Rheumatoid arthritis (RA) is an imperative, chronic, autoimmune and inflammatory disease of indistinct origin with its greatest impact on the joints of the body( Reference Aletaha, Martinez-Avila and Kvien 1 ). Recent evidence suggests that subjects with RA have significant changes in intestinal microbiota compared with healthy subjects( Reference Diamanti, Manuela Rosado and Lagana 2 ). In addition, RA subjects show a significant reduction in the quantity of Bifidobacterium species and lactic acid bacteria( Reference Abhari, Shekarforoush and Hosseinzadeh 3 ). Average homoeostatic model of assessment for insulin resistance (HOMA-IR) levels in patients with RA were reported to be 31 % higher than that in the healthy population( Reference Giles, Danielides and Szklo 4 ). Impaired insulin metabolism, increased indices of inflammation and oxidative stress play a significant role in the pathogenesis of RA( Reference Maruotti, d’Onofrio and Cantatore 5 – Reference Geronikaki and Gavalas 7 ), which in turn would result in an increased risk of fatal cardiovascular events by 50 %( Reference Avina-Zubieta, Choi and Sadatsafavi 8 ) and type 2 diabetes mellitus (T2DM) by 68 % in men and 46 % in women( Reference Su, Chen and Young 9 ).
Few studies have previously evaluated the effects of probiotic supplementation on clinical and metabolic parameters in RA subjects with conflicting findings. Our study among RA subjects has shown that probiotic administration for 8 weeks had beneficial effects on clinical symptoms, serum insulin and high-sensitivity C-reactive protein (hs-CRP) values, but did not influence insulin resistance and sensitivity, lipid profiles and other parameters of inflammation and oxidative stress( Reference Zamani, Golkar and Farshbaf 10 ). Furthermore, in an animal study, intake of probiotic Bacillus coagulans plus prebiotic inulin significantly improved the biochemical and clinical parameters of induced RA( Reference Abhari, Shekarforoush and Hosseinzadeh 3 ). Likewise, few animal studies in RA have demonstrated that treatment with probiotics was associated with decreased arthritic severity through reduced gut permeability( Reference Kano, Kaneko and Kaminogawa 11 , Reference Strowski and Wiedenmann 12 ). Synbiotics refer to nutritional supplements combining probiotics and prebiotics in a form of synergism( Reference de Vrese and Schrezenmeir 13 ). Previous studies have demonstrated that the synergistic effects of synbiotic supplementation on the intestinal and faecal microflora and immune system are significantly greater than the effects of either prebiotic or probiotic supplementation alone( Reference Frece, Kos and Svetec 14 , Reference Worthley, Le Leu and Whitehall 15 ). Synbiotic supplementation for 8 weeks among patients with T2DM also decreased inflammatory factors( Reference Akram, Tofighiyan and Rakhshani 16 ). In addition, synbiotic supplementation for 28 weeks among patients with the metabolic syndrome resulted in statistically significant improvements in insulin resistance indices, TAG, total- and HDL-cholesterol levels, whereas LDL-cholesterol levels remained unchanged( Reference Eslamparast, Zamani and Hekmatdoost 17 ).
Synbiotic supplementation might improve glucose metabolism, lipid profiles and inflammatory factors through the modification of gut flora, the reduction of endotoxin levels, elevation of faecal pH via the production of SCFA( Reference Compare, Coccoli and Rocco 18 ) and the reduction of pro-inflammatory cytokine production( Reference Li, Yang and Lin 19 ). Given the anti-inflammatory effects of synbiotics, we hypothesised that synbiotic supplementation might help RA patients to control their clinical signs, biomarkers of inflammation and oxidative stress, and insulin resistance. This research was, therefore, performed to determine the effects of synbiotic supplementation on clinical and metabolic parameters in patients with RA.
