Introduction
Staphylococcus aureus is an important cause of serious community- and health care–associated infections worldwide [Reference Zarb1] and one of the most frequent causative agents of bloodstream infections [Reference Camargo2, Reference Nagao3]. Although the proper management of S. aureus bacteraemia (SAB) has been associated with better outcomes [Reference Lopez-Cortes4, Reference Widmer, Lakatos and Frei5], related mortality rates remain inappropriately high [Reference Nagao3, Reference Kaasch6]. Proper mortality risk stratification for this disease may aid the identification of subjects who should receive more intensive cares to improve outcomes. For this purpose, several specific risk factors, such as the source of bacteraemia, have been associated with an increased risk of mortality among patients with SAB [Reference van Hal7]. In addition to these risk factors, comorbidity and the severity of the infection are important confounding factors that must be controlled for properly in observational studies for adequate analysis of mortality risk [Reference Lesens8, Reference Melzer and Welch9]. Among comorbidity scores, the weighted Charlson comorbidity index (CCI) is used widely to assess the presence of chronic diseases; it has been validated extensively and demonstrated to reduce potential confounding in epidemiological research [Reference McGregor10, Reference Schneeweiss11]. However, due to advances in the management of chronic diseases in the past three decades, since the development of the CCI, the relationships of several conditions included in the classical CCI (cCCI) with mortality have become obsolete. Recently, Quan et al. [Reference Quan12] updated the conditions and weights included in the cCCI, simplifying the score and validating an updated CCI (uCCI) for the prediction of 1-year mortality after hospital discharge.
The use of uCCI may, therefore, simplify the comorbidity adjustment in analyses of risk factors for SAB mortality. The aim of this study was to compare the capacity of the uCCI compared with cCCI to predict 30-day mortality in patients with SAB.
Methods
This observational study was conducted at the University Hospital of Salamanca, Spain, a tertiary care centre serving a population of 400 000. All cases of SAB in patients aged ⩾14 years identified in the Microbiology Unit of our institution between August 2010 and April 2015 were included prospectively and followed. Clinical, microbiological and outcome information were recorded. The protocol was approved by the Ethics Committee of the University Hospital of Salamanca and patients gave written informed consent for inclusion.
Definition of terms
Clinically significant SAB was defined as the isolation of S. aureus from one or more peripheral venous blood-culture samples collected from a patient with associated relevant symptoms and signs of systemic infection [Reference Fowler13, Reference Naber14]. Primary bacteraemia was considered when the focus was unknown. All cases with identified foci were classified as secondary bacteraemia. Blood culture positivity for S. aureus after 72 h of appropriate antibiotic treatment was considered to represent persistent bacteraemia [Reference Fowler15]. Metastatic infection was defined as a confirmed S. aureus infection remote from the focus of bacteraemia that was not present at presentation [Reference Fowler15]. SAB was classified as nosocomial when it was identified ⩾48 h after admission; as health care related when a patient had been admitted for ⩾2 days in last 90 days, was on haemodialysis, was receiving intravenous treatment or wound care at home, or was residing in a nursing home; and as community-acquired in all other cases [Reference Friedman16]. Septic shock at presentation with SAB was defined by the presence of severe sepsis with hypotension that was not reversed by fluid resuscitation [Reference Dellinger17]. In case of repeated episodes of SAB for the same patient, we have only included the first episode of each hospital admission.
