variation in temperature at the edge itself. In the tropics and elsewhere changes in microclimate can be detected up to 50 m from the edge (Kapos, Reference Kapos1989; Matlack, Reference Matlack1993; Stevens & Husband, Reference Stevens and Husband1998). However, microclimate responses to edges are strongest when small forest fragments are bordered by open areas (Didham & Lawton, Reference Didham and Lawton1999). The magnitude of microclimatic gradients as a result of edges usually depends on the size of the clearing (Pohlman et al., Reference Pohlman, Turton and Goosem2007). Thus the absence of a microclimate gradient may be attributable to the clearing along the Paranaguá–Curitiba railway being narrow and linear (Laurance et al., Reference Laurance, Goosem and Laurance2009) in a well-preserved, forested landscape.
Edge effects on light intensity (Kapos, Reference Kapos1989; Matlack, Reference Matlack1993; Young & Mitchell, Reference Young and Mitchell1994; Cadenasso et al., Reference Cadenasso, Traynor and Pickett1997) can influence plant survival, reproduction and seed germination (Boardman, Reference Boardman1977). This interaction often favours pioneer or invasive plants (Bazzaz, Reference Bazzaz1979; Kapos, Reference Kapos1989; Brothers & Spingarn, Reference Brothers and Spingarn1992) and can cause an increase in plant density (Willson & Crome, Reference Willson and Crome1989; Camargo & Kapos, Reference Camargo and Kapos1995) and diversity (Harper et al., Reference Harper, MacDonald, Burton, Chen, Brosofske and Saunders2005). Here, however, if such edge conditions existed their influence on small mammals (through their influence on vegetation) was not measurable. Anthropogenic noise can be detrimental to wildlife for a variety of reasons, such as increased stress causing abnormal feeding and reproductive behaviours and other consequences that can influence fitness (Marler & Sherman, Reference Marler and Sherman1983; Andersen et al., Reference Andersen, Rongstad and Mytton1990; Awbrey & Bowles, Reference Awbrey and Bowles1990). We suggest that noise along the railway track is sporadic and relatively rare compared to other edge situations, and probably causes little disturbance. The impact of noise near roads depends on noise intensity and the rate and duration of traffic, and thus there is a greater impact on wildlife near larger roads (Kaseloo, Reference Kaseloo, Irwin, Garrett and McDermott2005). In Marumbi State Park trains usually pass every few hours and, although noise may be intensely loud (up to 120 dB) near the railway, infrequent passage probably minimizes any effects on small mammals. It is also possible that small mammals derive a benefit from the noise because it may perturb potential predators. If so, this effect was not at a scale that was detectable in this study. Thus, the effect of noise from trains is apparently insignificant at current rates of train traffic in the Park.
Small mammals did not respond to the edge
We found no clear influence of edge effects on the group of mammals studied, which were typical of well-preserved Atlantic Forest (Cerboncini et al., Reference Cerboncini, Rubio, Bernardi, Braga, Roper and Passos2014). Capture probability of the most common mammal in the study, the russet rice rat Euryoryzomys russatus, was independent of all edge characteristics. A few uncommon species were captured only near the edge (Fig. 5) but are not thought to be associated with edges or disturbances. Spiny rats Trinomys spp. are typical in well-conserved coastal Atlantic Forest (Vieira et al., Reference Vieira, Olifiers, Delciellos, Antunes, Bernardo, Grelle and Cerqueira2009), and Ihering's spiny rat Trinomys iheringi was found in the Park at the limits of its distribution (Cerboncini et al., Reference Cerboncini, Rubio, Bernardi, Braga, Roper and Passos2014). Similarly, the South American water rat Nectomys squamipes is often found in forest patches with riparian areas, regardless of the size or quality of the fragment (Pires et al., Reference Pires, Lira, Fernandez, Schittini and Oliveira2002; Viveiros de Castro & Fernandez, Reference Viveiros de Castro and Fernandez2004). The Brazilian gracile mouse opossum Gracilinanus microtarsus is a habitat generalist, as long as forest is available (Pardini et al., Reference Pardini, de Souza, Braga-Neto and Metzger2005), and therefore the fact that this species was captured only near the edge does not suggest an edge effect (i.e. changes in community near edges), given the small number of captures. Anthropogenic, permanent linear clearings such as train rights of way may be unsuitable, and even dangerous, for wildlife but appear to have little effect in the nearby forest.