Methods
Participants
This study, registered in the Iranian website (www.irct.ir) for registration of clinical trials (http://www.irct.ir: IRCT201611165623N94), was a randomised double-blind clinical trial that was conducted among fifty-four patients with RA referred to the Shahid Beheshti Hospital in Kashan, Iran, according to the 1987 American College of Rheumatology criteria( Reference Reneses, Pestana and Garcia 20 ), diagnosed at least 6 months ago with moderate and severe disease activity (disease activity score-28 joints: DAS-28>3·2), aged 25–70 years from September 2016 to December 2016. Disease activity was evaluated on the basis of DAS-28( Reference Pereira, McCartney and Gibson 21 ). Patients who have chronic renal failure, pregnant or lactating women, symptoms or personal history of CVD, diabetes mellitus, the consumption of antihyperglycaemic agents including metformin, patients unlikely to come for follow-up in the following 3 months and patients who are unable to read numbers and/or unable to mark the pain scale, taking probiotics, synbiotics, antioxidant and/or anti-inflammatory supplements such as vitamin E, vitamin C and n-3, and taking antibiotics were excluded from the study. The study protocol was approved by the research ethics committee of Kashan University of Medical Sciences (KUMS) (reference no. IR.Kaums.REC.1395·46) and informed consent was obtained from all subjects.
Study design
At first, participants were randomised into two groups for the intake of either synbiotic supplements (n 27) or the placebo (n 27) for 8 weeks. In the treatment group, participants received a synbiotic capsule containing Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum (2×109 colony-forming units/g each) plus 800 mg inulin. Because of the lack of evidence about the appropriate dosage of probiotics and inulin for RA patients, we used the above-mentioned doses of probiotics and inulin based on observed beneficial effects on markers of insulin metabolism in gestational diabetes (GDM) women( Reference Ahmadi, Jamilian and Tajabadi-Ebrahimi 22 ) and observed beneficial effects of probiotics on hs-CRP in patients with RA( Reference Zamani, Golkar and Farshbaf 10 ). In the current study, the duration of the intervention was selected on the basis of the observed beneficial effects of probiotic supplementation on inflammatory factors in patients with RA( Reference Zamani, Golkar and Farshbaf 10 , Reference Vaghef-Mehrabany, Alipour and Homayouni-Rad 23 ). Participants in the placebo group received a placebo containing starch but no bacteria. The appearance of the placebo was indistinguishable in colour, shape, size, packaging, smell and taste from the synbiotic capsule. Synbiotic supplements and placebos were produced by Tak Gen Zist Pharmaceutical Company. Quality control of synbiotic supplements was conducted in the laboratory of the Food and Drug Administration in Tehran. Randomisation assignment was performed using computer-generated random numbers. Randomisation and allocation were concealed from the researchers and patients until the final analyses were completed. The randomised allocation sequence, enrollment of patients and allocation to interventions were conducted by trained staff. Patients were requested not to change their routine physical activity, and not to take any supplements that might affect their nutritional status during the 8-week treatment. A 3-d food record and physical activity records were completed by all participants at baseline and at weeks 2, 5 and 8 of intervention. The dietary records were based on estimated values in household measurements. To obtain macronutrient and micronutrient intakes of participants based on these 3-d food diaries, we used Nutritionist IV software (First Databank) modified for Iranian foods. Physical activity was described as metabolic equivalents (MET) (h/d)( Reference Ainsworth, Haskell and Whitt 24 ). To determine the MET for each patient, we multiplied the time (h/d) reported for each physical activity by its related MET coefficient using standard tables( Reference Ainsworth, Haskell and Whitt 24 ). Compliance with the synbiotic intake was evaluated by asking patients to bring the medication containers.
Assessment of anthropometric measurements
Weight and height (Seca) were determined before and after the 8-week treatment in a fasting state, without shoes and in minimal clothing, by a trained staff member. BMI was calculated using the height and weight measurements (weight (kg)/(height (m))2).
Assessment of outcomes
The primary outcome end-points were inflammatory factors and DAS-28 in the current study. The secondary outcome end-points were insulin metabolism, lipid concentrations and biomarkers of oxidative stress.
Clinical assessment
At baseline and after the 8-week intervention, we collected data on: the number of tender and swollen joints on the basis of the twenty-eight-joint count, visual analogue scales (VAS) (0–100 mm) for pain and DAS-28. All clinical assessments were conducted blindly by a single experienced clinician.