Comorbidity evaluation
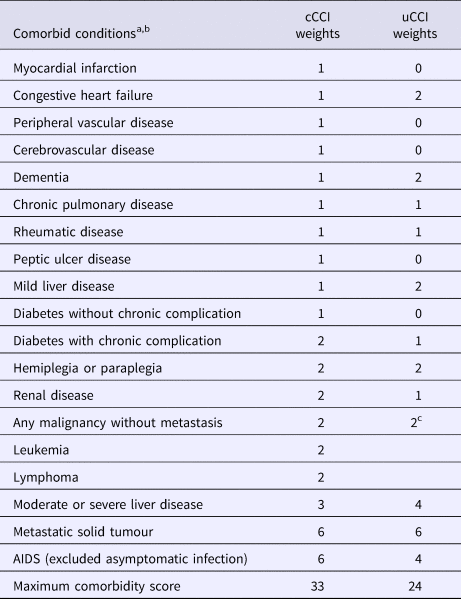
Comorbidity was evaluated with the cCCI [Reference Charlson18] and uCCI [Reference Quan12]. Both scores were registered at the presentation of SAB (Table 1); definitions of included comorbid diseases are listed in Supplementary Table S1 (available on the Cambridge Core website).
Table 1. Classical and updated Charlson comorbidity index weights
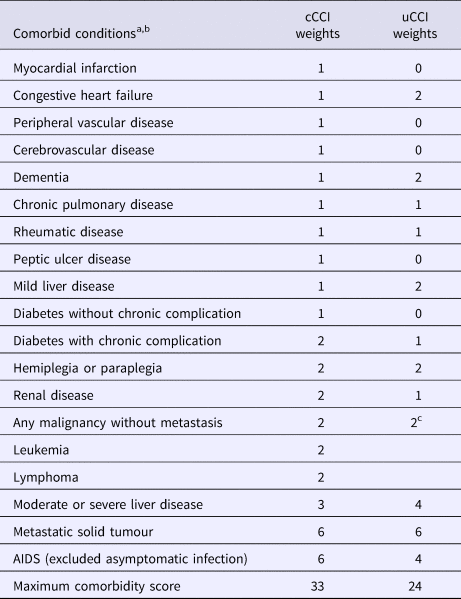
cCCI, classical Charlson comorbidity index; uCCI, updated Charlson comorbidity index. AIDS,acquired immunodeficiency syndrome.
a Definition of conditions are listed in Supplementary Table S1 (available on the Cambridge Core website).
b To calculate CCI the following comorbid conditions were mutually exclusive: diabetes with chronic complications and diabetes without chronic complications; mild liver disease and moderate or severe liver disease; and any malignancy and metastatic solid tumour.
c Including leukemia and lymphoma.
Microbiological methods
SAB was detected using the BACTEC 9240 automated blood culture system (Becton Dickinson, Franklin Lakes, NJ, USA). When the blood culture incubator device recorded blood culture positivity, a Gram stain was performed to check the growing microorganism. After confirming the presence of Gram-positive cocci by stain, the blood culture was plated onto blood agar, MacConkey agar and chocolate plates. Bacterial identification was performed directly from the blood culture with an automated system (Wider; Francisco Soria Melguizo, Madrid, Spain), based on biochemical and enzymatic tests. After 18–24 h of incubation, isolated colonies were subjected to catalase and coagulase tests (both were required to be positive for identifying S. aureus) and the identification was verified by MALDI-TOF mass spectrometry (Bruker Daltonics, Leipzig, Germany). Scores ⩾2 were considered to be reliable results.
Susceptibility testing was carried out using the broth microdilution test and disk diffusion method, according to the guidelines of the Clinical and Laboratory Standards Institute [Reference Wayne19]. The minimum inhibitory concentration of vancomycin was confirmed by E-test. Rapid detection of methicillin resistance (mec-A gene) was assessed by polymerase chain reaction (GeneXpert; Cepheid, Maurens Scopont, France).