The lack of correlation between the body condition of small mammals and distance from the edge also suggest little or no edge effect. Body condition is important for survival and reproduction (Millar & Hickling, Reference Millar and Hickling1990; Virgl & Messier, Reference Virgl and Messier1992; Zuercher et al., Reference Zuercher, Roby and Rexstad1999; Schulte-Hostedde et al., Reference Schulte-Hostedde, Millar and Hickling2001), and in female mammals is often associated with reproductive success (Atkinson & Ramsay, Reference Atkinson and Ramsay1995; Dobson & Michener, Reference Dobson and Michener1995; Wauters & Dhondt, Reference Wauters and Dhondt1995). The absence of an effect suggests there is no stress associated with the edge or associated noises. In contrast, a study in the Caribbean found the body condition of female rodents declined near roads (Fuentes-Montemayor et al., Reference Fuentes-Montemayor, Cuarón, Vázquez-Domínguez, Benítez-Malvido, Valenzuela-Galván and Andresen2009). Further study to determine which factors are detrimental to rodents near edges is necessary to compare the impacts of various types of edges.
Railway resources
Increased availability of resources may be one of the main reasons for increased diversity near edges (McCollin, Reference McCollin1998, Ries & Sisk, Reference Ries and Sisk2004; Ries et al., Reference Ries, Fletcher, Battin and Sisk2004). Food is often superabundant as a result of grain falling from trains but abundance of this resource had no measurable impact on small mammals. Although the number of small mammal species captured was greater near the edge, this is probably not a typical edge effect but a consequence of minor changes in the forest near the edge.
Rights of way and wildlife conservation
In Brazil roads are used more often than railways for commercial transportation. However, railways appear to have less impact on wildlife than do roads and highways. The construction of roads is usually followed by uncontrolled immigration and logging, hunting, agriculture and land speculation, which are the main causes of the destruction of tropical forests (Young, Reference Young1994; Laurance et al., Reference Laurance, Albernaz, Fearnside, Vasconcelos and Ferreira2004; Fearnside, Reference Fearnside2007). Increasing the use of railways for transport could be a useful conservation strategy, especially where it is necessary to transport agricultural or industrial products through important habitats.
Environmental edge effects in this landscape were minimal, with no measurable consequences for small mammals, except for infrequent encounters with some species only at the edge. It is important to note that the Paranaguá–Curitiba railway is > 100 years old and some species may have had sufficient time to adapt to the different environmental conditions created by the right of way. Whether newly constructed railways produce more intense edge effects remains to be investigated.
Acknowledgements
We thank the following for their invaluable help in the field: Talita V. Braga, Marcelo B. G. Rubio, Itiberê P. Bernardi, Jennifer de S. B. Pereira, Viviane Mottin, Bruna C. R. de Jesus, Luiz H. Varzinczak, Felipe L. S. Shibuya, Fernanda F. C. de Lima and Jaqueline P. Duarte. Our research was permitted by Instituto Ambiental do Paraná and the Park Manager Lothário H. Stoltz Jr, who also provided transportation and accommodation at the study site. Instituto Chico Mendes de Conservação da Natureza provided permits to capture and collect small mammals. Marcelo Passamani and Maurício O. Moura provided valuable suggestions for this article. A scholarship was granted to RASC by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, and the Conselho Nacional de Desenvolvimento Científico e Tecnológico provided support for FCP (303757/2012-4) and JJR (306963/2012-4).
Biographical sketches
Ricardo Cerboncini is interested in ecology, conservation and evolution of biodiversity. His research focuses on the ecology of rodents and marsupials in human-altered landscapes, and on the evolution of behavioural traits, especially breeding systems and parental care of birds. James Roper is interested in a broad variety of ecological and evolutionary questions, especially those related to population dynamics. Most of his research includes analysis of reproductive success and survival of terrestrial vertebrates. Fernando Passos works in wildlife biology and conservation. His research includes small and large mammals, and he has developed most of his studies based on the bats and primates of the Atlantic Forest.