Biochemical assessment
The 12-h fasting blood samples were obtained from participants at baseline and at week 8 of the treatment at the Kashan reference laboratory. The samples were stored at –80°C until analysis at the KUMS reference laboratory. Serum hs-CRP values were quantified by the use of a commercial ELISA kit (LDN) with a limit of detection (LoD) of 10 ng/ml, and with intra- and inter-assay CV 3·7 and 5·6 %, respectively. Plasma nitric oxide (NO) was determined by the Griess method( Reference Tatsch, Bochi and Pereira Rda 25 ). To quantify fasting plasma glucose (FPG), serum TAG, VLDL-, total-, LDL- and HDL-cholesterol values, we used available kits (Pars Azmun) with inter- and intra-assay CV <5 %. Serum insulin was assessed using an ELISA kit (Monobind) with an LoD of 0·114 µIU/ml, and the intra- and inter-assay CV 3·0 and 4·6 %, respectively. HOMA-IR, homoeostatic model assessment for β-cell function (HOMA-B) and the quantitative insulin sensitivity check index (QUICKI) were calculated according to suggested formulas( Reference Pisprasert, Ingram and Lopez-Davila 26 ). Plasma total antioxidant capacity (TAC) using the ferric reducing antioxidant power method developed by Benzie & Strain( Reference Benzie and Strain 27 ), GSH by the method of Beutler & Gelbart( Reference Beutler and Gelbart 28 ) and malondialdehyde (MDA) values using the thiobarbituric acid-reactive substance method( Reference Janero 29 ) were evaluated. All inter- and intra-assay CV for NO, TAC, GSH and MDA were <5 %. Measurements of lipid concentrations, insulin, biomarkers of inflammation and oxidative stress were performed in a blinded fashion, in duplicate, in pairs (pre-intervention/post-intervention) at the same time.
Statistical methods and sample size
Normal distribution of variables was assessed by histogram and the Kolmogorov–Smirnov test. The analyses were conducted in all randomised subjects according to the intention-to-treat (ITT) principle. Missing values were treated based on last-observation-carried-forward method (LOCF)( Reference Lachin 30 ). LOCF ignores whether the participant’s condition was improving or deteriorating at the time of dropout and instead freezes outcomes at the value observed before dropout (i.e. last observation). To establish changes in the general characteristics and daily dietary macronutrient and micronutrient intakes between the two groups, we used independent samples Student’s t test. Pearson’s χ 2 test was used for comparison of categorical variables. To evaluate the effects of synbiotic administration on insulin metabolism, lipid concentrations, biomarkers of inflammation and oxidative stress, we used one-way repeated measures ANOVA. The paired-samples t test was used to detect within-group differences. To assess confounders, we adjusted all analyses for baseline values, age and baseline BMI with the ANCOVA test. These analyses were also performed using ANCOVA. P<0·05 was considered statistically significant. All statistical analyses were conducted using the Statistical Package for Social Science version 17 (SPSS Inc.).
To calculate the sample size, we used the standard formula suggested for parallel clinical trials by considering type one error (α) of 0·05 and type two error (β) of 0·20 (power=80 %). We did not find a similar study about synbiotic supplementation in RA patients for determining the sample size based on main outcome (hs-CRP); therefore, the sample size was calculated based on synbiotic supplementation in pregnant women. Based on a previous study( Reference Taghizadeh and Asemi 31 ), we used an sd of 1581·6 ng/ml and a difference in mean of 13 100 ng/ml, considering hs-CRP as the key variable. Based on this, we needed twenty-three persons in each group. Assuming 20 % dropouts in each group, the final sample size was determined to be twenty-seven persons per group. Hs-CRP was used to estimate sample size because it is the most important variable in patients with RA. Furthermore, the largest sample size was obtained when we used this variable.
Results
At first, we invited sixty-five participants with RA; however, eleven subjects were excluded from the study as they did not meet the inclusion criteria (Fig. 1). During the intervention phase of the study, two patients were excluded in both the groups (withdrawn because of personal reasons (n 2)). However, as the analysis was conducted based on the ITT principle, all fifty-four patients with RA were included in the final analysis. On average, the rate of compliance in this study was high, such that >90 % of capsules were consumed throughout the study in both groups. No side effects were reported following intake of the synbiotic in RA patients throughout the study.

Fig. 1 Summary of patient flow.