Statistical analysis
Continuous variables are expressed as median (interquartile range) and categorical variables are expressed as frequencies (percentages). Univariate logistic regression and the χ 2 test were used to compare continuous and categorical variables, respectively. Predictors of 30-day mortality in the entire study population were assessed by logistic regression analysis. Relevant variables associated previously and consistently with SAB-related mortality and biologically plausible variables that had a potential statistical relationship with mortality (P values ⩽0.20 in univariate analyses) were entered into a multivariate logistic regression model (age, origin of bacteraemia, Pitt bacteraemia score, pulmonary focus, appropriate empirical treatment, persistent bacteraemia and CCI; see Supplementary Table S2, available on the Cambridge Core website). This analysis included adjustment for severity using PBS and comorbidity using the CCI in two different models (as a continuous variable and as a dichotomised qualitative variable (scores ⩽2 and ⩾3) [Reference Lesens8, Reference Cheong20, Reference Lu21]) or the uCCI in two different models (as a continuous variable and as a dichotomised variable (scores ⩽3 and ⩾4)). Variables with potential collinearity with PBS or CCI were not included in the model (e.g., shock, respiratory distress syndrome or comorbid conditions). Given the lack of previous studies, we performed simulations to establish a cut-off point for the uCCI that would best stratify the study population in terms of the probability of death within 30 days. After analysing the relationship between mortality and dichotomised uCCI using different cut-off points (2, 3 and 4), a cut-off value of ⩾4 was selected. The cCCI and uCCI were forced into the final models, regardless of their significance in univariate analysis. The Pitt bacteremia score (PBS) was dichotomised (⩽1 and ⩾2) and was used to adjust for disease severity. Associations are shown as odds ratios (ORs) with 95% confidence intervals (CIs). Global model fit, model calibration and predictive validity were evaluated using the Akaike information criterion (AIC) [Reference Akaike22], the Hosmer Lemeshow goodness-of-fit test [Reference Lemeshow and Hosmer23] and the area under the receiver operating characteristic (ROC) curve (95% CI), respectively. P values ⩽0.05 were considered to be significant. The SPSS software (version 20, IBM Corporation, Armonk, NY, USA) was used for statistical analysis.
Results
Descriptive results
In total, 257 episodes of SAB occurring in 239 patients during the study period were included in the analyses (Table 2). The median patient age was 78.2 (16.2) years and 167 (65%) patients were male. One hundred and three (40%) episodes of SAB were community acquired, 72 (28%) were health- care related and 82 (32%) were hospital acquired. Previous comorbid histories were remarkable for diabetes mellitus in 90 (35%) patients, congestive heart failure in 74 (29%) patients, oncologic disease in 71 (28%) patients and chronic kidney disease in 67 (26%) patients. The median cCCI score was 3 (3) and the median uCCI score was 2 (4); 161 (63%) cases had cCCI scores ⩾3 and 89 (35%) cases had uCCI scores ⩾4. The most common risk factors for SAB in this sample were a previous invasive procedure (n = 156 (61%)), the presence of any kind of vascular catheter (n = 148 (58%)), the presence of a urinary catheter (n = 106 (41%)), previous admission in the last 30 days (n = 83 (34%)), previous antibiotic treatment (n = 83 (32%)) and the presence of a central venous catheter (CVC; n = 77 (30%)). The focus of SAB remained unknown in 69 (27%) cases. The most frequently identified foci of SAB were pulmonary (n = 46 (18%)), vascular catheters (n = 40 (16%)), non–catheter-related endovascular (n = 37 (14%)) and cutaneous (n = 30 (12%)). SAB was associated at presentation with shock in 64 (25%) cases, acute respiratory distress syndrome (ARDS) in 49 (19%) cases and intravascular disseminated coagulation in 36 (14%) cases. The median PBS was 1 (4) and 100 (39%) cases had PBS >2. Seventy-nine isolates were methicillin-resistant S. aureus. According to the susceptibility of the isolates, 180 (70%) cases received appropriate empirical antibiotic treatment. During follow up, 33 (13%) cases were admitted to the intensive care unit, 28 (11%) cases required mechanical ventilation and 65 (25%) patients died within 30 days.
Table 2. Main characteristics of patients with Staphylococcus aureus bacteraemia according to 30-days mortality
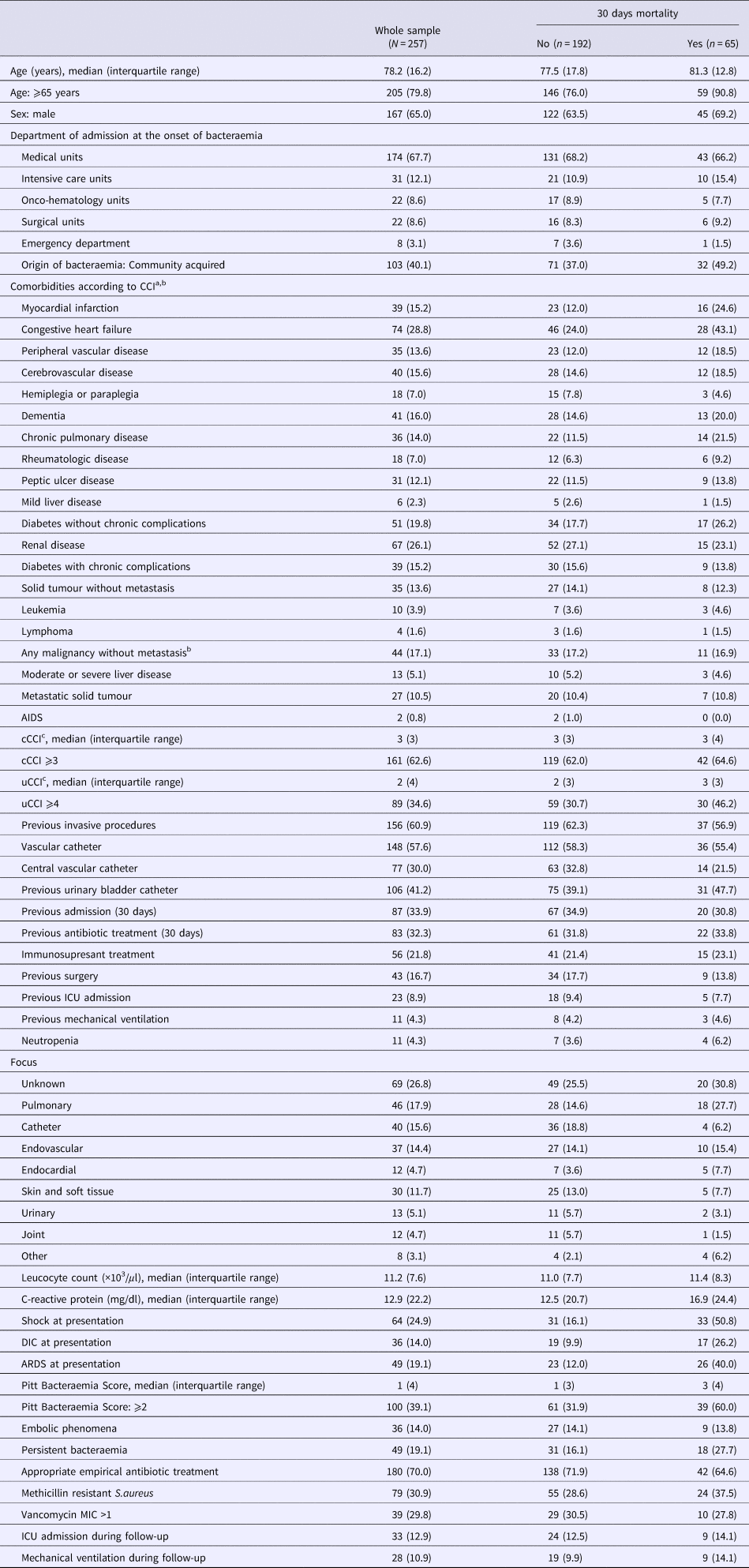
AIDS, acquired immunodeficiency syndrome. cCCI, classical Charlson comorbidity index; uCCI, updated Charlson comorbidity index. ICU, intensive care unit. DIC, disseminated intravascular coagulation. ARDS, acute respiratory distress syndrome. MIC, minimum inhibitory concentration.
a Comorbidities were defined according to comorbidity Charlson index criteria (see Supplementary Table S1, available on the Cambridge Core website).
b Including leukemia and lymphoma.
c To calculate CCI the following comorbid conditions were mutually exclusive: diabetes with chronic complications and diabetes without chronic complications; mild liver disease and moderate or severe liver disease; and any malignancy and metastatic solid tumour.