Distributions of sex, mean duration of RA, age, height, weight and BMI at baseline and after the 8-week intervention of the participants were not significantly different between the synbiotic and placebo groups (Table 1).
Table 1 General characteristics of study participants (Mean values and standard deviations; numbers and percentages)

RA, rheumatoid arthritis
* Obtained from independent t test.
† Pearson’s χ 2 test.
Comparison of dietary intakes of the study participants throughout the study revealed no significant changes in macronutrient and micronutrient intakes between the two groups (Table 2).
Table 2 Dietary intakes of study participants throughout the study (Mean values and standard deviations)
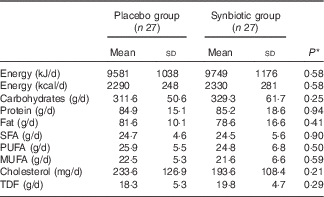
TDF, total dietary fibre.
* Obtained from independent t test.
After the 8-week intervention, compared with the placebo, synbiotic supplementation resulted in a significant reduction in serum hs-CRP levels (–1427·8 (sd 3267·2) v. +2833·4 (sd 5639·7) ng/ml, P=0·001). In addition, compared with the placebo, synbiotic supplementation improved DAS-28 (–1·6 (sd 0·8) v. –0·3 (sd 0·5), P<0·001) and VAS pain (–30·4 (sd 18·7) v. –11·5 (sd 15·9), P<0·001) (Table 3). In addition, a significant elevation in plasma NO levels (+0·8 (sd 4·4) v. –2·6 (sd 4·5) µmol/l, P=0·008), and significant reductions in insulin values (–13·8 (sd 26·4) v. +4·2 (sd 28·2) pmol/l, P=0·01), HOMA-IR (–0·5 (sd 1·0) v. +0·1 (sd 1·1), P=0·03) and HOMA-B (–9·4 (sd 17·9) v. +3·3 (sd 18·9), P=0·01) were observed following supplementation with synbiotic compared with those following the placebo. Compared with the placebo, synbiotic supplementation also resulted a significant increase in plasma GSH (+36·6 (sd 63·5) v. –58·5 (sd 154·4) µmol/l, P=0·005). Patients who received the synbiotic experienced borderline statistically significant improvement in plasma MDA (P=0·07) compared with the placebo. We did not observe any significant effect on other glucose homoeostasis parameters, lipid profiles and other biomarkers of oxidative stress after synbiotic administration.
Table 3 Disease activity score-28 joints (DAS-28) and metabolic status at baseline and after the 8-week intervention in patients with rheumatoid arthritis that received either synbiotic supplements or placebo (Mean values and standard deviations)

hs-CRP, high-sensitivity C-reactive protein; VAS, visual analogue scales; FPG, fasting plasma glucose; HOMA-IR, homoeostasis model of assessment-estimated insulin resistance; HOMA-B, homoeostasis model of assessment-estimated B cell function; QUICKI, quantitative insulin sensitivity check index; TAC, total antioxidant capacity; MDA, malondialdehyde.
* Obtained from paired-samples t test.
† Obtained from repeated measures ANOVA test.
Baseline levels of plasma NO (P<0·001) and DAS-28 (P=0·004) were significantly different between the two groups. Therefore, we adjusted the analyses for the baseline values of biochemical parameters, age and baseline BMI. When we adjusted the analysis for baseline values of biochemical parameters, age and baseline BMI, plasma NO levels (P=0·17) became non-significant, whereas serum LDL-cholesterol (P=0·02) became statistically significant, and other findings did not alter (Table 4).
Table 4 Adjusted changes in metabolic variables in patients with rheumatoid arthritis that received either synbiotic supplements or placebo (Mean values with their standard errors)

hs-CRP, high-sensitivity C-reactive protein; DAS-28, disease activity score-28 joints; VAS, visual analogue scales; FPG, fasting plasma glucose; HOMA-IR, homoeostasis model of assessment-estimated insulin resistance; HOMA-B, homoeostasis model of assessment-estimated B cell function; QUICKI, quantitative insulin sensitivity check index; TAC, total antioxidant capacity; MDA, malondialdehyde.