Differences according to 30-day mortality
Compared with survivors, patients who died within 30 days of SAB episodes were significantly older (median age 81.3 vs. 77.5 years) and significantly more of these patients had histories of myocardial infarction (25% vs. 12%), congestive heart failure (43% vs. 24%) and/or chronic pulmonary disease (22% vs. 12%) (Table 2 and Supplementary Table S3 for univariate analysis, available on the Cambridge Core website). Patients who died had higher mean cCCI and uCCI scores,and greater frequencies of cCCI scores ⩾3, but these differences were not significant. The frequency of uCCI score ⩾4 was significantly greater in this group than in survivors (46% vs. 31%). Patients who died were significantly more severely ill according to PBS ⩾2 points (60% vs. 32%); presented with significantly greater frequencies of shock (51% vs. 16%), ARDS (40% vs. 12%) and intravascular disseminated coagulation (26% vs. 10%); and had a significantly greater frequency of persistent bacteraemia (28% vs. 16%).
Relationships of classical and updated CCI scores with 30-day mortality
According to the cCCI, patients with score ⩾3 had significantly greater frequencies of non–community-acquired bacteraemia (67% vs. 48%), invasive procedures (66% vs. 53%), previous admission (39% vs. 25%), previous immunosuppressant treatment (26% vs. 15%) and, as expected, associated comorbidities (Table 3 and Supplementary Table S4 for univariate analysis, available on the Cambridge Core website). Crude 30-day mortality did not differ significantly between patients with cCCI scores ⩾3 and those with scores <3. According to the uCCI, patients with score ⩾4 had significantly greater frequencies of previous immunosuppressant treatment (32% vs. 17%), ARDS at presentation (26% vs. 16%) and, as expected, associated comorbidities. Crude 30-day mortality was significantly greater among patients with uCCI scores ⩾4 than among those with uCCI scores <4 (34% vs. 21%).
Table 3. Main characteristics of patients with Staphylococcus aureus bacteraemia according to categorised classical and updated Charlson Comorbidity Index