* Obtained from ANCOVA adjusted for baseline values+age and baseline BMI.
Discussion
In this research, we assessed the effects of synbiotic supplementation on clinical and metabolic parameters in patients with RA. We found that synbiotic supplementation for 8 weeks among patients with RA had beneficial effects on hs-CRP, DAS-28, VAS, NO, insulin levels, HOMA-IR, HOMA-B and GSH levels; however, it did not affect other glucose homoeostasis parameters, lipid profiles and other biomarkers of oxidative stress. However, when the analysis was conducted without the ITT approach, no significant changes were seen in our findings.
In unadjusted analyses, the current study indicated that synbiotic supplementation for 8 weeks among subjects with RA had beneficial effects on serum hs-CRP, DAS-28 and plasma NO levels compared with the placebo. When we adjusted the analyses for baseline values of biochemical variables, age and baseline BMI, the change in plasma NO levels was not significantly different between the groups. Data on the effects of synbiotic supplementation in RA subjects are limited. We have previously shown that probiotic intake for 8 weeks among RA subjects improved DAS-28 and serum hs-CRP values, but did not influence plasma NO levels( Reference Zamani, Golkar and Farshbaf 10 ). In addition, supporting our findings, supplementation with L. casei among patients with RA for 8 weeks resulted in a significant decrease in VAS( Reference Vaghef-Mehrabany, Alipour and Homayouni-Rad 23 ). In an animal study, B. coagulans plus inulin significantly improved inflammatory factors and clinical parameters in induced RA( Reference Abhari, Shekarforoush and Hosseinzadeh 3 ). Furthermore, few animal studies reported that probiotics including Lactobacillus GG and L. casei had anti-RA effects( Reference Baharav, Mor and Halpern 32 , Reference So, Kwon and Lee 33 ). In another study, supplementation with synbiotics in adults with non-alcoholic fatty liver disease over 28 weeks reduced inflammatory factors( Reference Eslamparast, Poustchi and Zamani 34 ). However, the intake of synbiotic supplements did not affect serum CRP levels among men with a low serum enterolactone concentration after 6 weeks( Reference Holma, Kekkonen and Hatakka 35 ). Pineda et al.( Reference Pineda Mde, Thompson and Summers 36 ) observed that probiotic supplementation among patients with active RA for 30 d had no significant effect on inflammatory cytokines and the Health Assessment Questionnaire. The exact mechanisms of the beneficial effects of synbiotics on clinical symptoms and inflammatory factors in RA patients are unknown. The up-regulation of IL-18 protein expression produced by SCFA( Reference Kalina, Koyama and Hosoda 37 ) and increased production of the methylketone family in the gut following supplementation with synbiotics( Reference Vitali, Ndagijimana and Cruciani 38 ) might result in its anti-inflammatory effects. SCFA may lower serum hs-CRP levels through blocking the enzymatic synthesis of hepatic CRP. CRP is synthesised by the liver in response to releasing factors by fat cells such as IL-6( Reference Kinoshita, Onoda and Imai 39 ). In a study by Hegazy & El-Bedewy( Reference Hegazy and El-Bedewy 40 ) it was observed that the consumption of probiotics by patients with ulcerative colitis for 8 weeks significantly ameliorated the inflammation by decreasing concentrations of IL-6, expression of TNF-α and NF-κB. Likely, decreasing concentrations of IL-6 indirectly cause a decreasing production of CRP.