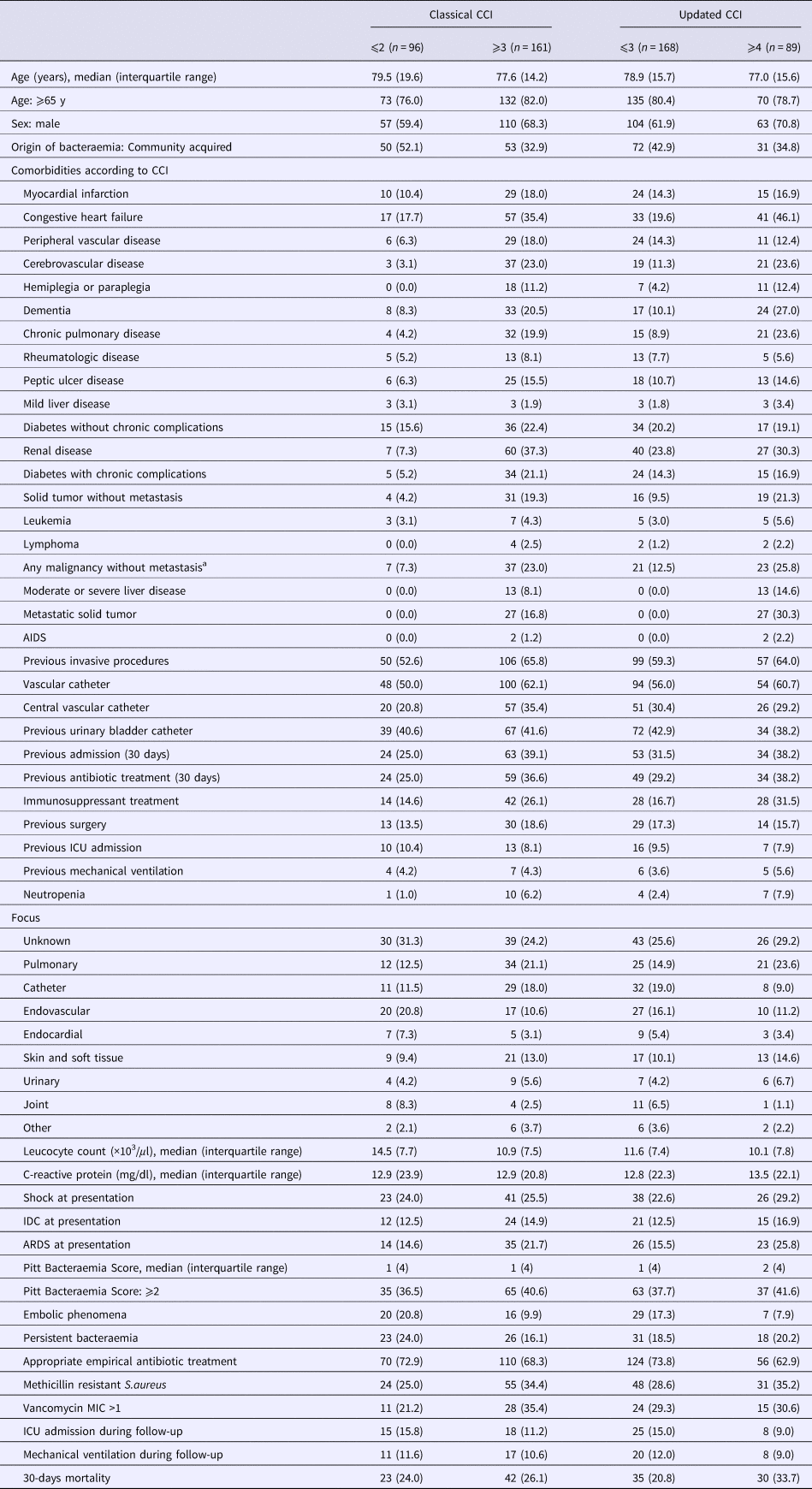
cCCI, classical Charlson comorbidity index. uCCI, updated Charlson comorbidity index. AIDS, acquired immunodeficiency syndrome. ICU, intensive care unit. DIC, disseminated intravascular coagulation. ARDS, acute respiratory distress syndrome. MIC, minimum inhibitory concentration.
a Including leukemia and lymphoma.
Adjusted models of 30-day mortality
The following variables were entered into the multivariate models: age, the origin of SAB, pulmonary foci, Pitt bacteremia score and uCCI or cCCI. The results of stepwise logistic regression analyses comparing the performance of the cCCI and uCCI are shown in Table 4: the cCCI and uCCI were included as continuous (models 1 and 3, respectively) and dichotomous (models 2 and 4, respectively) covariates in the multivariate models. In models 1 (ORm1 1.10, 95% CI 0.97–1.26) and 2 (ORm2 1.08; 95% CI 0.57–2.05), the cCCI score was not significantly associated with 30-day mortality. Age (ORm1 1.04, 95% CI 1.01–1.06; ORm2 1.04, 95% CI 1.01–1.06, respectively), pulmonary focus (ORm1 2.11, 95% CI 1.00–4.45; ORm2 2.27, 95% CI 1.09–4.73) and PBS (ORm1 3.52, 95% CI 1.91–6.52; ORm2 3.45, 95% CI 1.87–6.36, respectively) were independent and significant risk factors for 30-day mortality in both models; community acquisition of SAB was also a significant risk factor in model 1 (ORm1 1.86, 95% CI 1.00–3.44). In model 3, the continuous uCCI score showed a non-significant trend as a 30-day mortality risk factor (ORm3 1.10, 95% CI 0.96–1.26). In model 4, the dichotomous uCCI score was a significant predictor of 30-day mortality (ORm4 1.98, 95% CI 1.05–3.74); other risk factors significantly associated with mortality in this model were age (ORm4 1.04, 95% CI 1.01–1.06) and PBS (ORm4 3.42, 95% CI 1.85–6.34). In general, the calibration and predictive power of all models were good, although those of model 4 (dichotomous uCCI) were slightly better (lowest AIC and highest C statistics).
Table 4. Performance of the classical and updated Charlson Comorbidity Index in predicting 30-days mortality in Staphylococcus aureus bacteremia
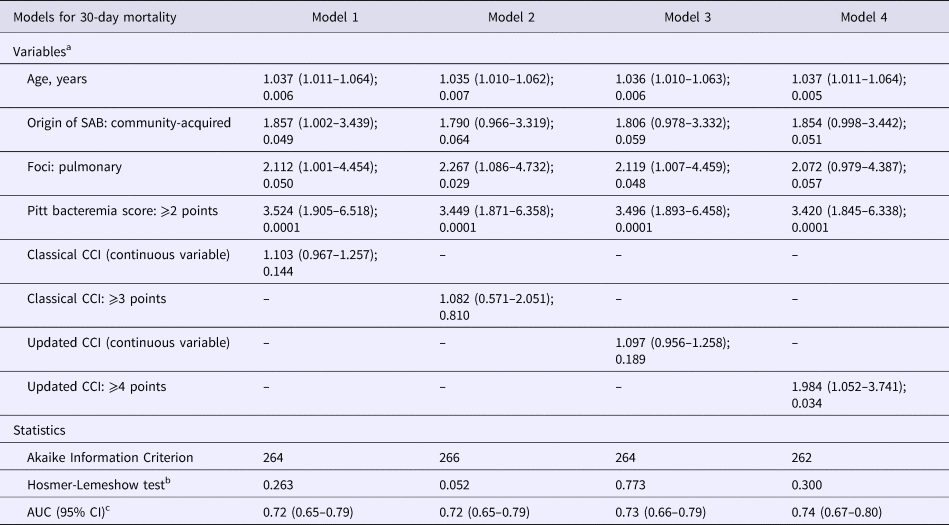
SAB, Staphylococcus aureus bacteremia. CCI, Charlson comorbidity index. AUC, area under the curve.
a Data denote Odds ratio (95% confidence interval); P value from Wald test.
b Data denote P value from Hosmer and Lemeshow goodness of fit test.
c Data denote the area under the receiver operating characteristic curve (95% confidence interval).
Discussion
The most notable finding of this study is that the uCCI, a more up-to-date, refined and parsimonious prognostic mortality score than the cCCI, may serve better than the latter as an independent factor of mortality in patients with SAB. This is particularly useful for prospective observational studies designed to assess the relationships of risk factors with mortality, which usually require the introduction of several variables to adjust for comorbidity and severity. In these designs, the use of aggregate risk scores enables the simultaneous introduction of several variables into predictive regression models by summarising all of them in a single variable. These scores allow the researcher to obtain a single measure of comorbidity and prevent statistical overfitting [Reference Babyak24, Reference McGregor25]. The cCCI, published in 1987, has been used widely for that purpose [Reference Charlson18] and has been shown to be valuable in predicting mortality in different clinical situations, including SAB. However, its usefulness is currently limited for two main reasons. First, the estimated mortality for every condition included in the score is obsolete. For example, in the era of proton pump inhibitors and highly active antiretroviral therapy, the continued use of peptic ulcer disease as a predictor of mortality and the multiplication of the mortality risk by 6 in patients with acquired immunodeficiency syndrome are questionable practices. The second limitation is practical; considering the parsimony criterion, reduction of the number of variables included in the CCI is desirable. Quan et al. [Reference Quan12] updated the CCI by reducing the original 17 comorbidities to 12 and giving new weights to the retained conditions. Because of advances in chronic disease management and treatment, several diseases, such as myocardial infarction, peripheral vascular disease, cerebrovascular disease, peptic ulcer diseas and diabetes without chronic complications, are not included in the uCCI [Reference Quan12].