In unadjusted analyses, we found that synbiotic administration for 8 weeks in patients with RA decreased serum insulin concentrations, HOMA-IR and HOMA-B compared with the placebo, whereas it did not affect FPG, QUICKI and lipid concentrations. When we adjusted the analyses for baseline values of biochemical variables, age and baseline BMI, a significant change in serum total cholesterol was observed. Some researchers have documented the beneficial effects of synbiotic supplementation on insulin metabolism and lipid profiles among patients without RA in former studies. We have previously demonstrated that synbiotic administration for 6 weeks among subjects with GDM had favourable effects on insulin metabolism, TAG and VLDL-cholesterol concentrations, but did not affect FPG and other lipid fractions( Reference Ahmadi, Jamilian and Tajabadi-Ebrahimi 22 ). Moreover, insulin resistance was significantly improved following the intake of synbiotics among subjects with the metabolic syndrome after 28 weeks of treatment, but unchanged lipid profiles( Reference Eslamparast, Zamani and Hekmatdoost 17 ). The same findings were seen by others after the consumption of synbiotic supplements among patients with non-alcoholic steatohepatitis for 24 weeks( Reference Malaguarnera, Vacante and Antic 41 ) and in high-fructose-fed rats( Reference Yadav, Jain and Sinha 42 ). However, Schaafsma et al.( Reference Schaafsma, Meuling and van Dokkum 43 ) found that synbiotic administration in male volunteers for 3 weeks significantly reduced the total-, LDL- and LDL-/HDL-cholesterol, whereas it did not affect TAG levels. The absence of beneficial effects of synbiotics on lipid profiles in our study compared with that in other studies may be mediated by different study designs, different dosages of used probiotic and inulin, types and quality of used probiotic bacteria and inulin as well as duration of the intervention. Prior studies have reported that insulin resistance( Reference Chung, Oeser and Solus 44 , Reference Dessein and Joffe 45 ) and oxidative stress( Reference Nakajima, Aoki and Shibata 46 ), independently, may impair disease activity in subjects with RA. Therefore, synbiotics, because of their improving effects on insulin metabolism, anti-inflammatory and anti-oxidative actions, may be useful to decrease complications in subjects with RA. Synbiotics might improve insulin metabolism through the modification of gut flora and elevation of faecal pH( Reference Compare, Coccoli and Rocco 18 ), decreased production of pro-inflammatory cytokines( Reference Li, Yang and Lin 19 ) and modulating NF-κB( Reference Grimoud, Durand and de Souza 47 ).
This study demonstrated that taking synbiotic supplements for 8 weeks among subjects with RA significantly increased plasma GSH levels, but did not affect other biomarkers of oxidative stress compared with the placebo. In agreement with our findings, no significant effect on MDA, TAC, superoxide dismutase, glutathione peroxidase, and catalase activities following supplementation with L. casei among patients with RA for 8 weeks was observed( Reference Vaghef-Mehrabany, Homayouni-Rad and Alipour 48 ). Probiotic intake for 12 weeks among pregnant women increased plasma GSH and TAC, but did not affect MDA values( Reference Jamilian, Bahmani and Vahedpoor 49 ). Likewise, we have previously shown that synbiotic food consumption for 9 weeks among pregnant women resulted in a significant elevation in plasma GSH concentrations( Reference Taghizadeh, Hashemi and Shakeri 50 ). Probiotic administration among subjects with major depressive disorder for 8 weeks increased GSH values, but did not influence plasma TAC values( Reference Akkasheh, Kashani-Poor and Tajadadi-Ebrahimi 51 ). Synbiotic intake may decrease oxidative stress through improved inflammatory factors resulting from the production of SCFA in the colon( Reference Sadrzadeh-Yeganeh, Elmadfa and Djazayery 52 ), increased generation of NO( Reference Sobko, Huang and Midtvedt 53 ), and its impact on decreased biomarkers including oxidised LDL, 8-isoprostanes and GSH ratio( Reference Kullisaar, Songisepp and Mikelsaar 54 ).
This research had some limitations. Because of funding limitations, we did not assess the compliance through quantification of faecal-bacteria loads and SCFA. Moreover, further studies are needed to evaluate gene expression related to inflammatory markers and insulin to explore the plausible mechanism and confirm our findings.
Overall, our study demonstrated that synbiotic supplementation for 8 weeks among patients with RA had beneficial effects on hs-CRP, DAS-28, VAS, NO, insulin levels, HOMA-IR, HOMA-B and GSH levels; however, it did not affect other glucose homoeostasis parameters, lipid profiles and other biomarkers of oxidative stress.
Acknowledgements
The present study was supported by a grant from the Vice-chancellor for Research, KUMS and Iran.
Z. A. contributed in conception, design, statistical analysis and drafting of the manuscript. B. Z., S. F., H. R. G. and F. B. contributed to data collection and manuscript drafting. The final version was confirmed by all authors for submission.
None of the authors has any conflicts of interest to declare.