Whereas some previous studies of patients with SAB have linked the cCCI score with 30-day mortality [Reference Lesens8, Reference Melzer and Welch9, Reference Kim26], other reports have found no association between comorbidity assessed by the cCCI and mortality in the same situation [Reference Sharma, Szpunar and Khatib27]. This discrepancy could be due to differences in the characteristics of the patients included. In this study, the cCCI score was not an independent factor of mortality, which could be due, at least in part, to the presence of other factors that are better predictors of mortality in this sample, such as age and pulmonary foci. Indeed, our study has a greater frequency of older patients and high-risk mortality foci compared with other studies [Reference Lesens8, Reference Kim26]. In this setting, the finding of a significant association with the uCCI score, but not the cCCI score, may be explained by more careful selection of variables currently related to mortality in the former. This improvement seems to favour the uCCI score as an independent and significant predictive factor of mortality.
In this study, the performance of the cCCI and uCCI was compared using both continuous variables and cut-off points. The use of cut-off points makes it easier for clinicians to make decisions at patients’ bedsides. cCCI cut-off points have been used widely in previous research; the cut-off point used most frequently to identify patients with the greatest probability of dying is ⩾3 [Reference Lesens8, Reference Lu21]. To enable comparison with previous studies, we used the same cut-off point in this study but it failed to be a significant risk factor of mortality. In contrast, uCCI score ⩾4 was a good predictor of 30-day mortality. The use of uCCI is more feasible than cCCI because of simplicity, but we must also acknowledge that our study is limited by the fact that this uCCI cut-off point has not been used previously and by the lack of a replication or validation cohort. Indeed, few data are available for comparison, as the uCCI has been validated previously using hospital discharge databases [Reference Quan12] and to our knowledge has only been applied in a few cases to patients with infectious diseases, such as pneumococcal infections [Reference Cremers28], but not to those with staphylococcal infections. Thus, until further validation, the results of our work do not allow clinicians to use this score as a part of a prediction rule or index to predict mortality. Other prospective studies are needed to confirm the usefulness of the uCCI compared with the cCCI in the field of severe acute infections and, specifically, SAB. We have also to acknowledge, as a potential limitation of our paper, that developing clinical prediction models requires careful design (e.g., prespecification of a limited set of predictors) and that several aspects of our analysis such as dichotomisation of continuous predictors or a relatively small sample size may lead to suboptimal performance [Reference Gelman and Carlin29, Reference Steyerberg30]. Despite the need of a validation cohort and the potential limitations of our paper, the recent increase in published reports confirming the utility of the uCCI in cases of other infectious [Reference Morr31, Reference Ebara32] and non-infectious [Reference Jurisson33] diseases, and the findings of the present study, make it likely that the uCCI is applicable in cases of SAB.
In conclusion, the uCCI appears to be a better predictor than the cCCI of 30-day mortality among patients with SAB. It is also a more parsimonious and up-to-date index, which should favour its application in the clinical and research contexts of this disease.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268818002480.
Acknowledgements
This work was supported by the Castilla León Regional Health Authority (Gerencia Regional de Salud de Castilla y León; grants GRS881/A/13 to H.G.T.V. and INT/M/17/17 to M.M.) and by the Institute of Biomedical Research of Salamanca (IBI14/0001 to M.M.).
Conflict of interest
None.